Serosurveillance is a strategy of epidemiology that seeks to monitor the dynamics of disease transmission using serological tests that establish the interrelation of the prevalence or incidence of infection and the behavior of susceptibility or immunity in population groups 1. This can help to determine the level of antibodies required to achieve herd immunity, to identify groups of susceptible individuals, to evaluate the persistence and duration of protective antibodies, to determine the effectiveness of vaccination, to document the elimination of diseases, and to define the need to modify the primary schemes including reinforcements or modifying the frequency of their application 1-3.
Serosurveillance was promoted in the 1960s by the World Health Organization (WHO) by establishing three reference serum banks based on the experience of seroepidemiological studies conducted since the 1930s and later promoted in the post-war period 4-7. These programs have been part of the vaccination programs; however, due to the reemergence of diseases, the conformation of a global serological bank has recently been proposed 8.
The data used to monitor the elimination and control of diseases come from epidemiological surveillance and vaccination coverage, which can be limited by inaccuracies in the population estimation, low vaccination effectiveness, reduced duration of protection, changes in the sensitivity and specificity of surveillance over time, and presence of subclinical or subnotified cases, among other 9.
The strategies used by serosurveillance are the determination of antibodies with samples for convenience from residual sera available in laboratories or the use of probabilistic population samples through seroprevalence surveys 10.
In the present study, we analyzed the experience with pertussis serosurveillance using a probabilistic hospital approach in the metropolitan area (Valle de Aburrá) of Antioquia, identifying the capabilities, opportunities, and challenges for advances in the periodic and systematic use of this approach, and reviewing several publications on the subject.
In Colombia, pertussis vaccination began in 1980 with the DPT vaccine (diphtheria, pertussis, and tetanus) using a three-dose schedule (2, 4, and 6 months) and two vaccine boosters (18 months and 5 years). Subsequently, with the resurgence of the disease between 2010 and 2012, vaccination of pregnant women with the acellular vaccine began in 2013 in Antioquia and Bogotá, which was later extended to the whole country 11.
In Antioquia, the second most populated department of Colombia with more than 7 million inhabitants, we conducted a serosurveillance program in the metropolitan area (Valle de Aburrá) between 2015 and 2016, which estimated the prevalence of IgG PT antibodies against pertussis in mothers and the umbilical cord as a measure of antibody transfer. Additionally, the health status of the children was monitored during the first six months of life 12.
The purpose of describing the serosurveillance experience was to facilitate the extension of this approach to other regions of Colombia and similar countries.
Materials and methods
We described the experience of planning and conducting pertussis serosurveillance in the metropolitan area of Antioquia (Valle de Aburrá) between 2015 and 2016 including the methodological aspects, ethics committee approval, disclosure, data collection and management, collection of blood samples, and custody and conservation of information. The results of the pertussis seroprevalence study are shown in another publication 12.
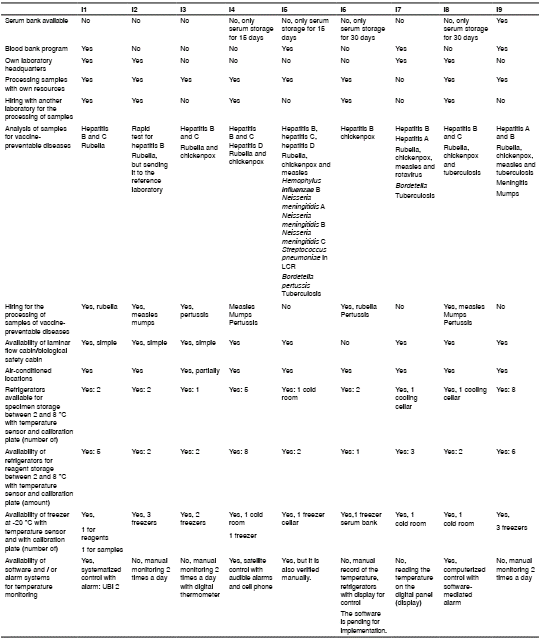
The capabilities to consolidate a serosurveillance program in eight randomly- selected hospitals and the Public Health Laboratory of Antioquia were analyzed after collecting the information with a structured interview. A questionnaire was designed to be answered by those in charge of clinical and public health laboratories. It included the following variables: Availability of locative and technological resources for the reception, processing, and conservation of blood samples; availability of hospital beds for delivery and postpartum care; previous experiences in serosurveillance and processing of samples from vaccine- preventable diseases, and the identification of opportunities and challenges related to the implementation of serosurveillance as a regular and systematic program.
We also analyzed the contributions and challenges regarding the orientation of vaccination and epidemiological surveillance of hospital-based serosurveillance in light of other published experiences of probabilistic and non-probabilistic serosurveillance programs.
Results
Description of the procedures for hospital serosurveillance
The target population for the survey included mothers who came to the hospital for labor, were 37 weeks or more of gestation and agreed to participate in the study. We did not include mothers of multiple gestations, with fever in the previous 72 hours (chorioamnionitis and sepsis), treated at the intensive care unit, and in the advanced stage of labor.
The study sample was made up of vaccinated and unvaccinated mothers against acellular pertussis during pregnancy (n=1,000). The size estimation of the sample was made according to the data of antibodies averages of vaccinated and unvaccinated pregnant women and their range or standard deviation according to reports from previous studies (minimum value of 2, maximum of 360, variance of 7,569 to 8,010, 95% confidence, and 80% potency) 13-15. The sample size was 500 vaccinated mothers and 500 unvaccinated mothers. However, due to the difficulty of recruiting unvaccinated mothers during the study period (two years after starting the vaccination of pregnant women against pertussis), unvaccinated mothers had to be recruited before vaccination and at the time of delivery in two of the randomly-selected hospitals.
The sampling was multi-stage and stratified by municipalities and by hospital conglomerates. The primary sampling units were hospitals in the municipalities of Valle de Aburrá. The secondary sampling units were all the mothers who came to hospitals for delivery care and met the inclusion criteria. The affixation of the sample was proportional to the childbirths attended within each hospital in the municipality.
One municipality had to be excluded because the public hospital chosen randomly had to suspend the childbirth service due to financial deficits. Furthermore, two selected private hospitals could not participate because the childbirth services were in the process of closing down.
For the random selection of hospitals, we considered the number of deliveries in 17 urban hospitals of the metropolitan area of Antioquia (Valle de Aburrá) and the data from nine municipalities of this sub-region including: Tdap vaccination coverage among pregnant women, incidence of pertussis, and distribution of the population according to the type of affiliation to the health social security system.
After signing the informed consent, mothers were surveyed on basic aspects including sociodemographic factors, antecedents of pregnancy, childbirth, vaccination, and disease. Other information sources were the daily delivery books (manual or electronic depending on availability), the national electronic registry of the expanded program of immunizations (PAIWeb), and hospitals’ medical records.
We prepared a procedure manual including a detailed description of participants selection and the procedure for obtaining informed consents; technical procedures for the randomization and identification of participants; data collection and completion of the survey; sample handling procedure and construction of the serum bank; follow-up of the newborn and the procedures related to the handling of the data, the analysis plan, and the mathematical modeling of prevention and control options.
The proposal was endorsed by the Ethics Committee of the Facultad Nacional de Salud Pública “Héctor Abad Gómez” of the Universidad de Antioquia (session 129-15 Oct., 2015) and the ethics or research committees of the health institutions selected.
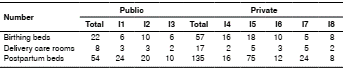
We sought the support of the directorates or medical councils, the personnel in charge of epidemiological surveillance, the hospitals’ infection control units, the immunization programs, and the quality control and occupational health units. The number of birthing beds, delivery rooms, and postpartum beds varied according to the public or private nature of the health institutions (table 1).
Table 1 Characteristics of hospitals participating in the pertussis serosurveillance, Valle de Aburrá, 2015-2016
All health institutions had data from mothers on manual or electronic medical records. The characteristics of the information systems, the clinical history, and the equipment of specific areas are summarized in table 2.
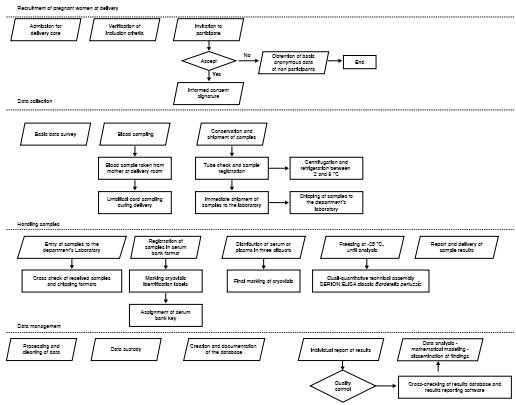
We took the samples for serosurveillance within the regular process of delivery care. We explored the procedures that were part of the route of inclusion and attention of mothers, as well as the technical laboratory procedures as explained by the health personnel (figure 1).
We checked on the physical space, required inputs, and technical personnel involved in all the stages of the clinical and laboratory processes. In this way, we minimized the interference in the procedures already established in these places, ensuring the fluidity and continuity of the serosurveillance program.
We disseminated the information on the disease, the vaccination, and the project using printed educational materials and a web page (www.epiteorica. com) addressed to health personnel, family, and visits.
We provided information to the mothers only when they were willing to listen to it according to their progress in labor. After providing the information, we obtained the consent and assent of the underage mothers and we conducted a brief survey. It was not possible to obtain blood samples from the children due to ethical and cultural objections; for this reason, we only carried out the clinical monitoring of the occurrence of pertussis during the first six months of age of the babies. The consent included the endorsement of later use of the sera collected for future investigations related to vaccine- preventable diseases. Besides, we obtained basic information from non- participating mothers.
The reagents to process the samples were selected taking into account the highest sensitivity and specificity reported in the literature 16. To ensure traceability of samples in all processes after they entered the laboratory, data were recorded in terms of collection, centrifugation, temperature and cooling time, transport, fractionation, and final inclusion in the serum bank. In each specimen, a code was assigned according to its type (G if it was from the mother and C if it was a cord), fraction number (F1, F2, F3 or FU if the sample was a single fraction), position in the cryobox (from A1 to J10), and date of entry to the serum bank.
Databases on the handling of samples from the serum bank, data on laboratory results (antibodies from the mother and the umbilical cord), and the survey of mothers and children followed for six months were created and combined as necessary. The results of the laboratory and the interpretation of their meaning were sent to each mother through a letter delivered by regular mail or with the support of the vaccination service staff in some cases. We defined the databases documentation, analysis, and custody procedures omitting personal identification information.
The analysis took into account: a) The inference of the results of the sample to the population by estimating the inverse probability of being chosen and the weighing of variability at each stage of random sampling. This allowed us to estimate the confidence interval of proportions and weighted averages; b) the estimation of the trans-placental transport ratio as that of umbilical cord blood to maternal antibodies, and c) the mathematical modeling of disease-transmission dynamics by formulating a system based on differential equations of the relevant states and parameters and the deduction of the reproductive number that allows for estimating the critical proportion of immunity to achieve control, herd immunity or disease elimination.
We systematized the data in Access™ (Microsoft) databases while surveys and consents were digitized for 15-year-period conservation.
In the survey regarding the capacity of the institutions to implement a serosurveillance program, we observed that none of them had any program. All participating laboratories had an area for sample reception and fractionation and processing with adequate lighting and ventilation, as well as areas for the conservation of samples and reagents for both refrigeration and freezing under controlled temperature conditions. All had air conditioning, except Institution 3, which had partial flow (table 2).
Only the department’s Public Health Laboratory had ultra-freezers at -70 °C with sensor and/or temperature monitoring using a system or specific software. However, the installed capacity of its three freezers was nearly 100% occupied.
All laboratories met the requirement of regular preventive maintenance of refrigerators and freezers with calibration support. They also had a power regulator system (supply plant) to support electrical faults or interruption in the light current.
The participating institutions agreed on the aspects necessary for the implementation and strengthening of a serosurveillance program, such as training in the subject, centralization of the program in a reference entity, and dissemination of information highlighting the usefulness and importance of the program for evaluating the impact of vaccination and the epidemiological characterization of public health events. They also mentioned the need to have regulations to support custody, sample handling, and accreditation systems, as well as the provision of equipment and suitable assets to give solid support to the program and carry out cost-benefit analyses. All of them expressed interest in participating in the serosurveillance program. The main challenges identified were funding to ensure program sustainability and health personnel under indefinite-term contracts with sufficient time for program activities.
Experiences of probabilistic population serosurveillance
México, the Netherlands, and the United States have documented the contribution of serosurveillance based on random population studies (table 3). In 1987, México conducted a national seroepidemiological survey and implemented a national serum bank, although it had advanced pertussis seroprevalence studies since the 1960s 17,18. In 1995 and 1996, the Netherlands conducted a study to evaluate the national serosurveillance immunization program including the creation of the national serum bank; the study was subsequently replicated in 2006 and 2007 19,20. In the United States, the National Health and Nutrition Survey (NHANES) conducted a serological study on antibodies against certain vaccine-preventable diseases in the 1960s and an annual program since 1999. This program received legal approval for data collection and management (The Public Health Service Act 42 USC 242k) 21-25.
Table 3 Studies on serosurveillance experiences according to the approach used (random or not sampling)
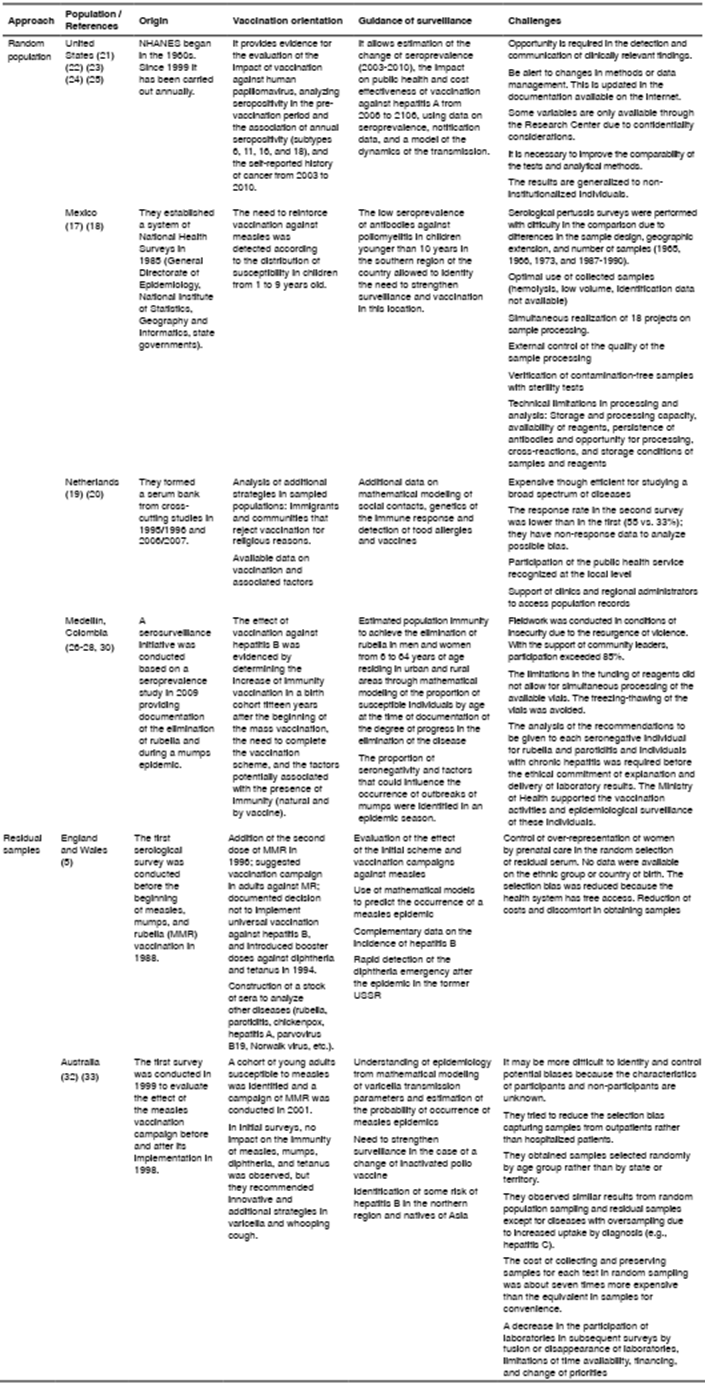
NHANES: National Health and Nutrition Examination Survey
Based on these experiences, a population seroprevalence study was conducted in 2009 in Medellín, which estimated the prevalence of antibodies in the population 26-30 and obtained detailed information on the history of vaccination and the biological and social factors that could influence the presence of antibodies and guide vaccination policies based on the evidence of local epidemiology, as well as the use of mathematical modeling to estimate the critical proportion of immunity and the basic and effective reproductive number indicating the level of achievement of disease elimination 27,31. The percentage of participation in this population-based seroprevalence study was 88,5% 26 during a time of armed conflict in the city, which implied high costs, greater efforts and additional time.
Experience with non-probabilistic institutional serosurveillance
In 1986 and 1987, England and Wales implemented a convenience sampling to obtain sera to monitor changes in the prevalence of antibodies before and after the measles vaccination program (table 3) 5. In the 1990s, Australia considered serosurveillance as an important component of its surveillance system for vaccine-preventable diseases and in that same year, serum samples were compiled from associated laboratories to evaluate measles vaccination strategies 32,33 (table 3).
In Europe, a serosurveillance network was formed in 1996 with the initial participation of six countries to standardize serological methods, which was started in some cases from residual samples available in laboratories and in others, from population studies 34 (table 3). A typical case was measles serosurveillance with samples obtained from the 1996-2000 and 2001-2003 periods in 18 countries in order to identify susceptible cohorts and adjust vaccination strategies accordingly. Also, these data were compared with the incidence reported in the surveillance and vaccination coverage. The analysis revealed that seven countries had a high susceptibility prevalence and low vaccination coverage, which led to the outbreaks in some of these countries 35.
Discussion
The uses of serosurveillance have been widely analyzed in the literature in terms of their contributions to vaccination and epidemiological surveillance programs and it is considered the most reliable strategy to monitor the population impact of vaccination 8,9.
The advantages and disadvantages of population and institutional serosurveillance approaches have been analyzed extensively 10,33.
In this study, we used a hospital approach, which had the advantage of obtaining estimates of the seroprevalence from the mother and the umbilical cord during labor and from probabilistic samples whose data can be inferred from the target population.
In our experience, the percentage of participation in population-based seroprevalence studies was high despite the resurgence of the armed conflict, which made household surveys risky. Providing detailed information on the usefulness of the study through different means such as household surveys, the use of newsletters, and telephone calls fostered participation. The participation of pregnant women in the determination of antibodies against pertussis at the time of delivery and the follow-up of the newborn was achieved using "active" recruitment strategies 36, such as the involvement of the family and the health personnel, showing respect for the process of childbirth care, the willingness of hospitals to participate, the support of the health secretaries, and the documentation of the research experience undertaken by the university team in charge of the project through different means (web page and others).
We also reviewed experiences of non-probabilistic institutional serosurveillance generally based on residual samples available in laboratories or blood banks from health services users. This approach requires less time, effort and investment than the population approach but it has limited possibilities of population inferences given its convenience sampling.
This study reported on the experience of a hospital serosurveillance approach during 2015 and 2016 based on the combination of the institutional approach and random selection of participating hospitals. This approach had the advantage of optimal handling of the samples in the hospitals’ laboratories and their subsequent transfer to a public health laboratory for processing. In addition, the risks of health personnel in charge of collecting the data areas affected by armed confrontations were reduced, as well as the costs and the time required for fieldwork. The existence of hospital admission records allowed for the analysis of differences between participating and nonparticipating mothers to identify and control a potential selection bias 12. The mathematical modeling constructed in local seroprevalence studies allowed to take advantage of the data collected in their course and in epidemiological surveillance for identifying disease control and elimination options 27,37.
However, several challenges remain and should be faced to advance in this type of initiatives. First, it is necessary to have a regulatory framework to support serosurveillance as a complement to epidemiological surveillance of vaccine-preventable diseases. Second, the hiring of indefinite term health personnel is required to ensure the sustainability of the serosurveillance program and the continuity of the activities at all levels. Third, training on the usefulness of serosurveillance and the construction of strategies for resolving difficulties is also required.
These challenges relate to the organization of health services provision. During the serosurveillance, we observed how some obstetric and gynecological services shut down or were reduced, as well as financial deficits which affected the quality of childbirth-related attention.
Hospital serosurveillance can be affected by health services coverage. Nevertheless, the coverage of hospital care during childbirth has increased 38), which may reduce this limitation. Other sources of information on hospital serosurveillance, such as blood banks, allowed us to estimate only the prevalence of diseases associated to the risk of transmission via transfusion and depend on the access to and the acceptance of health services. The sampling at the time of delivery is preferable to collect residuals derived from prenatal control such as umbilical cord samples, which is a practice allowed.
Ethical considerations restricted the collection of umbilical cord samples from the children, but they were monitored during the first six months of life for pertussis 12. Due to budgetary restrictions, we processed only pertussis antibodies, although three vials of serum were available. Other studies have analyzed the transfer of multiple diseases antibodies, such as diphtheria, tetanus, and meningitis 19,39, as well as the utility of influenza 40 and chickenpox 41 serosurveillance.
This study aimed at the identification of serosurveillance capacity in the participating institutions and the creation of a serum bank, as currently there are around 5,000 specimens available for further analysis, which is close to the adequate number required for monitoring population immunity in other countries (19) and responded to the current tendency of studying antibodies transfer from the mother to the umbilical cord or the newborn as recommended in mass immunization or epidemics management strategies, especially when seroprevalence or serosurveillance programs exist 42,43.
This paper described the serosurveillance activities carried out in 2015 and 2016 in the participating institutions, but given that epidemiological and vaccination strategies vary with time, they should be repeated in five or ten years to monitor changes in population immunity against diseases associated with transplacental transfer of antibodies 20.
Serosurveillance can guide vaccination and prevent the occurrence of epidemics and the resurgence of diseases at local and global levels 8. The experience documented in this work regarding hospital serosurveillance programs could be replicated in other places under similar conditions as an opportunity to strengthen public health laboratories and integrate vaccination and surveillance programs of vaccine-preventable diseases.