Dengue is the most important arboviral disease in humans in tropical and subtropical countries and it is considered endemic by the World Health Organization. It has been estimated that 390 million cases occur annually, 96 of which are clinically diagnosed as symptomatic dengue fever 1. Although most infections are asymptomatic, the more severe forms of dengue (formerly called hemorrhagic or shock syndrome) may result in organ failure or death 2 with around a half-million cases reported each year, an estimated average of 10,000 fatal cases per year (between 1990 and 2013), and a peak in 2010 (11,302 fatal cases) 2. Dengue incidence has seen a 30-fold increase in the last 50 years 3 making it a major public health concern currently.
In Colombia, dengue virus infection has an endemic and epidemic behavior with a steady increment in the last 20 years. The Colombian national surveillance system reported the highest historic peak of dengue cases in 2010 (157,000 cases) with 9.777 severe cases and a worrisome number of 217 fatalities (2.28% lethality rate) 4. During 2011, there was a decrease in dengue cases but the lethality rate increased to 3.75% 5. The burden of the 2010 dengue epidemic was 14-fold higher than that of 2011 or 2012 (57.017 vs. 3.989 disability-adjusted life years were lost, respectively). Additionally, the estimation of the 2010 epidemic costs rose to USD$ 65.5 million, almost fourfold higher compared to a regular endemic or epidemic year. Approximately 30% of these costs was linked to loss of income due to fatalities.
Dengue is caused by a virus from the Flavivirus genus of the Flaviviridae family and presents four antigenically different serotypes (DENV-1 to 4). Each serotype can produce asymptomatic infections or clinical signs and symptoms ranging from a mild febrile disease to a severe infection characterized by the imbalance of endothelial function leading to massive plasma leakage, severe hemorrhage, and multi-organ failure 6. The infection can be fatal and involve organs such as the liver, brain, spleen, lungs, and kidneys 7.
Dengue virus can infect different cell types and its pathological manifestations are variable 8,9. In the most severe cases, the damage in the vascular endothelium results in plasma extravasation and hemorrhage, liver function impairment with high transaminase levels, and histologic alterations 10-12.
The histopathological analyses of fatal cases indicate that the liver and spleen are the most affected organs during dengue virus infection. In the liver, it is common to find small necrotic foci, microvesicular steatosis, hyperplasia and apoptosis of Kupffer cells, lymphocyte infiltration in the portal tract and Councilman bodies (necrotic foci, acidophilic bodies, and pyknotic nuclei) 13-16, although intranuclear glycogen can be occasionally found 17.
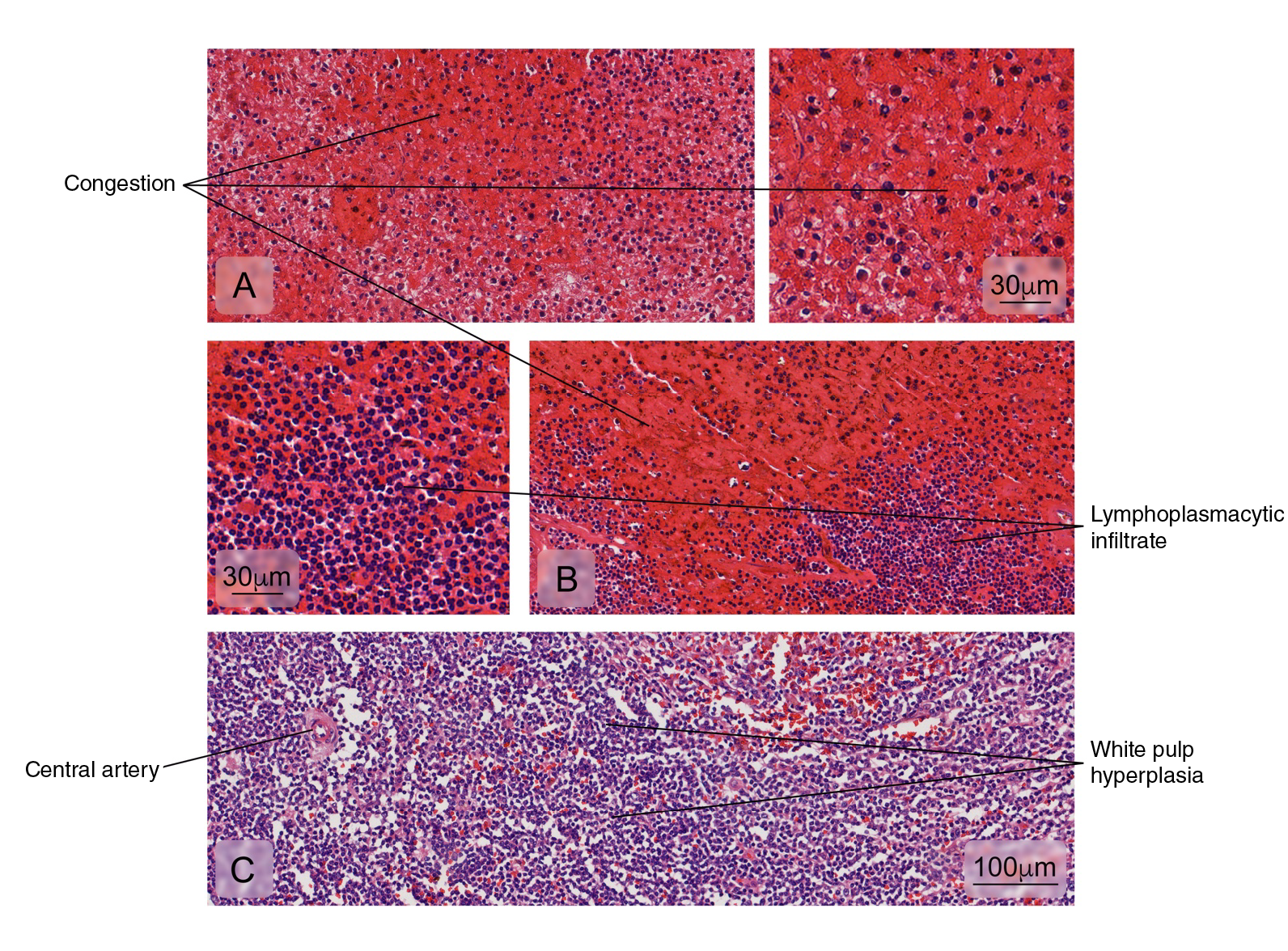
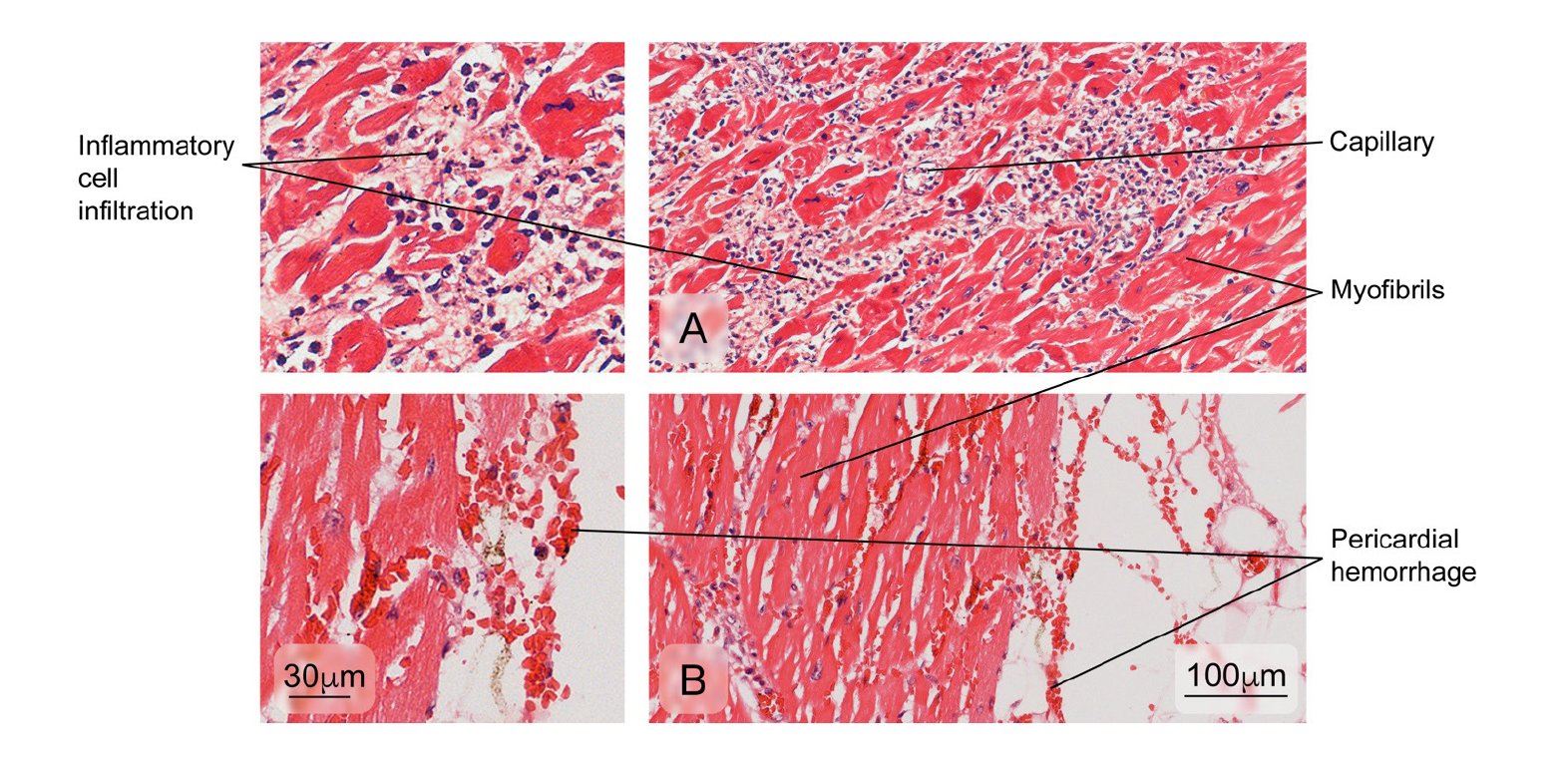
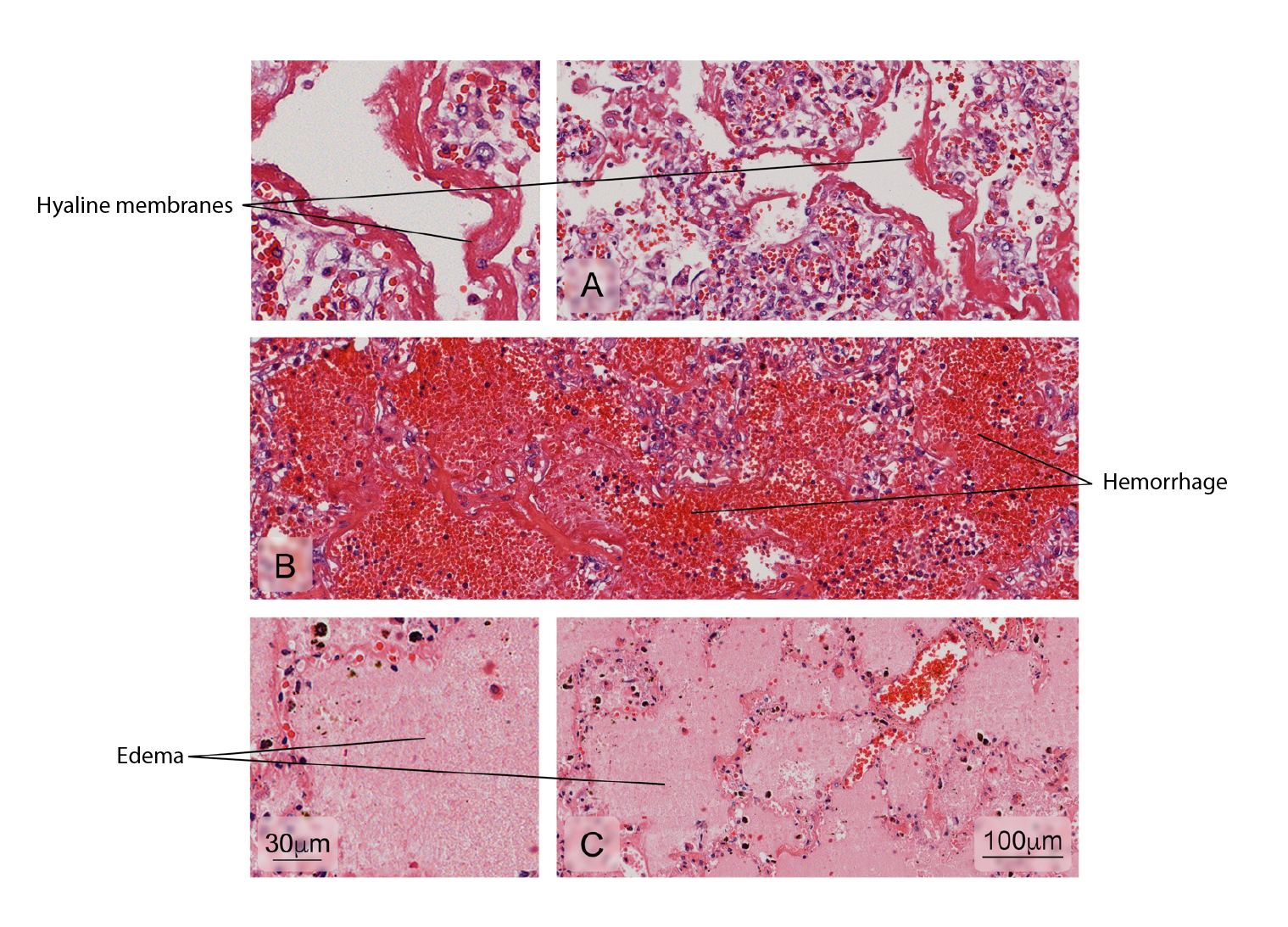
The histopathological analysis of the spleen usually shows interstitial edema and white pulp vascular and cellular congestion with reactive hyperplasia 13,18. Atypical alterations have also been reported in the kidneys, lungs, heart, and the brain with hemorrhage, edema, and leukocyte infiltrates but no specific morphological findings in each tissue 8,19-22. On the other hand, immunohistochemistry for DENV antigens has revealed different distribution patterns: from the location of antigens in a single organ per case to the presence of antigens in multiple organs 8,9,18,22,23.
In the present study, we aimed to describe, illustrate, and compare different histological alterations found in 95 fatal confirmed dengue cases using 87, 42, 32, 37, 22, and 16 samples of liver, spleen, kidney, lung, heart, and brain, respectively. We reviewed histopathological slides with hematoxylin and eosin staining from 95 cases belonging to the pathology archive at the Colombian Instituto Nacional de Salud. The confirmation of death due to dengue virus infection was done using molecular techniques and the analysis of the clinical history of the cases.
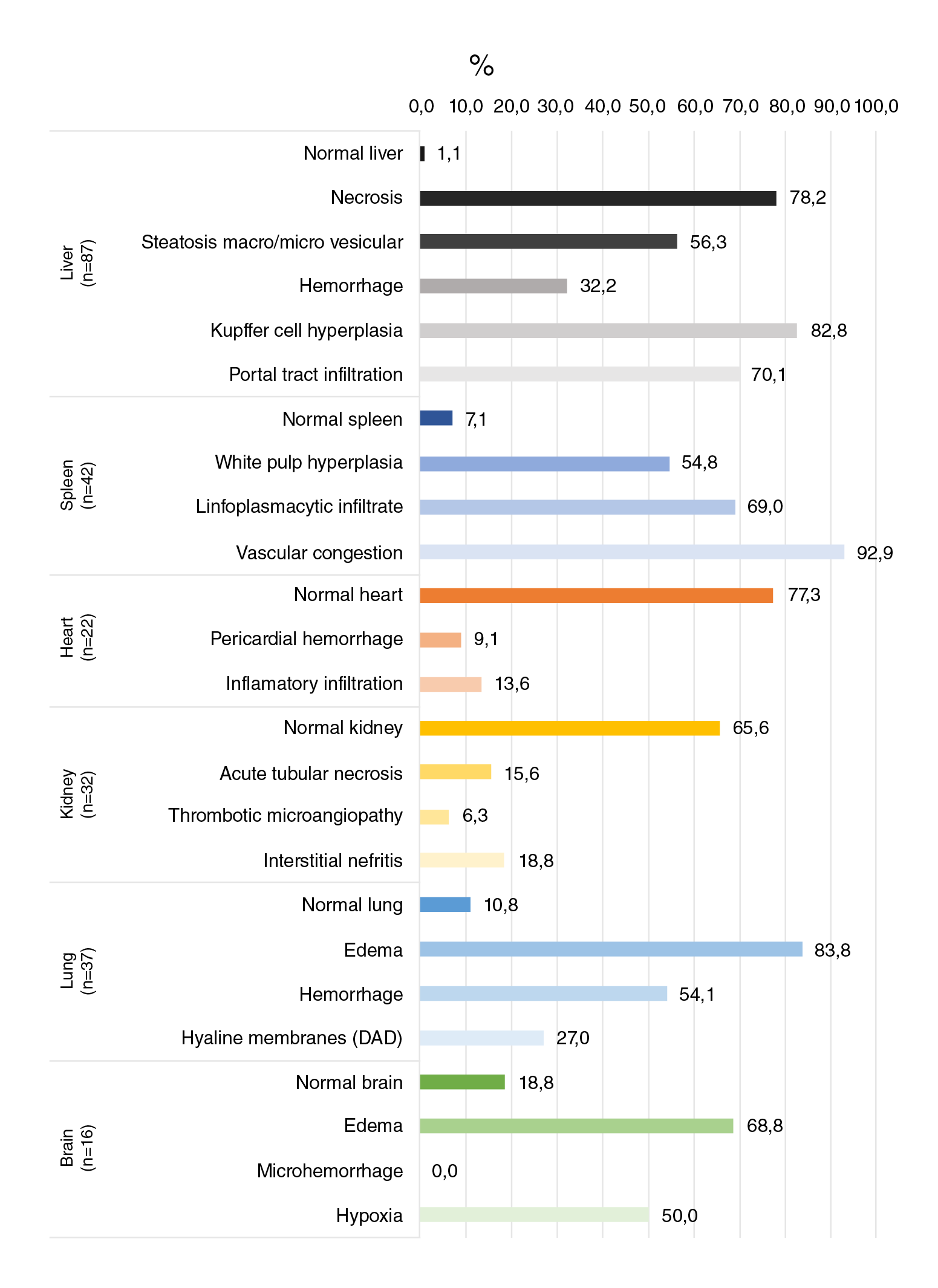
The main alterations found in the liver were necrosis (78.2%) and hyperplasia of Kupffer cells (82.7%) while in the spleen, reactive plasmacytosis (69%) and vascular congestion (92.9%) were the most frequent findings. Edema was the most common alteration in the lungs (83.8%) and the brain (68.8%). Interestingly, most heart (77.3%) and kidney (65.3%) tissues had a normal histopathological aspect without any other specific finding (figures 1-12). Figure 13 illustrates in more detail the frequencies of the alterations found in each tissue.
The organs and its histopathological alterations
The liver
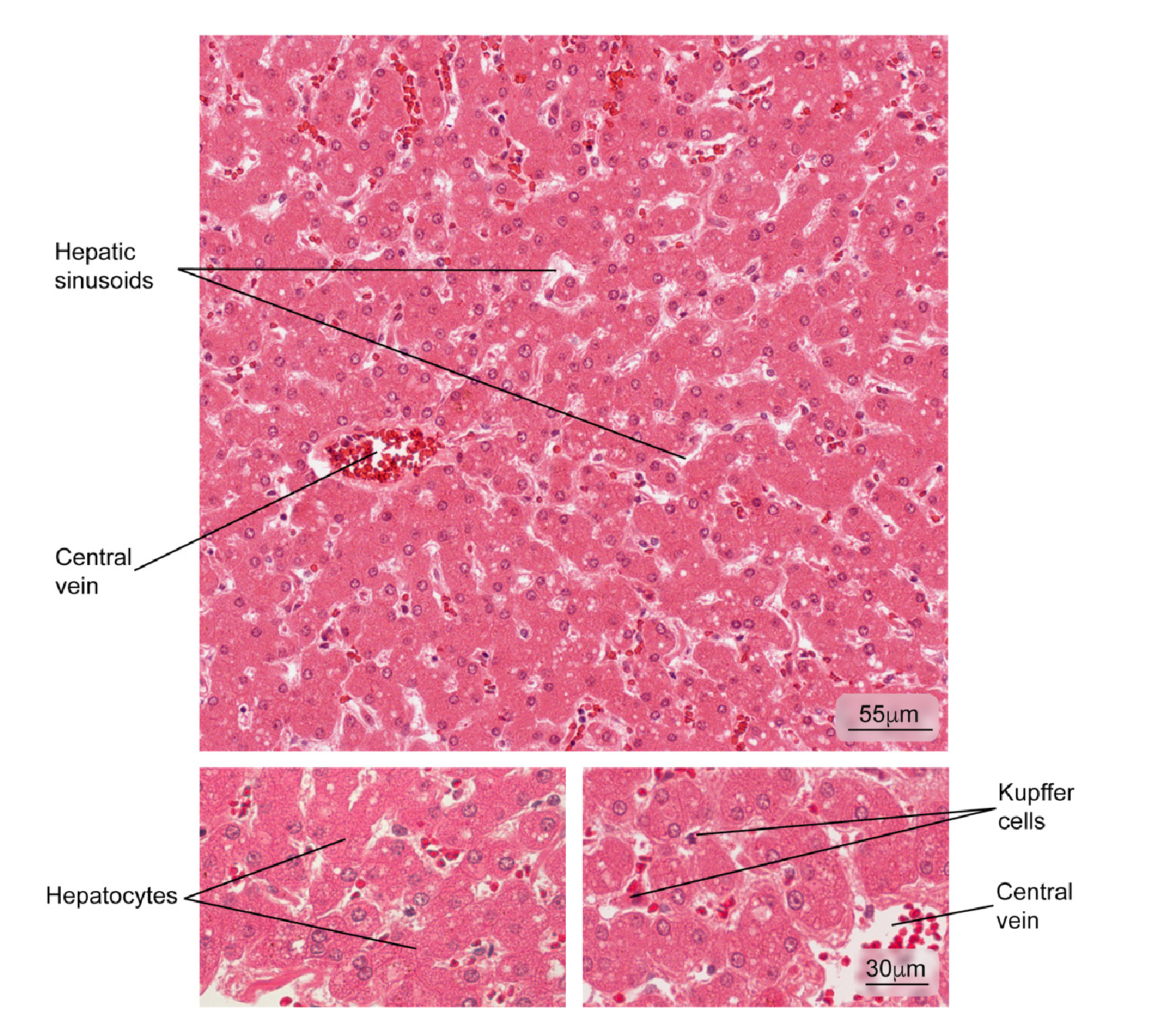
Figure 1 Normal liver tissue. Part of a hepatic lobule is observed; note the radial distribution of the hepatocyte plaques from the central vein. Some phagocytic cells (Kupffer cells) and hepatocytes in greater magnification are observed. Hematoxylin and eosin stain.
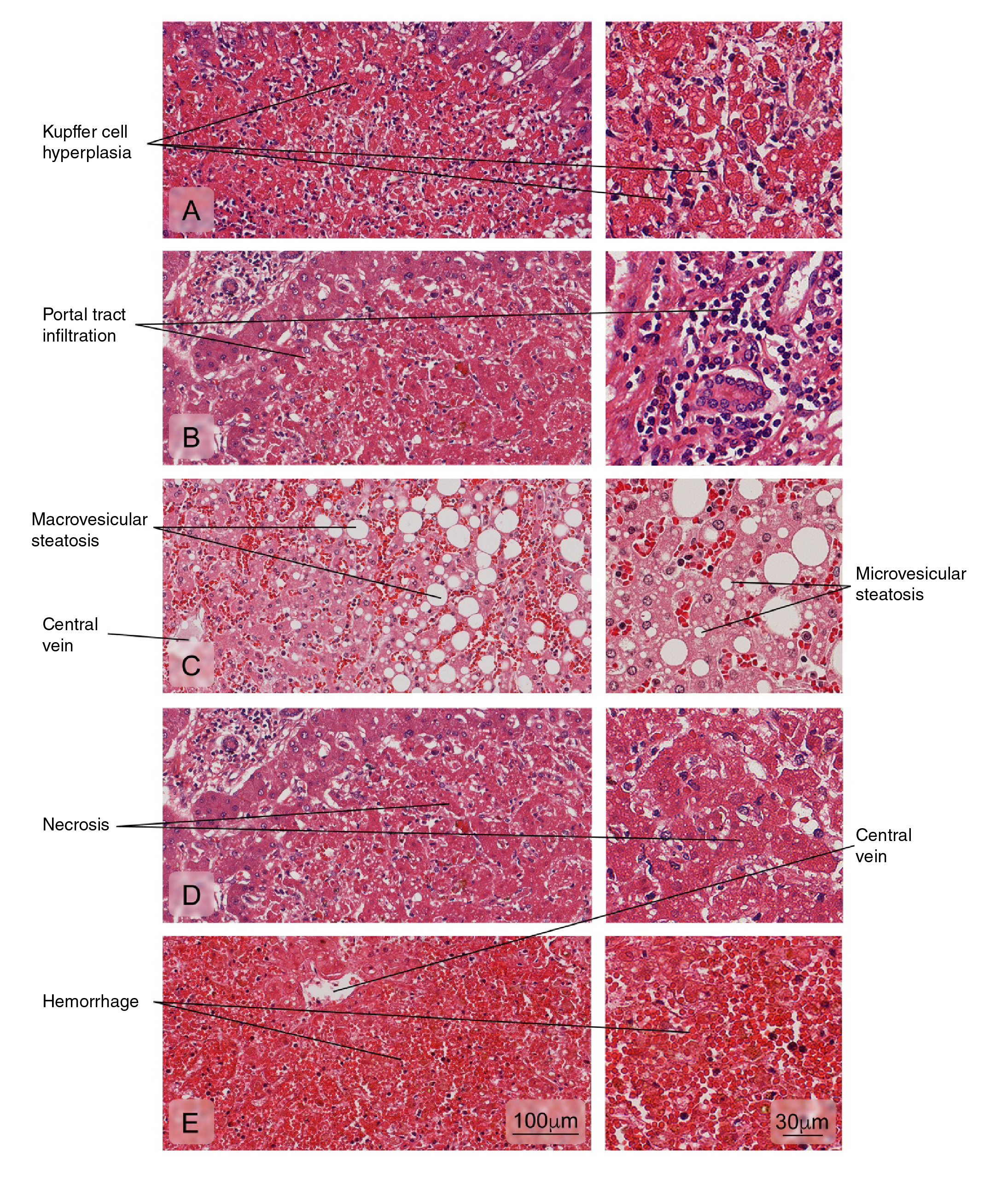
Figure 2 Alterations of the liver tissue showing: A) Kupffer cell hyperplasia; B) Portal tract leukocyte infiltration; C) Hepatic fatty degeneration (macro and microvesicular steatosis); D) An area of necrosis with loss of radial arrangement of hepatocyte plaques, a pale eosinophilic stain, and pyknotic or absent nuclei; E) Hemorrhages. Hematoxylin and eosin stain.
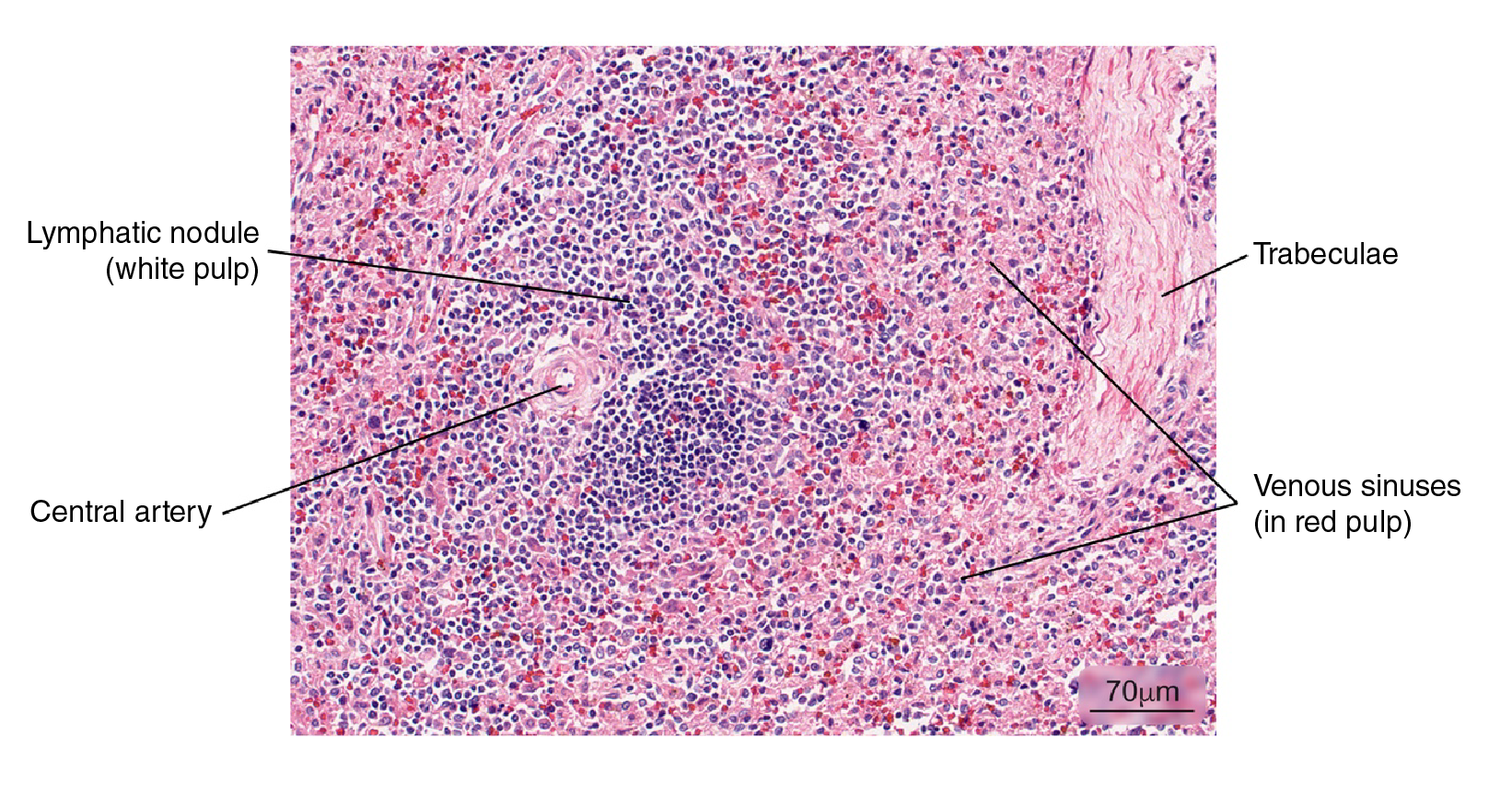
Figure 3 Normal splenic tissue. A small lymph node with a peripherally located central artery is observed. Connective tissue trabeculae are evident. Hematoxylin and eosin stain.
The kidney
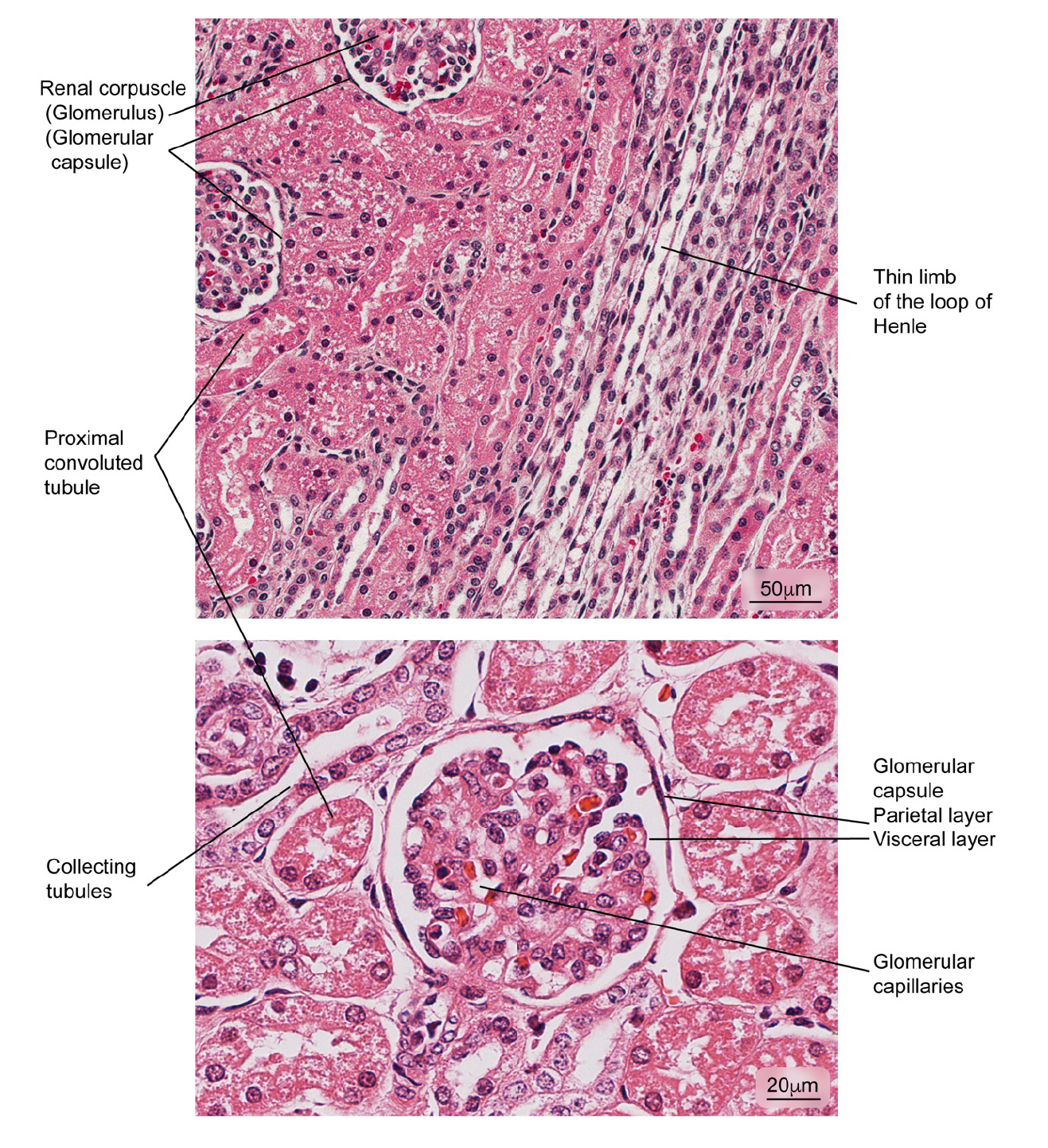
Figure 5 Aspect of normal kidney tissue. Renal corpuscles are observed in medullary areas and renal cortex; the glomerular capsule is shown in detail. Hematoxylin and eosin stain.
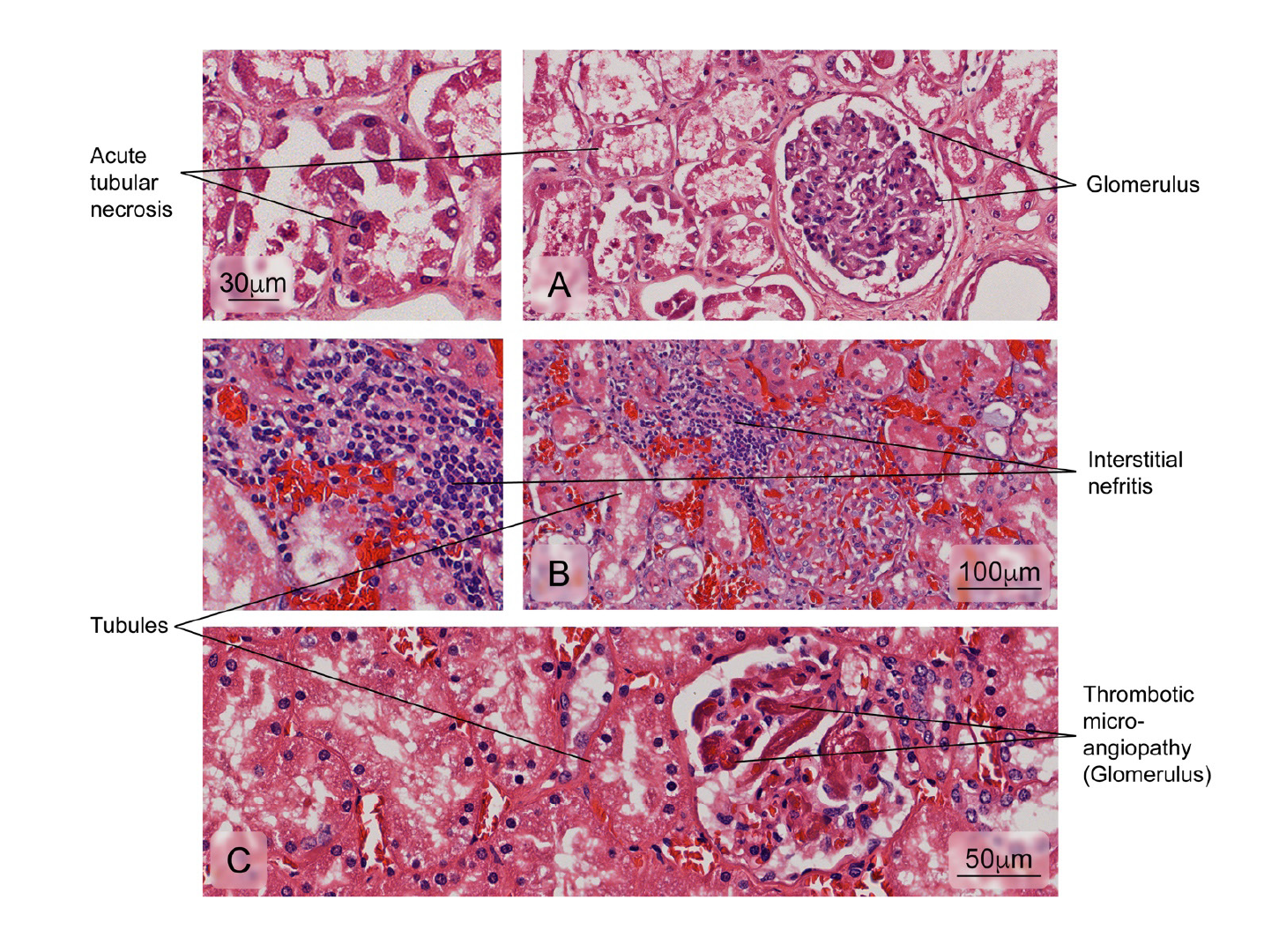
Figure 6 Alterations observed in renal tissue of cases. A) Necrosis of the tubular epithelium; B) Leukocyte infiltration of the interstitial space surrounding the renal tubules (interstitial nephritis), and C) Vascular lesions accompanied by intraluminal platelet thrombosis obstructing the vascular lumen (thrombotic microangiopathy). Hematoxylin and eosin stain. The heart
The heart

Figure 7 Aspect observed in patients with normal cardiac muscle tissue. The myocardium composed of cardiac muscle fibers, venules, and fibroblasts in the endomysium is observed. Hematoxylin and eosin stain.
The lung
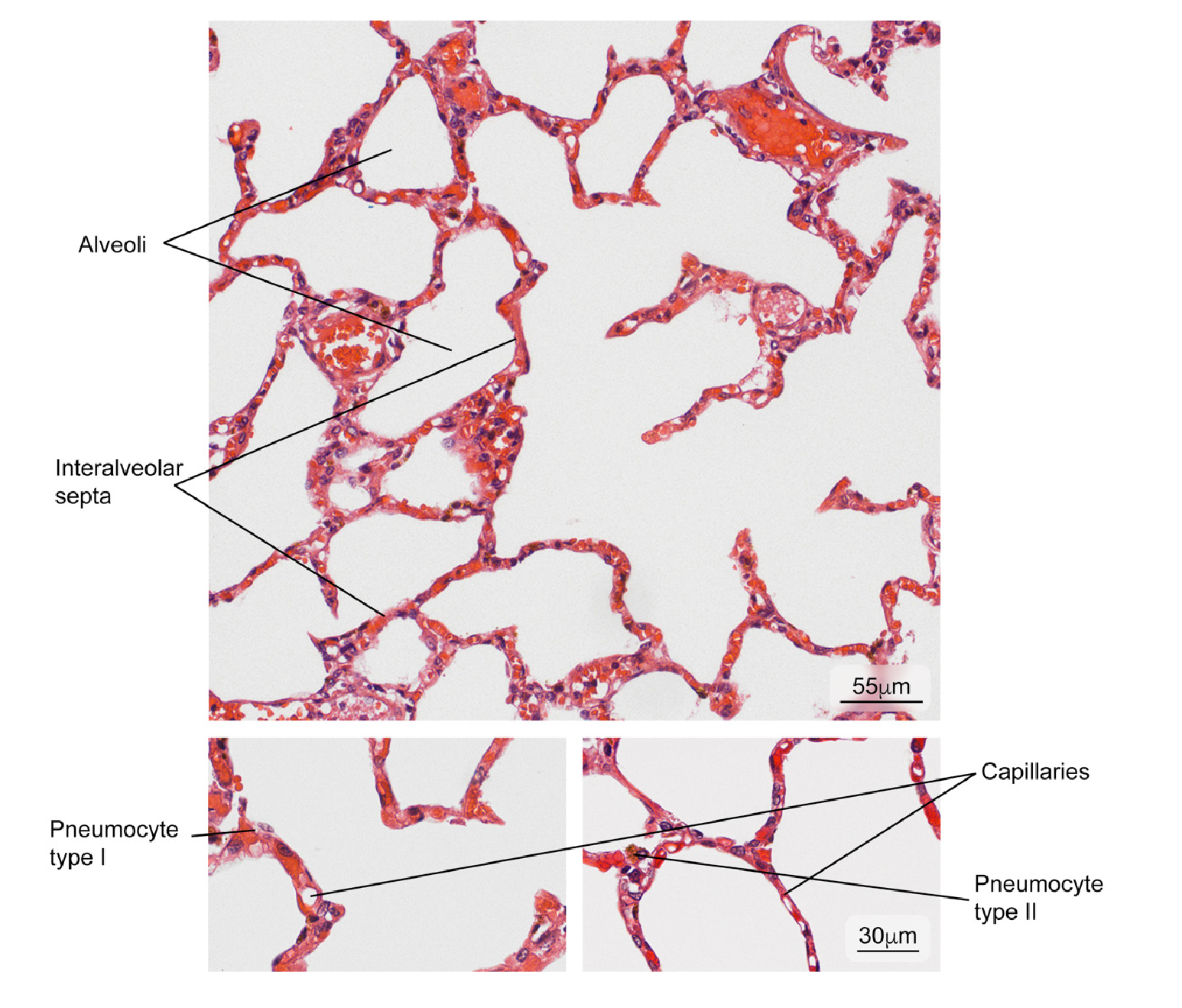
Figure 9 Normal lung tissue. Intrapulmonary structure with gaseous exchange (alveoli) and main cell types in the interalveolar septum (type I and II pneumocytes). Hematoxylin and eosin stain.
The brain
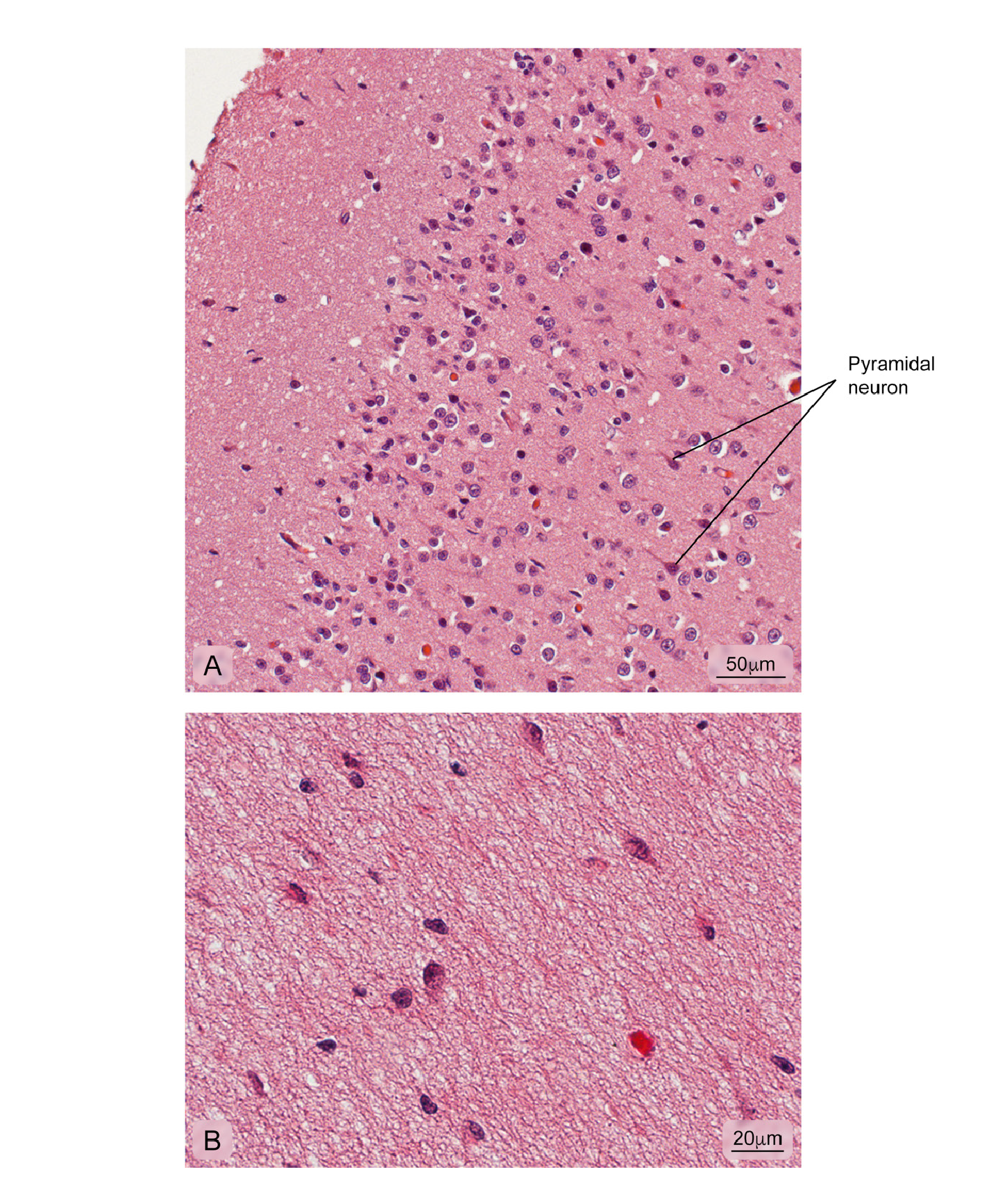
Figure 11 Normal cerebral cortex. A) Cerebral cortex outermost layer showing different kinds of neuronal bodies; B) More internal part of the cortex where the integrity of the neuropil is observed. Hematoxylin and eosin stain.
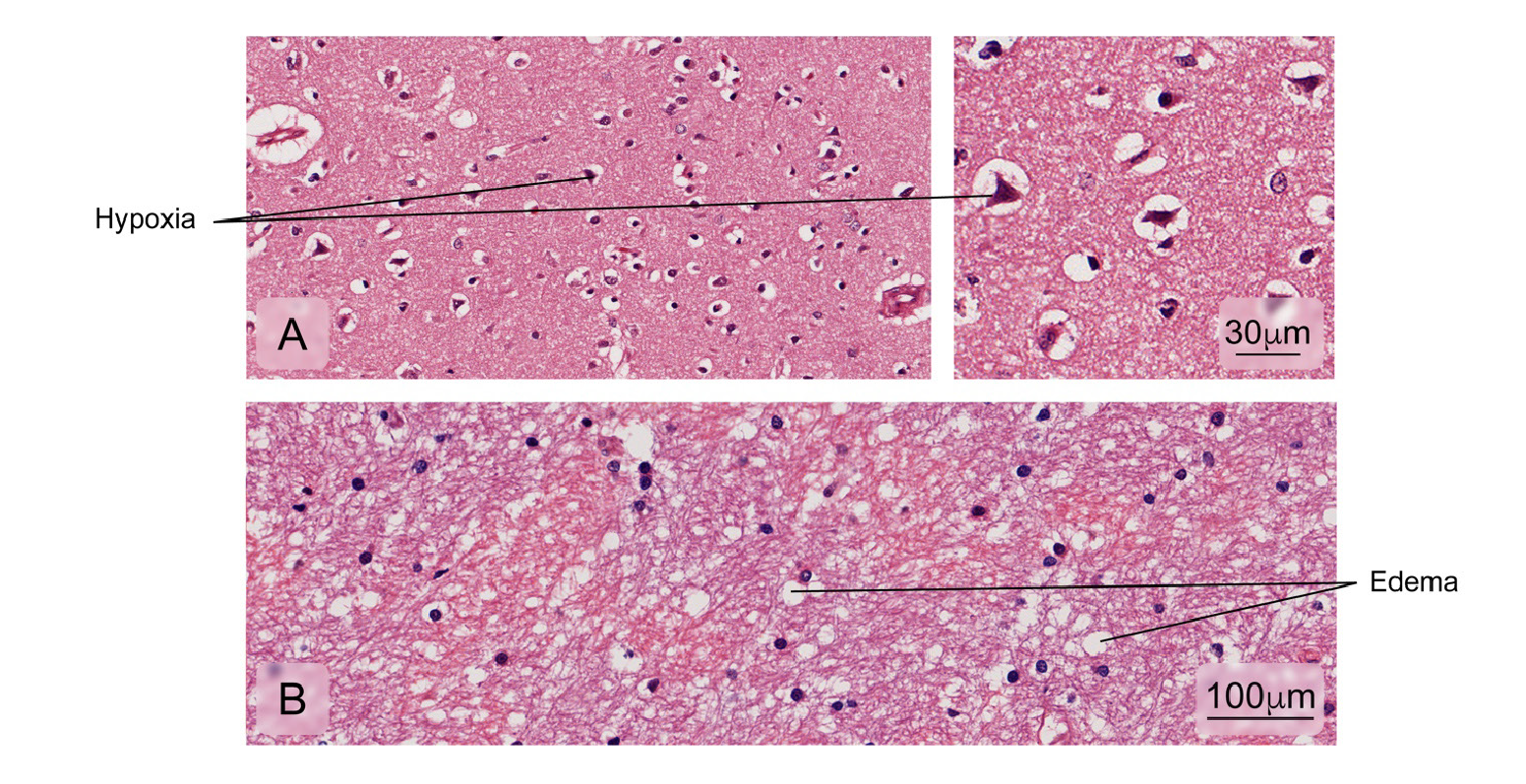
Figure 12 Alterations found in brain tissue-cerebral cortex. Some of the fatal cases presented:A) Decreased neuronal size due to retraction of the cytoplasm with nuclei pyknosis and hyperchromasia associated with hypoxic cortical changes; B) Edema. Hematoxylin and eosin stain.