Infertility is defined by the failure to achieve a clinical pregnancy after one year or more of regular unprotected sexual intercourse. Approximately 40-50% of all infertility cases are due to male factors 1. While reproductive technologies can counter male infertility, genetic defects may still be passed to the male’s offspring 2.
Medicinal plants are used for the development of new drugs, as well as for health care. For example, Trigonellae Semen, derived from Trigonella foenum-graecum L., is commonly used as a medicinal herb for the treatment of infertility in Korean medicine 2. Panax ginseng is a traditional medicinal plant for male infertility and rats treated with ginseng had had a significant increase in sperm count and sperm motility 3,4. Aspalathus linearis and Camellia sinensis have also a forceful positive impact on sperm factors 5. Decursin, extracted from Angelica gigas, has also shown a positive effect on sperm counts and motility in cryptorchidism-induced infertile rats 6. The protective effect of Crocus sativus L. on cadmium (Cd) toxicity in rat spermatogenesis has been reported as well 7. Besides, recent evidence suggests that the Ca2+ levels of mice sperm cells treated with P. ginseng extract increased significantly compared with the normal group inducing the expression of CatSper genes (involved in sperm motility and fertility) 8,9.
There is a growing body of literature on the therapeutic effects of cruciferous plants like broccoli largely attributed to their high content of glucosinolates. Broccoli is well known worldwide for its anti-cancer effects 10 and its antioxidant properties, which make it the finest natural active substance for scavenging free radicals 11,12, as an antigenotoxic agent 13, a reducer of fasting blood glucose in type 2 diabetes patients 14, a protection agent of the cardiovascular 15 and central nervous systems, against diabetic nephropathy and neuropathy 16, beneficial in the restoration of skin integrity 17, against Helicobacter pylori infection 18, and for the improvement of social interaction in patients with autism 19.
Heavy metal pollutants such as cadmium and lead are considered sources of significant environmental damage as they do not have any biological functions and can be extremely toxic even at low concentrations 20. The toxic effect of heavy metals on living organisms’ health are expressed in diseases and conditions like cancer, infertility, nephritis, hair loss, brain damage, cardiovascular disease, low blood pressure, and paralysis. However, kidney and lung damage, fragile bones, and calcium deregulation in biological systems have been determined as specific toxicological effects of cadmium 21.
The effect of broccoli on spermatogenesis and related gene expression in mouse testes has yet to be determined. The genetic bases and molecular mechanisms underlying spermatogenesis and its molecular regulation are not fully understood yet. In this context, in the present study, we analyzed the impact of broccoli extract on sperm count and the expression of Arl4a gene (involved in spermatogenesis) 22, of Catsper1 and Catsper2 genes (involved in sperm motility) 23, as well as that of Sox5 and Sox9 genes (transcription factors involved in the Sertoli cells and sex development) 24.
Materials and methods
Experimental animals
The study was approved by the Ethics Committee at the University of Mazandaran, Iran (IR.UMZ.REC.1399.002). We purchased NMRI male mice (5-6 weeks old between 25-30 g of weight) from the Pasteur Institute (Tehran, Iran), which were kept for one week in polycarbonate cages for adaptation to their new environment until reaching the desired conditions. They were kept under standard conditions (12 h light/dark cycle at 24 ± 2°C) with free access to drinking water and standard pellets. Once every two days, the cages were cleaned, the remaining food was collected, and fresh food was provided. Finally, they were anesthetized intraperitoneally with ketamine hydrochloride (100 mg kg−1) and xylazine (5 mg kg−1).
Preparation of the broccoli aqueous extract
Two-month broccoli was collected from a local greenhouse, immediately frozen and transferred to the plant physiology laboratory. Broccoli flowers were grinned in liquid nitrogen to a fine powder, dissolved in boiling water, and boiled for 3 minutes (1 g ml-1). Then, the suspension was filtered through one layer of filter paper and the extraction was centrifuged at 10,000g for 10 minutes at 4°C and stored at −20°C for further experiments 25.
Experimental design
Thirty-six NMRI male mice were divided randomly into the following six groups, with six animals per group: 1) control; 2) cadmium, 3 mg/kg of mouse body weight; 3) orally treated mice with 200 broccoli aqueous extract (1 g ml-1); 4) orally treated with 400 µl of broccoli aqueous extract; 5) orally treated with 200 broccoli aqueous extract plus cadmium, and 6) orally treated with 400 µl of broccoli aqueous extract plus cadmium. The mice in the control group only received distilled water. In the cadmium group, mice were injected intraperitoneally only with cadmium chloride (3 mg/kg of mouse body weight). Four of the groups were orally administered 200 and 400 µl of the extract (1 g ml-1/60 g of mouse body weight) with and without intraperitoneally injection of cadmium chloride. The extract was administered every day for 48 days and cadmium on the last day of the treatment. On the 49th day of the experiment, all animals were sacrificed and samples were collected. The sperm was rapidly detached from the epididymis and used to determine the parameters. Mice testis were separated, immediately frozen in liquid nitrogen, and stored at -80°C for subsequent RNA extraction.
Evaluation of cauda epididymal sperm count and motility
We used the seminal plasma aspirated from the caudal part of the mice epididymis for the semen analysis. The epididymis was put in a solution containing 1 ml of PBS buffer (pH=7) and then cut into small pieces. We incubated the tissue homogenate at 37°C for 10 minutes to release the sperm in the solution. We placed 10 μl of the sperm on a clean slide coverslip for sperm count and motility evaluation 26. Total sperm counts were determined with a hemocytometer under a light microscope at 200X magnification. Spermatozoa were collected with a micropipette and placed on a slide for motility estimation and classification into motile and non-motile sperms. Motility was expressed as the percentage of sperm showing movement (fast forward, slow movements, and movement in place); data were expressed as percentages 26.
Sperm viability
Viability was assessed by eosin-nigrosin solution 26: A 10 µl sample of the sperm suspension was placed on a glass slide, mixed with 10 µl eosin, and after drying at laboratory temperature observed under a light microscope (400X). With this procedure, the head of the dead spermatozoa absorbs eosin and becomes red, but live spermatozoa appear colorless as the plasma membrane remains intact. Live spermatozoa were counted in five fields of vision at random and their percentage was recorded.
Semi-quantitative RT-PCR
RNA extraction and cDNA synthesis. Total RNA was extracted from the mice testis using RNX plus (EX6101) solution following the manufacturer’s protocol (SINACLON, Iran). The RNA pellet was dissolved in RNase-free water (DEPC treated water). Total RNA was then treated with DNAaseI (SINACLON, Iran) to destroy possible genomic DNA contamination followed by heat treatment (65°C for 10 minutes) to inactivate the enzyme and then stored at −80°C for future cDNA synthesis. We checked the purity and integrity of the total RNA extracted at 260/280 nm ratio using a Thermo Scientific NanoDrop spectrophotometer and visualized it on 1% agarose gel. We used a commercial cDNA synthesis kit (2-steps RT-PCR kit, Vivantis, Malaysia) following the manufacturer’s instructions. Briefly, 1 µg of total RNA, 0.5 µl M-MuLV Reverse Transcriptase (200 U/µl), 2 µl of 10X Buffer M-MuLV, 1 µl Oligo d(T)18 (40 µM), 1 µl of 10 mM dNTPs were added to a 0.2 ml microcentrifuge tube. The reaction mixture was adjusted to the final 20 µl volume with nuclease-free water.
Polymerase Chain Reaction (PCR)
Primer sequences, GeneBank ID, and amplification product sizes are summarized in table 1. Primers were synthesized (Metabion, Germany) and samples were normalized with the β actin gene as a reference gene for its constitutive expression. Synthesized cDNA was amplified by PCR reaction performed by using 2x PCRBIO Taq Mix Red in a reaction volume of 12.5 μl containing 6.25 μl 2x PCRBIO Taq Mix Red, 0.5 μl of each of the forward and reverse primers (10 pM), 1.5 μl cDNA, and 3.75 μl H2O. PCR conditions for Catsper1, Catsper2 and Sox9 were: 94°C for 2 minutes followed by 36 cycles (94°C for 30 s, 57°C for 30 s, and 72°C for 1 min as an extension), and a final extension at 72°C for 10 min. For Sox5, the conditions were: 94°C for 2 minutes followed by 32 cycles (94°C for 30 s, 60°C for 30 s, and 72°C for 1 minute as an extension), and a final extension at 72°C for 10 minutes. For Arl4a and β actin, the conditions were: 94°C for 2 minutes followed by 30 cycles (94°C for 30 s, 57°C for 30 s, and 72°C for 1 minute as an extension) and a final extension at 72°C for 10 minutes.
Table 1 Sequence of the designed primers used for RT-PCR
| Gene | Function | Accession number | Primer sequences | Product size | Optimized cycle number* | Reference | |
|---|---|---|---|---|---|---|---|
| CatSperl | Sperm motility | NM_ | 139301.3 | Forward: 5' 'TCGGAGAACCACAGAGAAGAG-3' | 566 bp | 36 | 27 |
| Reverse: 5' CACACACCGGGAATATCTTC-3' | |||||||
| CatSper2 | Sperm motility | NM_ | 153075.3 | Forward: 5' TGGCCACAGAGCAGTATTTG-3' | 513 bp | 36 | 27 |
| Reverse: 5' TGTCAGGCTGTTGCTTTGTC-3' | |||||||
| Arl4a | Spermatogenesis | NM | 007487.3 | Forward: 5' CAGGCTGCAGTTCAACGAAT-3' | 377 bp | 30 | Current study |
| Reverse: 5' - AATGCCAAGGAGTCGATGAG-3' | |||||||
| Sox5 | Transcription factor | NM | 001113559.2 | Forward: 5' CCCCACATAAAGCGTCCAATG-3' | 196 bp | 32 | 28 |
| Reverse: 5' - TCTCCAGGTGCTGTTTGCTGAG-3' | |||||||
| Sox9 | Transcription factor | NM | 011448.4 | Forward: 5' GAAGCTGGCAGACCAGTACC-3' | 479 bp | 36 | 29 |
| Reverse: 5' - CTGCTCAGTTCACCGATGTC-3' | |||||||
| β actin | Reference gene | NM | 007393.1 | Forward: 5' 'GGGAAATCGTGCGTGACAT - 3' | 385 bp | 30 | 27 |
| Reverse: 5' TCAGGAGGAGCAATGATCTTG -3' | |||||||
The number of PCR cycles was determined to obtain a detectable signal without reaching saturation.
We determined the number of PCR cycles for each gene to obtain a detectable signal without reaching saturation. For the electrophoresis of amplified products, we used 1% agarose gel and the amplified cDNA fragment was visualized and photographed under UV light. The band intensities were semi-quantitatively analyzed using the E-capt software (Vilber Lourmat, France) and normalized against that of β actin. The resulting data were expressed as means and standard deviation (SD) of at least three PCR replicates.
GeneMANIA in silico analysis to study protein-protein interaction
We utilized a large set of protein-protein interaction databases within the GeneMANIA package 30 to build a protein-protein interaction network. This package comprises 244 databases/articles that collectively utilize experimentally proved physical protein-protein interactions in humans. The Arl4a gene was used as input for the GeneMANIA algorithm. Although we used “mice” in the study, we selected “Homo sapiens” as the organism in the GeneMANIA input setting because protein-protein interaction in “human” is more complete than in “mouse” and, thus, it provided a more comprehensive insight into Arl4a biological functions.
Results
Impact of the broccoli extract on the body and testis weight with and without Cd-toxicity
No significant difference was evident between groups regarding body and testis weight (table 2).
Table 2 Body and testis weight in NMRI male mice treated with 200 and 400 µl of broccoli extract (BE) of (1 g ml-1) with and without Cd (1 mg kg-1 of mouse weight). Data represent the mean of six mice in each group (± SD). The different letters indicate a significant difference among groups (p ≤ 0.05; Oneway ANOVA; Tukey´s HSD all-pairwise comparisons as a post-hoc test).
| Sample | Control | Cd | BE (200 μl) | Cd+ BE (200 μl) | BE (400 μl) | Cd+ BE (400 μl) |
|---|---|---|---|---|---|---|
| Body weight (g) | 47.7±3.9a | 39.7±5.2a | 38.7±4.8a | 44.8±5.7a | 40.9±1.5a | 45.2±5a |
| Testis weight (g) | 0.17±0.03a | 0.16±0.04a | 0.13±0.01a | 0.15±0.03a | 0.14±0.02a | 0.16±0.02a |
Impact of the broccoli extract on sperm parameters with and without Cd- toxicity
Figure 1 displays sperm microscopic images in NMRI mice treated with cadmium and the broccoli extract while figure 2 shows the experimental data on sperm parameters: we observed a positive significant impact of the extract on the sperm count in a dose-dependent manner (figure 2A). The sperm count in the 400 µl extract-treated group was 1.5, i.e., two-fold higher than in the 200 µl extract-treated and the control groups (figure 1, A, C and E). Additionally, we found a clear benefit of the extract in the prevention of Cd-toxicity in the sperm count. Likewise, the sperm count significantly increased in the extract (almost 2.5 fold) and CD-treated (2.7 fold) groups (200 and 400 µl) compared to the mice treated only with Cd (figure 1B, D, F).
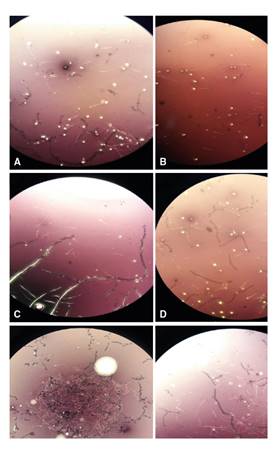
Figure 1 Microscopic images of sperm in NMRI mice treated with Cd and BE. A: Control; B: Cd; C: 200 µl of BE (1 g ml-1); D: 200 µl of BE (1 g ml-1) plus Cd (3 mg kg−1 of mouse body weight); E: 400 µl of BE (1 g ml-1); F: 400 µl of BE (3 g ml-1) plus Cd (1 mg kg−1). 4X
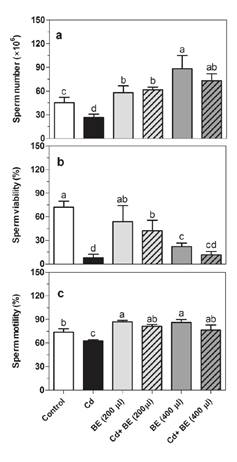
Figure 2 Sperm count (a), sperm viability (b), and sperm motility (c) in NMRI male mice treated with 200 and 400 µl of BE (1 g ml-1) with and without Cd (1 mg kg−1 of mouse weight). Data represent the mean of six mice in each group (± SD). Different letters indicate significant differences among groups (p ≤ 0.05; Oneway ANOVA; Tukey´s HSD all-pairwise comparisons as a post-hoc test).
Furthermore, sperm viability was clearly reduced in Cd-treated mice compared with the control group (figure 1A, B; figure 2B). When Cd-treated mice were administered 200 and 400 µl (1g/ml) broccoli extract, the sperm viability increased 5.3 and 1.5 fold, respectively (figure 2B).
However, sperm viability hardly changed in mice treated with 200 µl extract and acutely decreased in mice treated with 400 µl extract (figure 2B). Additionally, sperm motility significantly decreased in Cd-treated mice (figure 2C), but their treatment with both 200 and 400 µl extract led to an increase in sperm motility. Likewise, the mice treated with both extract doses exhibited higher sperm motility than those in the control group.
Impact of the broccoli extract on Catsper1, Catsper2, and Arl4a gene expression with and without Cd-toxicity
Figure 3 shows the relative gene expression by semi-quantitative RT-PCR while in figure 4 no significant difference in the Catsper1 gene expression among groups is evident.
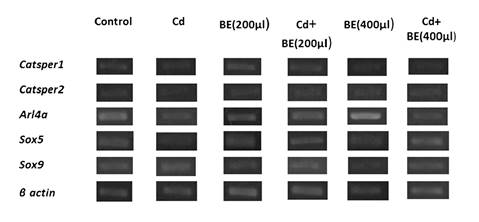
Figure 3 Relative gene expression determined by semi-quantitative RT-PCR. The expression of genes corresponds to the ratio of the target gene divided by the reference gene (β actin).
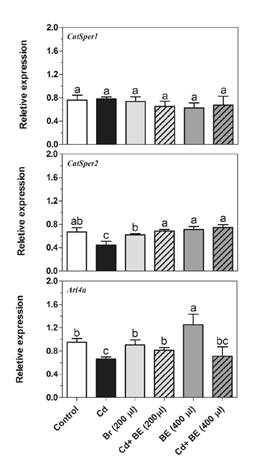
Figure 4 Transcript levels of Catsper1 and Catsper2 (genes involved in sperm motility) as well as Arl4a (gene involved in sperm count) in NMRI male mice. For experimental details, see legend in Figure 1. Relative gene expression was determined by RT-PCR compared to β actin as reference gene. Data represent the mean of three mice in each group (± SD). Different letters indicate significant differences among groups (p ≤ 0.05; Oneway ANOVA; Tukey´s HSD all-pairwise comparisons as a post-hoc test).
Catsper2 gene expression in Cd-treated mice was significantly lower than in the other groups (Figure 4). However, there was no significant difference among extract-treated groups with and without Cd-toxicity and a closer look at the graph indicated that the Catsper2 gene expression in the groups treated with 200 and 400 µl extract plus Cd was significantly higher than in the mice treated only with Cd (figure 4).
The Arl4a gene mRNA level was up-regulated (1.8 fold) in the group treated with 400 µl extract (figure 4) while its expression was clearly down-regulated in the group treated only with Cd but higher in the two groups treated with 200 and 400 µl extract plus Cd than in the mice treated only with Cd (figure 4).
Impact of the broccoli extract on the gene expression of transcription factors SOX5 and SOX9 with and without Cd-toxicity
Figure 5 provides the experimental data on the gene expression of Sox5 and Sox9 transcription factors. Following the intraperitoneal administration of Cd, we detected a significant decrease in the Sox5 gene expression. However, no significant differences were found in Sox5 gene expression between extract- treated mice (200 and 400 µl) and the control group while it was significantly up-regulated in the extract plus Cd-treated mice compared with those treated only with Cd. Furthermore, Sox9 gene expression appeared to be unaffected by Cd. Likewise, none of the extract-treated mice groups, both with and without Cd, showed significant differences in the Sox9 mRNA level (figure 5).
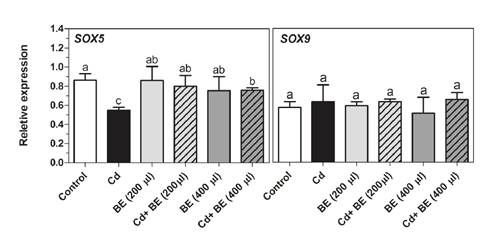
Figure 5 Transcript levels of Sox5 and Sox9 (transcription factors involved in sperm motility) in NMRI male mice. For experimental details, see legend in Figure 1. Relative gene expression was determined by RT-PCR compared to β actin as reference gene. Data represent the mean of three mice in each group (± SD). Different letters indicate significant differences among groups (p ≤ 0.05; Oneway ANOVA; Tukey´s HSD all-pairwise comparisons as a post-hoc test).
Discussion
We found few publications on the association between spermatogenesis and broccoli extract 31,32. In such context, we conducted the present study to determine the effect of this extract on the sperm factors and the expression of some genes whose relation to spermatogenesis has already been reported.
The most obvious and interesting finding (figure 2A) was the significant dose-dependent increase in sperm count among extract-treated groups even in the presence of Cd. Surprisingly enough the Arl4a gene expression also showed a similar increase in sperm count in extract-treated groups with and without Cd. ARL4 is a 22-kDa GTP-binding protein abundant in testes of pubertal and adult rodents. In mouse Arl4-null mutants (Arl4 -/-) the inactivation of the Arl4 gene caused a significant reduction of testis weight and sperm count (30% and 60%, respectively) 22 and, in general, the association between testis weight and the Arl4a gene has been reported in the literature 33.
Genome-wide mapping in house mice revealed a significant association between SNPs located in the Arl4a gene and relative testis weight. However, no significant difference between body and testis weight among the six groups in our study was found. Similarly, Zhou, et al. did not find significant effects on body and testis weight in mice treated with broccoli seed extract (0.3, 1, and 3 g/kg body weight/day) for 30 days 34 and the broccoli seed extract LD50 in rats was >10 g/kg of body weight/day.
Although the role of the Arl4a gene in mouse spermatogenesis was reported in 2002 22, very little is currently known about its biological functions and spermatogenesis-related mechanisms. In this sense, we conducted an in silico analysis to find further evidence regarding its role in spermatogenesis and its binding proteins using the GeneMANIA algorithm. As shown in figure 6, Arl4a was bound to Spatc1l through the protein-protein interaction. Previously, it had been shown that Spatc1l maintains the integrity of the sperm head-tail junction and that Spatc1l knockout mice developed male sterility owing to the separation of sperm heads from tails 35. The Kpna2 gene also presented protein-protein interaction with Arl4a and it has been reported that it is a key mediator of nucleocytoplasmic transport in the embryonic testis. Kpna2 mRNA was identified in pachytene spermatocytes and round spermatids 36 and Kpna2 amount markedly increased in both cells 37. Golga2, another protein physically binding to Arl4a, also plays a role in spermatogenesis. Han, et al. showed that the inactivation of Golga2 caused male infertility in a mouse model. In Golga2 −/− mice, acrosome and round sperm heads, characteristic features of human globozoospermia, were absent 38 and the cytoskeleton was disorganized in testes.
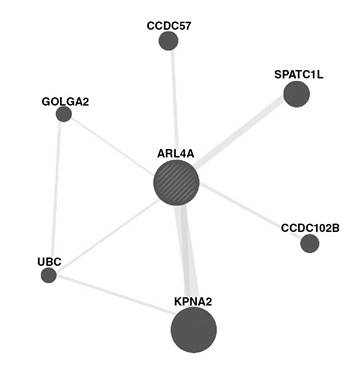
Figure 6 Protein-protein interaction between human ARL4A protein and six other proteins based on the GeneMANIA algorithm
During spermatogenesis, the ubiquitin-proteasome pathway (UPP) plays a key role in facilitating the formation of condensed sperm and, therefore, deficient UPP blocks spermatogenesis. Ubiquitination occurs in different cell types during spermatogenesis, especially in spermatocytes differentiating into round and elongated spermatids and, even, in mature sperm in the epididymis 39. As shown in figure 4, ubiquitin C (UBC), one of the ubiquitination enzymes, can also physically bind to Arl4a.
Some studies have focused on the association between CCDC102B (coiled-coil domain containing protein 102b) and spermatogenesis. This protein is active in the assembly of the centrosome linker to maintain centrosome cohesion as it contributes to holding the duplicated centrosomes together and prevents centrosome separation. The regulation of the connection and/ or disconnection of two centrosomes is involved in several cellular processes, such as Golgi and cilia positioning 40. On the other hand, CCDC57 is another protein whose functionality has not been well-documented and it may play a role in the centrosome as it has a coiled-coil domain 41.
Interestingly, it was found that Arl4 has two separate promoters in the rat. Jacobs, et al., showed that the mRNA transcription is under the control of the downstream promoter in most tissues while the upstream promoter seems to drive specifically the expression of Arl4 in adult testis 42. In fact, they recorded tissue-specific alternative splicing and promoter use in the Arl4 gene. These findings provide insights into the molecular control of Arl4a protein in spermatogenesis. Apparently, it has several biological functions in different tissues and organelles, and its expression is regulated developmentally.
Broccoli accumulates selenium, consequently reducing the risk of several cancers 43,44; besides, it is required for spermatogenesis and male fertility 45. Selenium significantly increased the Catsper1 and Catsper 2 gene expression in adult male mice 46 and, therefore, it can enhance sperm mobility. The results of our study indicated that Catsper2 was also up-regulated with the dose-dependent broccoli extract; however, no significant difference was detected in the Catsper1 mRNA levels. We also observed an obvious increase in spermatogenesis using the extract, which is consistent with the selenium enrichment of broccoli and its positive impact on this process. Our finding is in line with those reported by Raeeszadeha, et al. who observed that the number of spermatogonia, primary spermatocytes, spermatids, and sperm significantly increased with the hydro-alcoholic extract of broccoli (300 mg/kg) in male mice after 42 days of treatment 32.
As reported in other studies, we found that sperm count, motility, and viability were reduced in Cd-treated mice while the Catsper2 gene was down-regulated 47,48. However, our findings diverge from those reported by Mohammadi, et al. who observed a reduction of Catsper1 mRNA level in Cd-treated mice 48. Of course, Catsper1 gene expression is complex, its promoter is bidirectional and regulates the lncRNA (Catsper1au) expression. Indeed, Catsper1au is expressed in adult male mouse testis and can regulate gene expression during spermatogenesis 49. It may be that broccoli contains compounds that bind directly or indirectly to lncRNA regulatory proteins or binds to the Catsper1au target site or some other unknown phenomenon involved in Catsper1 gene regulation.
Previous studies have established that both Sox5 and Sox9 transcription factors interact with the Catsper1 promoter in HEK-293 cells 50. As shown in figure 5, the gene expression in Sox9 was similar to that in the Catsper1 gene, and no differences were observed among treatments in any of them, which agrees with Mata-Rocha, et al.’s findings. However, our findings indicated that Sox5 gene expression was dramatically reduced in Cd-treated mice as compared with the control. A deeper look revealed that in the 200 and 400 µl extract-treated mice, Sox5 mRNA level was slightly higher than in the Cd plus 200 and 400 µl extract-treated mice may be due to the other transcription factors or to the miRNA, e.g., miR-195, which targets Sox5 3’ UTR and impairs its expression 51.
Quercetin and kaempferol are the predominant flavonoids in commercial broccoli 52. Quercetin attenuates Cd-induced oxidative damage and apoptosis in granulosa cells from chicken ovarian follicles 53. In spite of the antioxidant properties of quercetin, a significant concentration-dependent conflicting effect on sperm viability and motility has already been observed 54,55, which is somewhat in line with our findings. Indeed, we observed that the broccoli diet affected positively the sperm count; however, it negatively impacted sperm viability, though increasing sperm motility slightly (figure 2). Raeeszadeh, et al., determined that the total antioxidant IC50 of broccoli hydro-alcoholic extract is 278±14 µg/ml 32, a finding that probably explains the negative impact of broccoli extract on sperm viability and highlights the complexity of its antioxidant properties. Additionally, it is reported that the treatment with quercetin markedly inhibited nickel-induced global hypermethylation and DNA hypomethylation of the nuclear factor E2-related factor 2 (Nrf2) promoter 56, which may subsequently affect its downstream target genes. Kaempferol also inhibits DNA methylation by suppressing DNA methyltransferases 57.
It has been previously observed that kaempferol leads to the downregulation of DNMT3 (a kind of DNA methyltransferase) and modulates DNA methylation in cancer 58. Indeed, kaempferol alters 103 DNA methylation positions (hypo-methylation and hyper-methylation) associated with bladder cancer genes 57. Likewise, sulforaphane, another predominant ingredient in broccoli, increased Nrf2 expression, a transcription factor, in prostate tumor cells through epigenetic regulation in mice 59. By the activation of Nrf2/ARE signaling pathways, sulforaphane prevented testicular damage in Cd-treated mice 60. Sulforaphane also reduced Cd-induced toxic effects on human mesenchymal stem cells and showed a significant recovery of cell viability 61. Despite supportive evidence on the impact of sulforaphane as an anticancer agent, the fact is that it also interferes with the T cell-mediated immune response 62 turning it into a double-edged sword.
There is a growing number of reports on the epigenetic regulation of broccoli ingredients and the stable and reversible mechanism regulating gene expression. Further research is needed to determine which compounds in broccoli extract stimulate spermatogenesis without a negative effect on sperm viability. Future studies on lower dosage would allow finding a suitable concentration of broccoli to avoid its negative effect on sperm viability and use it as a medicinal plant for male infertility.
In future studies, experiments using real-time PCR would offer a more precise view of gene expression alterations. Probably, Homo sapiens-based protein-protein interaction instead of Mus musculus do not reflect exactly what happens in mice; however, this in silico analysis definitely would be informative as it supports the role of Arl4a in the spermatogenesis process. Our results are promising for the pharmaceutical industry in its efforts to produce medications for infertile men.














