In Venezuela, dengue is the most important arboviral disease affecting humans, and its incidence and prevalence rise annually 1. Until now, there is not a vaccine to avoid DENV infections and, therefore, vector control is the only way to restrain these disease risks 2.
Arboviruses such as DENV have to go through a series of critical steps that demand their interplay with different tissues, which lasts for days or weeks until transmission can occur 2-4. These tissues represent barriers that restrict virus growth through, among others, immune molecules with antipathogenic activity that belong to a very complex system of highly regulated pathways called the innate immune system of mosquitos. Toll, IMD, JAK/STAT, and RNAi are the primary immune signaling pathways (2-5.
Different strategies to diminish viral transmission have been considered, among them, the use of genetically engineered vectors and natural symbionts like Wolbachia6,7. Any strategy to control dengue transmission should consider the interactions between viruses and mosquitoes, especially, their innate immune system.
Toll and IMD pathways produce effector molecules such as the antimicrobial peptides, low molecular-weight proteins well known for their action against bacterial and fungal infections, although there is less information regarding their effect on viral infections. Reports suggest that dengue virus infection is controlled by the toll pathway in mosquitoes 8 and that together with the IMD pathways they upregulate the Sindbis 9 and the DENV-2 viruses in mosquitoes 10,11. However, other studies have evidenced the inhibition of toll's innate immune response in salivary glands infected by DENV-2 with 3'UTR substitutions associated with high epidemiological fitness and enhanced production of infectious saliva 12.
In our study, we found that the expression of defensin A and cecropin A genes, two antimicrobial peptide genes mediated by the toll pathway, was significantly reduced in Ae. aegypti mosquitoes infected with DENV-1 suggesting that the infection progresses by suppressing the toll pathway.
Materials and methods
Mosquito collection
We collected Ae. aegypti mosquitoes as larvae from Maracay, Venezuela, and then obtained their F1 generation.
Dengue virus, bacteria, and infection processes
We used a DENV-1 isolate (LAR23644) recovered from a patient in Maracay in 2007 for the infection assays. Viruses were serially passaged in Ae. albopictus C6/36 cells, the infected supernatants were then harvested, titered via plaque-forming assay, and frozen at -80 °C. The viral titer was 4,8 x 105 PFU/ml. For oral infection experiments, we mixed viral stocks 1:1 with human red blood cells washed with PBS and fed to mosquitoes (sugar starved for 24 h) via membrane feeders. Some groups of mosquitoes were fed only on human red blood cells. Immediately post-feeding, fully engorged specimens were transferred to new cages held under standard rearing conditions and provided with sucrose.
At different times after feeding, ≈30 mosquitoes were collected each time. At early times (5 and 24 hours) we checked if the virus was inactivated by some antiviral defense mechanism present in the mosquitoes' guts while at later times (10 and 15 days), we aimed at detecting viral replication in their bodies.
To determine the percentage of virus infection, dissemination, and potential transmission in the vector, 50 individual mosquitoes' abdomen (fed with the virus similarly as before and collected 15 days later) were dissected to check for infection, their legs and wings for dissemination, and their salivary glands for potential transmission. It is known that the only way to measure transmission is by analyzing the saliva of the mosquitoes, for which the viruses in these last tissues are potentially transmittable 13. Prior to their lysis, all the tissues were washed three times with 200 μl of PBS to discard any contamination. Mosquitoes were stored at -80 °C.
Similar infections were carried out with E. coli cultured in OD600 0.8, pelleted, washed, and resuspended in PBS. The bacteria culture was mixed with human red blood cells in equal proportions, and then we applied the methodological procedure used for viral infection.
Detection and typing of dengue viruses in Aedes aegypti
RNA extraction, detection, and typing of dengue viruses in pools of whole bodies or in dissected samples of Ae. aegypti were performed according to Urdaneta, et al.14.
Quantitative RT-PCR (qPCR) for measuring gene expression
Gene expression was determined by relative quantification relating the qPCR signal of the defensin A or cecropin A gene transcript in a mosquito group fed on virus or bacteria mixed with human red blood cells and that of a control group (calibrator) fed only on human red blood cells. qPCR was conducted in a reaction volume of 25 μl in a 96-well plate containing 0.5 μg of template based on the initial RNA concentration and 200 nM forward and reverse primers using real-time Go Taq qPCR™ (Promega Corporation, USA) on a 7500 Real-Time PCR System™ (Applied Biosystems, Massachusetts, USA) using the following program: 2 minutes of preincubation at 95 °C followed by 40 30-s cycles at 95 °C and one minute at 60 °C. The designed specific primers used were: Defensin A gene (sense: 5'-AACTGCCGGAGGAAACCTAT-3'; antisense: 5'-TCTTGGAGTTGCAGTAACCT-3') and cecropin A gene (sense: 5'-CGAAGTTATTTCTCCTGATCG-3'; antisense: 5'-AGCTACAACAGGAAGAGCC-3'). To normalize the data, we used the α-tubulin gene (sense: 5'-GCGTGAATGTATCTCCGTGC-3'; antisense: 5'-AGCTACAACAGGAAGAGCC-3') s an endogenous reference.
We assessed a-tubulin, defensin A, and cecropin A primer pairs and we found the following for each: The observed efficiency was near to 100% (figure 1S), the amplification specificity was displayed through the production of a unique peak in the melt-curve analysis (figure 2S), which was corroborated by sequencing the PCR products from each gene in both directions using the PCR primers (data not shown). The sequencing reactions were performed with the ABI PRISM BigDye Terminator™, version 3.1 Cycle Sequencing Kit on an Applied Biosystems genetic analyzer, Model ABI 3130XL. Therefore, the 2-∆∆Ct method of relative quantification was used to appraise relative gene expression.
We used the control and virus-infected pool samples (=30 mosquitoes/ pool) at different times after feeding (5 h, 24 h, 10 days, and 15 days) in the qPCR reaction (a total of 8 pools: ≈240 mosquitoes). The control values were very close at all times, so we took their average as the calibrator. Each qPCR experiment was repeated three times with three replicates of each one. Similar experiments were carried out with E. coli. The average and standard deviation (SD) of the CTs from the three replicates were determined and the average was only approved if the SD was <0.38 15. Repeatability and reproducibility were calculated by a percent coefficient of variance (% CV) within and between assays respectively (tables 3S).
We calculated N-fold copy numbers of the Ae. aegypti defensin A and cecropin A gene transcripts relative to the control in each assay using geometric means for the three experiments.
Results
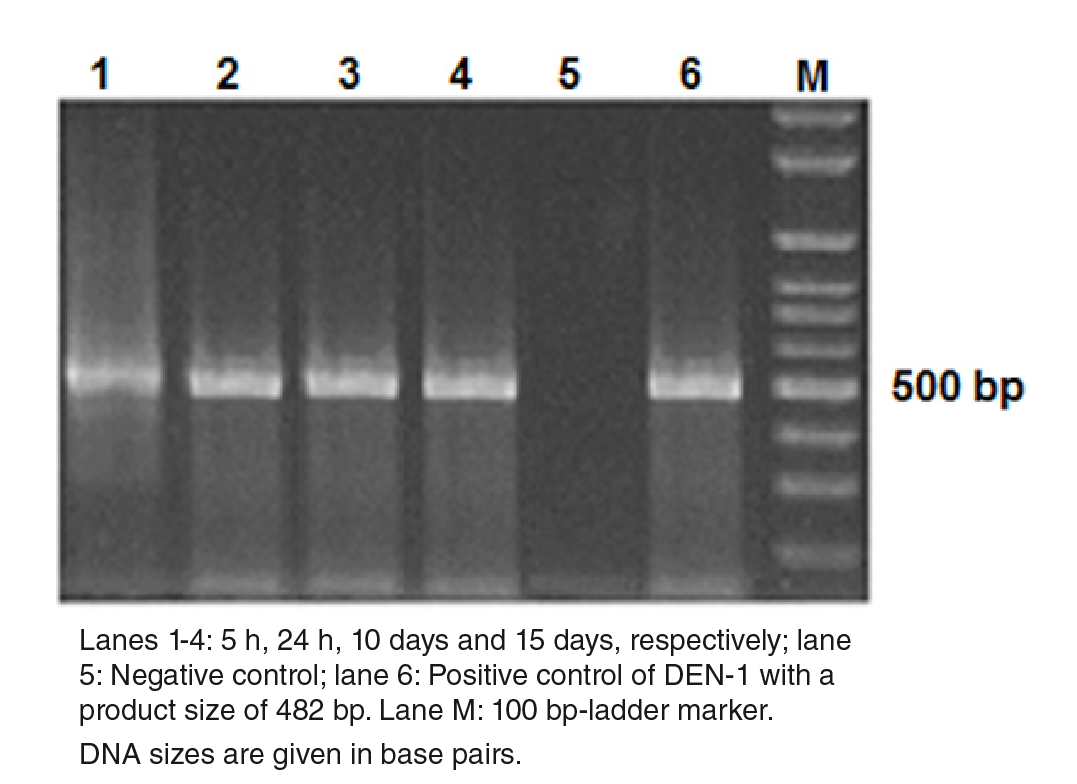
Stability and replication of DENV-1
We determined whether the DENV-1 was stable at early post-infection (dpi) times (5 and 24 hours) and replicated at later ones (10 and 15 days) in the mosquitoes using RT-PCR amplification followed by agarose gel electrophoresis analysis of the products. Figure 1 shows the presence of DENV-1 with the cDNA band at the 482 bp position at all time points under study. The replication was further corroborated in the dissected samples of 50 individual mosquitoes with 70% and 100% viral infection and a dissemination efficiency 15 days post-infection. Regarding the virus present in the salivary glands, it also replicated (45%) and evidenced potential transmission efficiency (Table 9S).
Inhibition of defensin and cecropin mRNA by DENV-1
The relative expression levels of defensin A and cecropin A genes in DENV-1-infected Ae. aegypti mosquitoes as compared to the calibrator are shown in figure 2A with both mRNA detectable in control mosquitoes; however, a significant decrease in abundance occurred at all time points measured with at least five to eight-fold fewer amounts of defensin and cecropin mRNA, respectively, in mosquitoes infected with DENV-1.
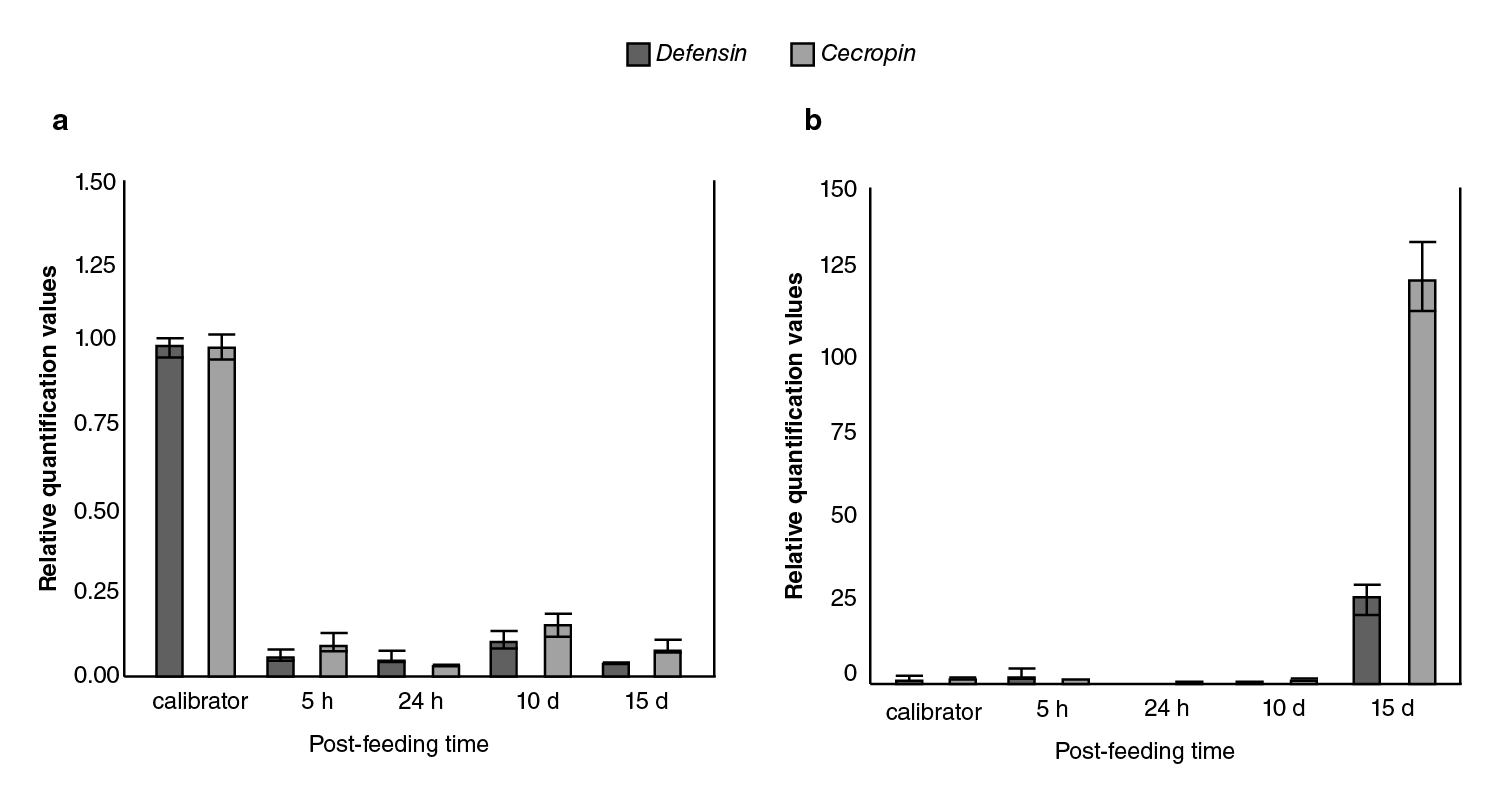
Figure 2 Comparison of immune responses to DENV-1 and Escherichia coli bacteria in field-collected Aedes aegypti. Averaged data from three independent real-time qPCR experiments were used to assess the expression of each of the selected immune genes in the Aedes aegypti mosquitoes infected with the DEN-1 virus (a) or Escherichia coli bacteria (b) with the host α-tubulin as an internal reference control to normalize the data. For each pathogen, the control values for both genes at all the time points were very similar. In every case, the average of all these values was used as the calibrator. The 2-∆∆CT method was used to calculate fold change for each gene.
Induction of defensin and cecropin mRNA by bacteria
The response of the field population of Ae. aegypti mosquitoes to bacteria was contrary to the previously described viral response given that, as expected, the bacterial infection did not produce down-regulation in any of the genes (figure 2B); however, the induction of transcripts occurred at later times (15 days).
Discussion
There is a discrepancy regarding the reaction of mosquitoes' immune system in the presence of DENV-1. The response may be stimulation 2,8,10,11 or suppression in vivo9,16-18 and in vitro19,20. Such discrepancy probably depends on the viral strain used, the genetic history of the vector, and the mode of transmission 3,21. We found a reduced expression of defensin A and cecropin A genes using the F1 generation of wild mosquitoes infected with DENV-1. Similar results were reported with DENV-2-infected field Ae. aegypti populations 6.
The specific molecular mechanism by which DENV acts remains uncharacterized. The virus may be able to knock down the expression of some factor needed to induce the expression of defensin and cecropin mRNA, similar to the role reported for the /AeFaDD protein in Ae. aegypti22. Alternatively, the DENV may directly target and inhibit the transcription of both genes.
The suppression of the innate immune responses of mosquitoes found in this study was time-independent contrary to other reports using similar times: 1,2,7, and 14 days 17, which implies that DENV may exert continuous immunomodulatory activity in mosquitoes, or for some period of time. This is critical for defining the vector competence of local mosquitoes, as well as dengue transmission intensity in a particular area.
As expected, the bacterial infection did not produce down-regulation of the defensin and cecropin genes 23,24; however, the induction of the transcripts occurred at later times (figure 2B). These data could indicate that the capacity of these wild Ae. aegypti mosquitoes to mount a highly effective production of defensin and cecropin to control invading bacteria would take the time probably required to inactivate bacterial growth factors.
In conclusion, DENV-1 inhibits the expression of defensin A and cecropin A genes in a wild Ae. aegypti population from Maracay city in Venezuela. The way the virus participates in this inhibitory mechanism and the viral effector molecules acting in it are still to be determined.