Diarrhea is a major global health issue causing 1.3 million deaths each year 500.000 of which occur in children less than five years of age 1. Shigellosis is endemic in most low- and middle-income countries and is the most important cause of bloody diarrhea worldwide 2. Recent estimates attribute to Shigella spp. approximately 125 million diarrhea episodes annually leading to approximately 160,000 deaths one-third of which are associated with young children 1,3. The Global Enteric Multicenter Study revealed that Shigella spp. was the most prevalent causative agent in children aged 2 to 5 years who experienced diarrhea and suggested that the induced burden may be twice as high as previously estimated 4.
Shigella genus is divided into the following four species and into multiple serotypes dependent on O-antigen and biochemical differences: Shigella dysenteriae (serogroup A, 15 serotypes), Shigella flexneri (serogroup B, 19 serotypes), Shigella boydii (serogroup C, 20 serotypes), and Shigella sonnei (serogroup D, 1 serotype) 5. The pathogen spreads by direct contact with an infected person or by ingesting contaminated food or water. The infective dose can be as low as 10 microbes; its global importance arises from its wide distribution and water quality concerns that make it an important risk for public health 6. Shigella sonnei and S. boydii usually cause relatively mild illness with watery or bloody diarrhea while S. flexneri is the chief cause of endemic shigellosis in low- and middle-income countries 7.
Many observations have concluded that Shigella species are geographically stratified based on the level of economic development in each country. Shigella flexneri is the primary infectious species in low- and middle-income countries and S. sonnei rates increase with economic development. Shigella boydii is usually restricted to Bangladesh and South-East Asia rarely occurring outside these regions while S. dysenteriae type 1 (Sd1) outbreaks occur sporadically 8. Emerging multidrug-resistant Shigella isolates have exacerbated the public health impact of shigellosis leading to increased morbidity, mortality, and treatment costs 2,9.
Given that Shigella is a major contributor to the global diarrhea burden, vaccination can be an effective strategy to prevent the disease. However, there are at least 50 recognized Shigella serotypes and their distribution differs among geographical regions hindering vaccine development 10.
Public health significant microorganisms in Colombia include Shigella spp. and Salmonella spp. These species are part of the surveillance of acute diarrheal diseases by public health laboratories and the Laboratorio de Microbiología at the Instituto Nacional de Salud. Given the need to better understand the behavior of Shigella species at a national level, we report here the age of the population affected, the temporal and geographical characteristics of the isolates, and the antimicrobial resistance profiles of Shigella species recovered in the country from 1997 to 2018.
Materials and methods
Strains recovered from patients were collected in hospitals or public health laboratories and then sent to the Laboratorio de Microbiología at the Instituto Nacional de Salud to confirm their biochemical identification following the standardized procedures and to serotype them using commercial polyclonal and monoclonal typing antisera (Eurobium) in the framework of the acute diarrheal disease surveillance program 11.
Antimicrobial resistance was determined using the Kirby-Bauer test against tetracycline (30 μg), chloramphenicol (30 μg), nalidixic acid (30 μg), amoxicillin/clavulanic acid (10 μg), and ciprofloxacin (5 ug). Additionally, we obtained the minimal inhibitory concentrations (MIC) for ampicillin (8-16 μg/ ml), cefotaxime (2-32 μg/ml), ceftazidime (1-16 μg/ml), ciprofloxacin (1-2 μg/ml), gentamicin (4-86 μg/ml), and trimethoprim-sulfamethoxazole (2/38 μg/ml) with the Microscan® (Siemens) automated system following the recommendations of the Clinical and Laboratory Standards Institute yearly updated 12. Statistical significance (p value) was calculated with Epilnfo 7.
Results
We studied 5,251 isolates of Shigella spp. recovered in 29 Colombian departments. Bogotá, the country's capital city, sent the highest number of isolates (n=3,257, 62%). Another five states recovered more than one hundred isolates each: Antioquia (n=814; 15%); Valle (n=205; 3.9%); Norte de Santander (n=187; 3.6%); Narino (n=157; 3%), and Boyacá (n=146; 2.8%). The additional 485 isolates were recovered from the other 23 departments.
The majority of isolates were obtained mainly from stools (96.4%; 5,063/5,251) of patients whose ages ranged from newborns to 92 years. The children under one year of age contributed 452 isolates (8.6%), and the patients between 2 and 5 years contributed 2,059 isolates (39.2%); 1,498 (28.5%) isolates were recovered from 6 to 14-year-old patients while 904 isolates (17.2%) were recovered from those over 15 years (Table 1).
Table 1 Age and sample distribution
| Age in years | ||||||
|---|---|---|---|---|---|---|
| Sample type | <1 | 2-5 | 6-14 | >15 | ND | Total |
| Stool | 435 | 2,012 | 1,469 | 834 | 313 | 5,063 |
| NC | 6 | 24 | 15 | 18 | 18 | 81 |
| Blood | 8 | 10 | 4 | 36 | 6 | 64 |
| Urine | 2 | 11 | 4 | 13 | 30 | |
| Other | 1 | 2 | 6 | 3 | 1 | 13 |
| Total | 452 | 2,059 | 1,498 | 904 | 338 | 5,251 |
NC: Information not collected
Two species were predominant representing together 96% of the strains: 2,896 (55.1%) isolates were S. sonnei and 2,191 (41.7%) were S. flexneri. Other species included S. boydii and S. dysenteriae with 103 and 14 isolates each. Some of the isolates had some of the typical characteristics of Shigella but it was not possible to identify them at the species level and, therefore, they were labeled as non-serotypeable Shigella. Non-serotypeable Shigella accounted for approximately 47 isolates, none of which were agglutinated with any antisera of the established Shigella serovars.
During the first three years of surveillance (1997-1999), S. flexneri was the dominant species decreasing after 2002 when S. sonnei became the most dominant and continued to be for 15 years, until 2016, when it became again the second most common strain (figure 1).
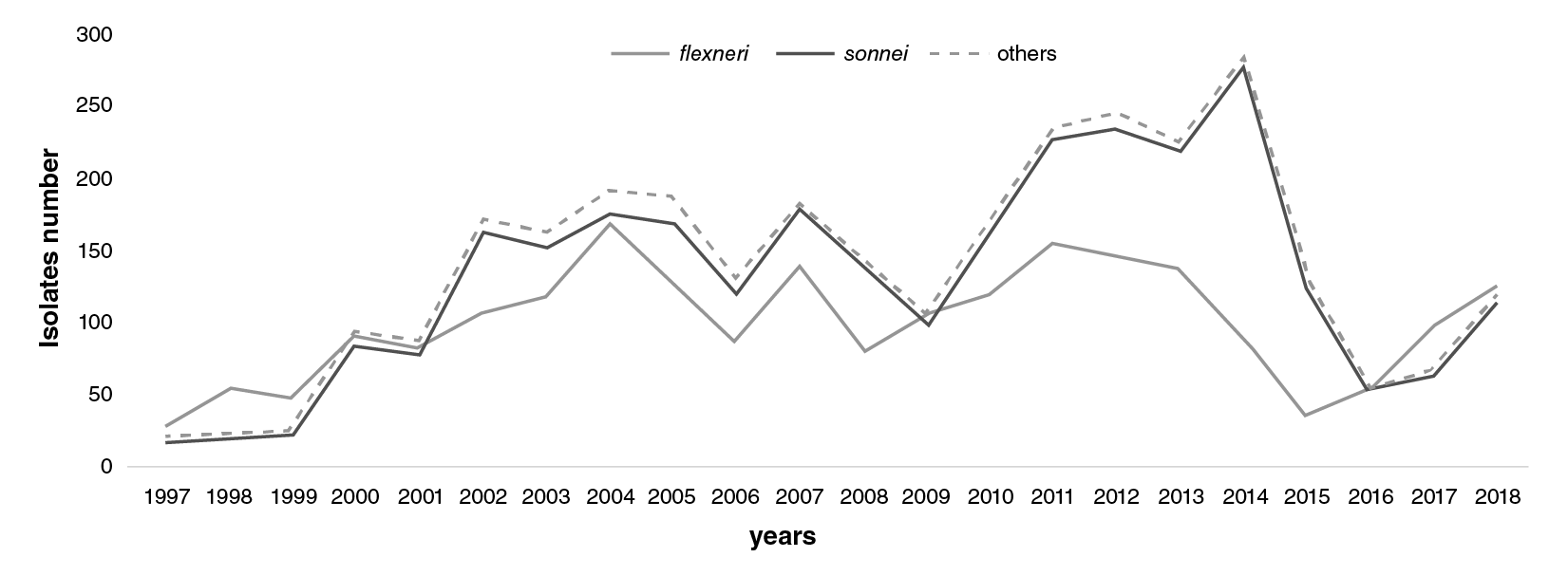
Figure 1 Distribution of Shigella spp. isolates in Colombia from 1997 to 2018. S. fkxneri (grey), S.sonnei (black), and other species (dashed line). This information was based on the annual Shigella surveillance system in Colombia.
The highest resistance level was found for tetracycline (88.1%) followed by trimethoprim-sulfamethoxazole (79.3%), and ampicillin (65.5%); 50.8% of isolates were resistant to chloramphenicol and 43.6% to amoxicillin/ clavulanic acid. Less than 1% of the isolates were resistant to cephalosporins (cefotaxime and ceftazidime), gentamicin, and ciprofloxacin (table 2).
Table 2 Antimicrobial resistant isolates distribution in different Shigella species
| Shigella bspecies | Antibiotics | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AMC | TE | C | NA | CAZ | CTX | AMP | SXT | CIP | GM | |
| boydii | 12.3 | 83.5 | 10.7 | 3.13 | 2.47 | 0.97 | 76.7 | 51.5 | 0 | 1.06 |
| dysenteriae | 0 | 41.7 | 7.14 | 11.1 | 0 | 0 | 28.6 | 42.9 | 0 | 0 |
| fbxneri | 60.7 | 94.3 | 80.8 | 2.34 | 0.22 | 0.37 | 83 | 67.8 | 0.41 | 0.27 |
| Shigella spp. | 12.5 | 63.8 | 27.7 | 9.76 | 0 | 0 | 61.4 | 65.9 | 2.17 | 0 |
| sonnei | 32.8 | 83.9 | 29.4 | 7.15 | 0.4 | 0.43 | 52 | 89.6 | 0.49 | 0.37 |
| Total | 43.6 | 88.1 | 50.8 | 5.13 | 0.36 | 0.41 | 65.5 | 79.3 | 0.46 | 0.34 |
AMC: Amoxicillin/clavulanic acid; TE: Tetracycline; C: Chloramphenicol; NA: Nalidixic acid; CAZ: Ceftazidime; CTX: Cefotaxime; AMP: Ampiclllin; SXT:Trlmethoprim-sulfamethoxasole; CIP: Ciprofloxacin; GM: Gentamlcin
Geographical distribution, age, and antimicrobial resistance of species
Shigella sonnei
Geographical distribution: S. sonnei represented more than half of the Colombian Shigella spp. isolates evaluated (2,896, 54.7%); 66% (1,975/2,896) of the isolates under study were recovered in Bogotá: 58 isolates detected in 2001 and 101 in 2002; 16% of isolates were recovered in Antioquia while the remaining 17.8% were from the other 25 departments where the recovery increased between 2011 and 2014 as the highest number of isolates for this species came from those states in the last few years (figure 2).
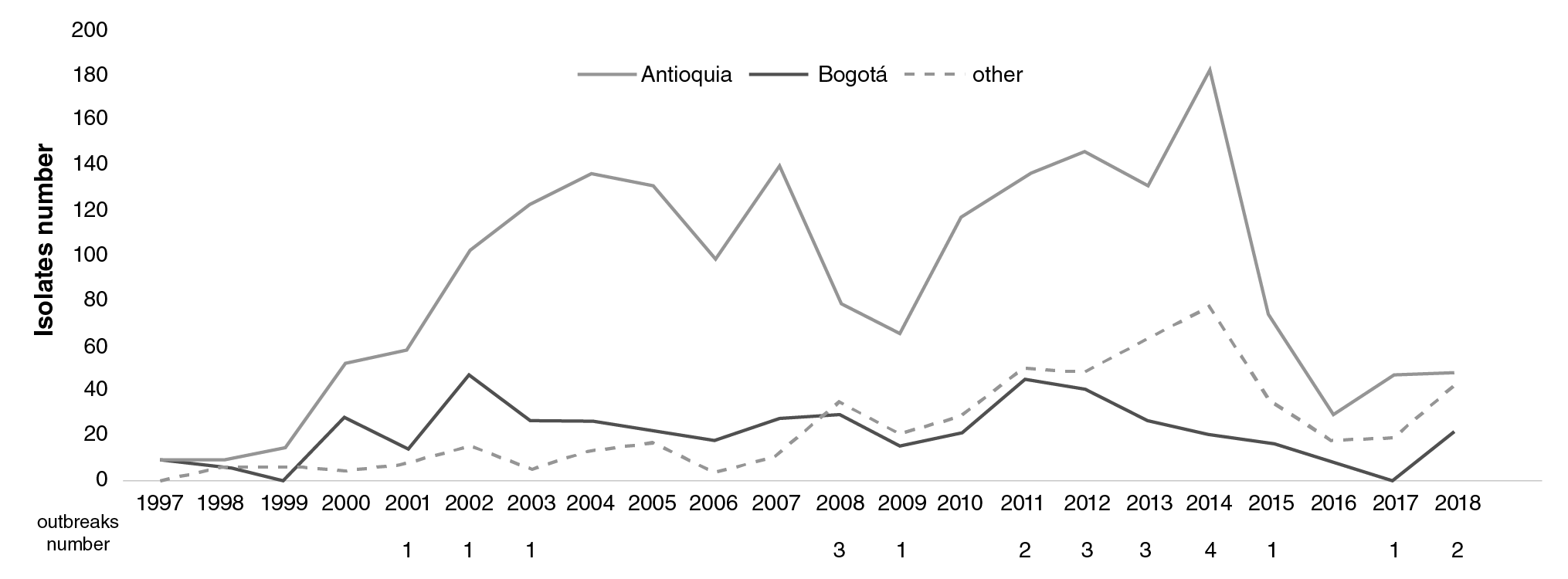
Figure 2 Comparison of the number and distribution of Shigella sonnei isolates in Antioquia (black), Bogotá (grey), and other regions (dashed line) in Colombia from 1997 to 2018
There were 23 outbreaks in nine different departments (Antioquia, Bogotá, Boyacá, Cundinamarca, Narino, Norte de Santander, Santander, Sucre, and Valle) with 80 related isolates. The outbreak that grouped most cases 12 occurred in Narino in 2008. The isolates recovered in the other outbreaks registered there varied.
Age: Almost half of the isolates (48%, 1390/2896) were recovered from children under 6 years of age. In the 6 to 14-year age group, S. sonnei was the most frequently recovered (32.6% of isolates; 943/2896) (table 3).
Table 3 Age range and Shigella species distribution Shigella species
| Shigella species | ||||||
|---|---|---|---|---|---|---|
| Age in years | boydii | dysenteriae | flexneri | Shigella spp. | sonnei | Total |
| <1 | 6.8 | 0.0 | 11.2 | 9.9 | 6.7 | 8.6 |
| 2 to 5 | 30.1 | 28.6 | 37.2 | 34.1 | 41.3 | 39.3 |
| 6 to 14 | 34.0 | 7.1 | 23.5 | 15.4 | 32.6 | 28.5 |
| >15 | 22.3 | 57.1 | 22.4 | 30.8 | 12.5 | 17.2 |
| NC | 6.8 | 7.1 | 5.7 | 9.9 | 7.0 | 6.5 |
NC: Information not collected
Antimicrobial resistance: S. sonnei showed the highest resistance to trimethoprim-sulfamethoxazole (89.6%; 2,530/2,825) and to tetracycline (more than 80%; 2,278/2,714). Resistance levels for nalidixic acid, chloramphenicol, amoxicillin/clavulanic acid, and ampicillin were 7% (184/2,574), 29.4% (803/2,733), 32.8% (785/2,391), and 52% (1469/2827), respectively (table 2); 14 isolates were resistant to ciprofloxacin, 12 to cefotaxime, 10 to ceftazidime, and 7 to gentamicin.
Shigella flexneri
A total of 321/2,191 isolates (14.65%) were detected in Antioquia with increases in 2000, 2006, and 2012. Another 627 (28.6%) isolates were recovered in 25 departments representing an increase as of 2016. Eleven outbreaks of S. flexneri were notified (figure 3) with 37 related isolates in five departments (Amazonas, Bogotá, Cundinamarca, Meta, and Narino).The most important outbreak occurred in Cundinamarca in 2001 when 14 isolates were related to food consumed in a school (Figure 3).
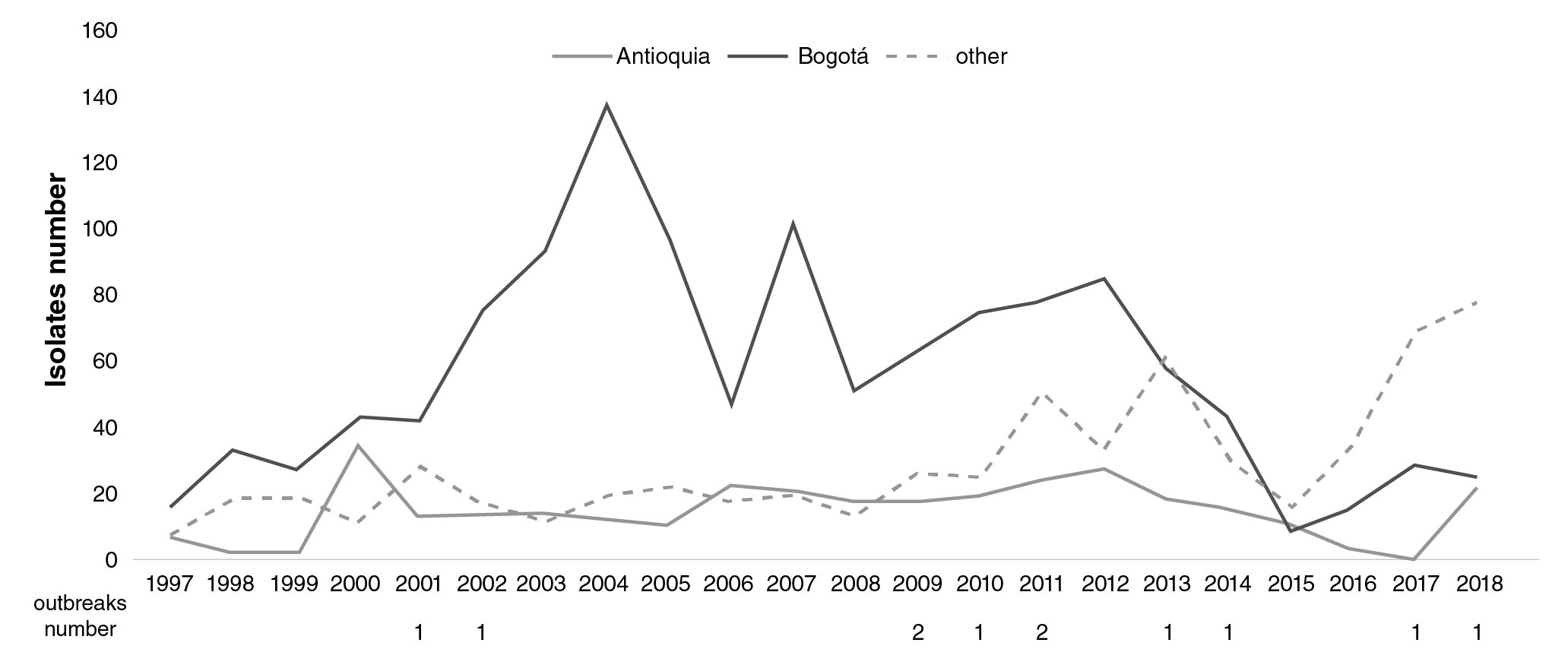
Figure 3 Comparison of the number and distribution of Shigella fkxneri isolates in Antioquia (grey), Bogotá (black), and others regions (dashed line) in Colombia from 1997 to 2018
Age: The number of S. flexneri isolates by age groups was similar. However, almost half of the isolates (48.5%) were recovered in under six-year-old children (table 3).
Antimicrobial resistance: S. flexneri highest resistance rates were to tetracycline (94.3% of isolates), ampicillin (83%), chloramphenicol (80.8%), and trimethoprim-sulfamethoxazole (67.8%) (table 2). Less than 10 isolates were resistant to ciprofloxacin, cefotaxime, ceftazidime, and gentamicin.
Other species
Geographical distribution: Out of a total of 164 isolates, 103 were S. boydii, 14 were S. dysenteriae, and 47 were non-serotypeable Shigella isolates. The highest number of S. boydii isolates was recovered in 2004 and 2006, the largest number being 42/103 (41%). Shigella dysenteriae only appeared in seven of the 22 years surveilled. The highest number of S. dysenteriae isolates 6 was recovered in 2018 from Arauca 3, Bogotá 2, and Norte de Santander (figure 4). Shigella dysenteriae was not related to outbreaks.
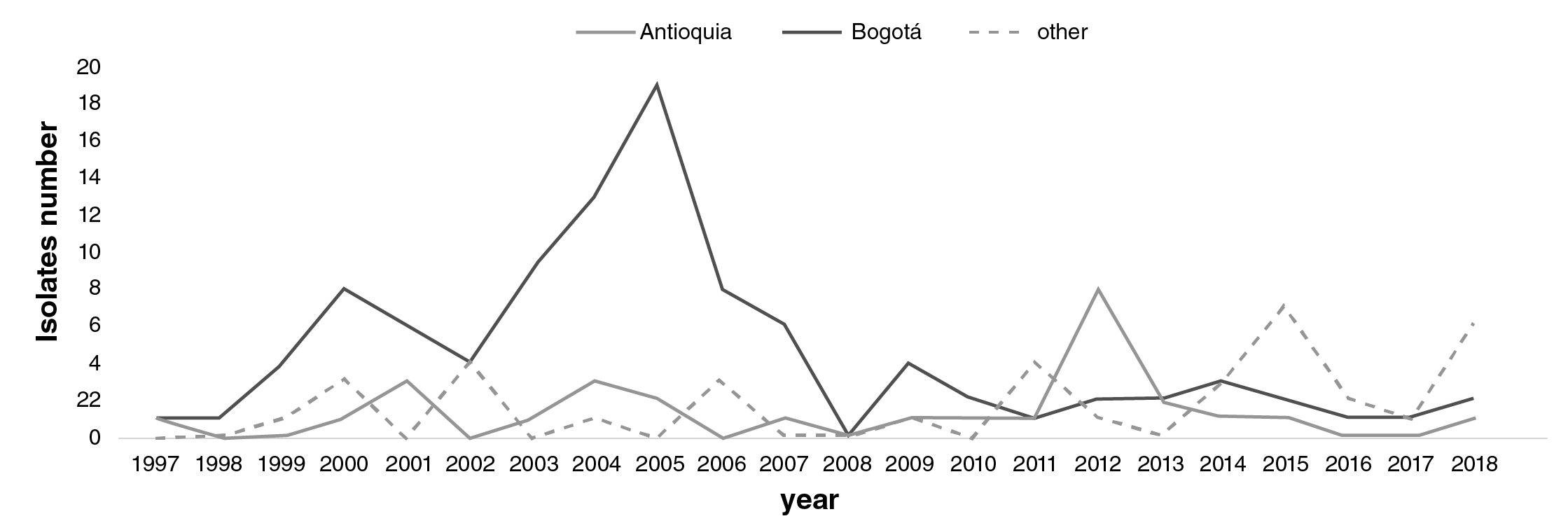
Figure 4 Comparison of the number and distribution of Shigella species different to sonnei and fkxneri in Antioquia (black), Bogotá (grey), and other regions (dashed line) in Colombia from 1997 to 2018
Age: S. boydii was recovered mostly in under 14-year-olds (71%) while S. dysenteriae was recovered mostly from the ≥15-year-old population. Non-serotypeable isolates were recovered in patients of all ages (table 3).
Antimicrobial resistance: S. boydii exhibited resistance to all evaluated antibiotics except ciprofloxacin. More than 75% of S. boydii isolates were resistant to ampicillin and tetracycline and half of these were resistant to trimethoprim-sulfamethoxazole.
Shigella dysenteriae was resistant to five of the ten evaluated antibiotics with a lower resistance rate than other species. However, the highest percentage of resistance to nalidixic acid was found in S. dysenteriae when compared with other evaluated species (11.1%).
Non-serotypeable isolates showed resistance to seven of the evaluated antibiotics; characteristically, this group had the greatest resistance rate to ciprofloxacin (2.1%) (table 2).
Antimicrobial resistance evaluated per year and species
Beta-lactam antibiotics
Amoxicillin/clavulanic acid: S. sonnei resistance began to appear in 2006 when 24.6% of the isolates were resistant; eleven years later, in 2017, this resistance rate reached its highest level of almost 66% (p<0.05). In 2018 the resistance level significantly decreased (11.6%; p<0.05) (Table 4).
During the first five years of surveillance of S. flexneri, no resistance was recorded. Nonetheless, between 2002 and 2005, rates ranged from 22% to 40.9%; a year later, in 2006, they had increased significantly to 71.3% (p<0.05), and then they were stable until 2018 when there was a significant decrease to 35.2% (p<0.05). This was also observed in S. sonnei isolates. In the other species, resistance was detected in nine of the 22 years under surveillance and the highest resistance level was detected in 2017 (50%) (Table 4).
Ampicillin: Since surveillance started in 1997 and for 12 years, the resistance in S. sonnei has remained above 50%. In 2010, resistance rates increased significantly to 71.5% (p<0.05) and were stable until 2018 when they decreased significantly to 25.9% (p<0.05). In contrast, S. flexneri resistance rates to ampicillin remained high in the same period of time ranging from 62.7% to 92.8% (table 4). There were no resistant isolates from other species only in 2008, 2015, and 2017 (table 4).
Table 4 Antimicrobial resistant isolates distribution per year in different Shigella species
Cefotaxime and ceftazidime: Resistance to cefotaxime was found in 21 isolates: 12 S. sonnei, 8 S. fbxneri, and 1 S. boydii isolate while we detected 16 resistant isolates to ceftazidime: 10 S. sonnei, 4 S. fbxneri, and 2 S. boydii isolates.
Tetracycline: From the very first year of S. sonnei surveillance, we found resistance levels in more than 90% of the isolates which decreased to 70% as of 2013 reaching the lowest levels in 2018 with 41.2%. Shigella flexneri isolates showed more than 90% resistance during 17 of the surveillance years registering the lowest rates (83.3%) in 2018. Resistance rates in other species fluctuated: in some years there were no resistant isolates (2008 and 2017) while resistance levels reached 100% in 1997, 1998, 2000, 2002, 2006, and 2007 (Table 4).
Trimethoprim-sulfamethoxazole: In S. sonnei, resistance remained constant during the study period ranging from 75.9% to a maximum of 96.6% in 2006 and 2015, respectively. In S. flexneri resistance levels exceeded 65% until 2010 and then decreased to 48.8% in 2018. Regarding other species, resistance rates varied more: in 1998, 2008, and 2010, isolates were completely sensitive while in 2017 resistance rates reached 100% (table 4).
Quinolones and fluoroquinolones: Resistance to nalidixic acid in S. sonnei was higher than in other species remaining stable (below 10%) until 2012 with a maximum level in 2017 (38.1%). In 2016 alone, S. flexneri resistant isolates exceeded 20% while in other years this level did not exceed 6% (table 4). Ciprofloxacin-resistant isolates amounted to only 24: S. sonnei14, S. flexneri9, and S. boydii1.
Phenicols: The highest resistance rate was detected in S. flexneri with more than 70% of isolates during surveillance years. In S. sonnei isolates, resistance rates were less than 30% until 2008, when they began to increase until reaching 60.3% in 2012. For other species, resistance levels varied during the surveillance years (table 4).
Aminoglycosides: Gentamicin was evaluated within the aminoglycoside class and we detected 12 resistant isolates: seven were S. sonnei, four S. flexneri, and one S. boydii.
Resistance profiles
The most frequent resistance profile was detected in 1,001/5,043 (19.8%) isolates and it corresponded to multidrug resistance (resistance to three or more antibiotic classes: (TE-C-AMC-AMP-SXT) (Table 5). Resistance rates were similar in S. flexneri and S. sonnei (Table 5).
Table 5 Antimicrobial resistance profiles distribution in different Shigella species
| Shigella species | ||||||
|---|---|---|---|---|---|---|
| Resistance profiles | Flexneri n (%) | sonnei n (%) | boydii n (%) | dysenteriae n (%) | Shigella spp. n (%) | n isolates |
| TE-C-AMC-AMP-SXT | 555 (55.4) | 442 (44.2) | 3 (0.3) | 0 | 1 (0.1) | 1,001 |
| TE-SXT | 125 (13) | 825 (86) | 4 (0.4) | 1 (0.1) | 4 (0.4) | 959 |
| TE-AMP-SXT | 90 (16.9) | 402 (75.6) | 33 (6.2) | 2 (0.4) | 5 (0.9) | 532 |
| TE-C-AMP-SXT | 410 (77.5) | 109 (20.6) | 3 (0.6) | 1 (0.2) | 6 (1.1) | 529 |
| TE-C-AMC-AMP | 374 (83.5) | 73 (16.3) | 0 | 0 | 1 (0.2) | 448 |
| SXT | 16 (5.3) | 277 (92) | 3 (1) | 0 | 5 (1.7) | 301 |
| AMP-SXT | 27 (17.5) | 120 (77.9) | 2 (1.3) | 1 (0.6) | 4 (2.6) | 154 |
| TE-C-AMP | 141 (91.6) | 8 (5.2) | 4 (2.6) | 0 | 1 (0.6) | 154 |
| TE-C-SXT | 128 (87.7) | 17 (11.6) | 0 | 0 | 1 (0.7) | 146 |
| TE-AMC-AMP-SXT | 30 (21.1) | 111 (78.2) | 1 (0.7) | 0 | 0 | 142 |
| Other profiles (n=78) | 245 (36.2) | 377 (55.7) | 40 (5.9) | 1 (0.8) | 14 (2.1) | 677 |
| Total | 2,141 | 2,761 | 93 | 6 | 42 | 5,043 |
AMC: Amoxicillin/clavulanic acid; TE: Tetracycline; C: Chloramphenicol; NA: Nalidixic acid; CAZ: Ceftazidime; CTX: Cefotaxime; AMP: Ampicillin; SXT: Trimethoprim sulfamethoxasole; CIP: Ciprofloxacin; GM: Gentamicin
Other resistance profiles were specific for each species. The most frequent in S. flexneri were TE-C-AMP (91.6%), TE-C-SXT (87.7%), TE-C-AMC-AMP (83.5%), and TE-C-AMP-SXT (77.5%). Interestingly, the main S. sonnei resistance profile was to SXT solely (92%; 277/301) followed by resistance to two antibiotics: TE-SXT (86%; 825/959). Furthermore, compared with S. flexneri, fewer multidrug-resistant isolates from other species were detected. TE-AMC-AMP-SXT: 78.2%; AMP-SXT: 77.9%, and TE-AMP-SXT: 75.6% were the most frequent resistance profiles (table 5).
Discussion
Diarrheal diseases continue to cause morbidity and mortality in low- and middle-income countries. Estimates from 195 countries reveal that global diarrhea mortality among individuals older than 5 years has been dominated by Shigella spp. 13. Additionally, Shigella spp. was the leading cause of diarrheal mortality among people older than 70 years 14,15.
In this report, most of the strains were isolated from under 6-year-old children. Shigella sonnei resistance profile included trimethoprim-sulfamethoxazole_solely, which indicates that age could be an important factor in acquiring Shigella infection in Colombia. The findings we present were supported by a prospective surveillance study also performed in Colombia to determine the incidence of Shigella spp. in acute gastroenteritis that revealed that 23% of the isolates were associated with hospitalization and outpatient care in single infections and coinfections among children between 24 and 59 months of age 16. As described in the Global Enteric Multicenter Study (GEMS), Shigella spp. burden increased proportionally with age and became the second most common pathogen identified among 12 to 23 months children and the leading pathogen among 24 to 59 months ones 17.
The dominant species was S. flexneri in the first three years (1997 to 1999) and in the last three years of surveillance (2016 to 2018). Since 2000 and for 16 years (2000 to 2015), the dominant species was S. sonnei in all the departments under study. This 'replacement phenomenon' has been documented in many countries in different regions of the world such as Asia and it supports the emergence of S. sonnei replacing S. flexneri as the most frequent agent of shigellosis in economically transitional states 18,19.
Shigella sonnei and S. flexneri were the most common isolates coinciding with reports at global level. For example, reports on the incidence of several Shigella species in countries of the Americas evidenced their presence in an urban community in Ecuador and in a Chilean periurban area (20,21). Additionally, the current global epidemiological burden for shigellosis is attributed to these two species: S. flexneri, conventionally associated with low-and middle-income countries, and S. sonnei with high-income regions 19 .
Noticeably, regions that had undergone significant industrialization reported increasing cases of S. sonnei compared to low- and middle-income areas where S. flexneri levels have remained high 22. It has been suggested that this shifting trend may be due to an improvement of overall nutritional status 23, socioeconomic level, and sanitation conditions 24. It has also been suggested that such shift is mediated by cross-immunity. In less-developed countries, repeated ingestion of Plesiomonas shigelloides bacteria through consumption of untreated surface waters may stimulate cross-protection against S. sonnei with O antibodies mediating protection 25.
The appearance of S. flexneri in our study as the most common species during the last three years of surveillance could be an indicator of a decline in the quality of water supply and good sanitation in Colombian communities. It has been shown that an increase in the provision of clean water and sanitation possibly disrupts S. flexneri traditional transmission route 26.
Regarding the other species evaluated in our report, S. boydii and S. dysenteriae, we observed fewer isolates, which agrees with other worldwide reports 10,17,27, but outbreaks of both species have been reported 28,29.
We recovered S. dysenteriae isolates sporadically, one or two strains during the 23 years of surveillance. However, in 2018, six isolates were recovered from three different departments, two of them located in borders (Arauca and Norte de Santander); isolates of these species had not been previously reported in these states. According to previous reports, their presence may indicate the possibility of an epidemic in these or other regions within Colombia 30.
These isolates were evaluated for the presence of Shiga toxin and were negative. Some did not react with any Shigella serogroup/serotype-specific antisera. Other studies have found similar results with strains classified as untypable by serotyping in many other countries 31-33.
Regarding the antimicrobial resistance profiles of species under study, we found the highest resistance levels to tetracycline, trimethoprim-sulfamethoxazole, and ampicillin, a trend also observed in Africa, Asia, and other South American countries where ampicillin resistance rates were high for almost all Shigella species. In most of the studies in Africa and Asia, Shigella serogroups developed resistance to tetracycline, chloramphenicol, and trimethoprim-sulfamethoxazole 34-36.
Additionally, resistance levels to amoxicillin/clavulanic acid reached almost 50% in our study, which was higher than the Latin American mean reported by the Antimicrobial Surveillance Program where 27% of the isolates were resistant to this antibiotic but less frequent than the 71% reported in Europe 35.
Resistance rates to cefotaxime, ceftazidime, gentamicin, and ciprofloxacin were less than 1%. In general, the antimicrobial resistance level for these antibiotics is similar across Latin America where ceftriaxone is active against all Shigella spp. and fluoroquinolone-resistant isolates are limited 35,37.
The antimicrobial resistance against specific species revealed that S. sonnei had the highest resistance level to trimethoprim-sulfamethoxazole. Indeed, S. sonnei resistant isolates were found in all surveillance years. The resistance to amoxicillin/clavulanic acid reached its highest rate in 2017 and abruptly decreased by more than 50% during 2018, which was unexpected considering the behavior of the isolates reported herein.
Only 6% of S. flexneri isolates were susceptible to tetracycline, which means that more than 90% of the population is resistant. Likewise, ampicillin and chloramphenicol resistance were higher in S. flexneri isolates than in all other evaluated species. A similar situation has been revealed in other parts of the world with high rates of resistance to at least one of the common antibiotics such as ampicillin, tetracycline, and chloramphenicol 38,39.
Contrary to reports of S. flexneri resistance to gentamicin, nalidixic acid, and ciprofloxacin 34 in other regions, our resistant isolates did not reach high resistance levels. A similar situation occurred for cephalosporin where resistance was less than 1%, similar to that reported by Lima, et al.40.
The S. boydii isolate we analyzed here exhibited resistance to all antibiotics evaluated except for ciprofloxacin. Interestingly, this species had the highest resistance rates to cephalosporin and gentamicin. Regarding tetracycline, ampicillin, and trimethoprim-sulfamethoxazole, the trend was similar to previous reports 34.
We found the highest percentage of resistance to nalidixic acid in S. dysenteriae isolates, similar to those in India and Bangladesh where an alarming 82% resistance in this species was reported 41,42. In Egypt, Wasfy, et al., found over 50% resistance levels to chloramphenicol, tetracycline, and ampicillin 43,44. However, compared to other studies 31,45, in ours the isolates had lower levels of resistance to other antibiotics evaluated and were completely sensitive to amoxicillin/clavulanic acid, ceftazidime, cefotaxime, and ciprofloxacin.
An increase in fluoroquinolone resistance in S. flexneri and S. sonnei isolates in high-income countries has been reported and, although nalidixic acid resistance was common among S. sonnei isolates, the trend of fluoroquinolone resistance is slowly increasing 46,47. Oral ciprofloxacin can achieve high concentrations in serum and stool and has activity against Shigella isolates. However, ciprofloxacin should not be prescribed for children 48.
In Colombia, quinolones are not generally recommended for shigellosis treatment, which may have contributed to the low levels of resistance observed. Macrolide azithromycin is widely used in children and is recommended as an alternative therapy for the treatment of shigellosis in adults infected with multidrug-resistant isolates 49; however, in vitro azithromycin susceptibility testing is not routinely performed by the country's Shigella surveillance system.
Our laboratory-based surveillance of Shigella spp. determined that children under five years of age were affected by all of them. These findings should be considered by funders and public health officials to make evidence-based decisions for effective protection and prevention including attainable vaccines. Characteristically, an increased frequency of multidrug resistant S. flexneri was observed. Following international epidemiological trends, S. sonnei is likely to become more prevalent on a global scale as countries increase their level of development and sanitation. Combating the spread of global antibiotic resistance will be greatly benefited by focusing on S. sonnei surveillance while more localized efforts are needed to combat S. flexneri resistance.














