Snake bites and cerebrovascular attacks (CVA) are complex events whose pathogenesis is not yet fully understood, and CVA is considered one of the most severe complications of snake bites 1.
Bothrops spp. vipers belong to the Viperidae family, which comprises about 200 species of snakes. Russell's viper (Daboia ruselli), Echis carinatus, Bitis arietans, Crotalus spp., Sistrurus spp., Bothrops spp., and Bothropoides spp. are among the most dangerous species for humans. These snakes are mostly found in the Americas, Europe, Africa, and Asia 2-4.
Bothrops spp. snakebites produce most of the envenomation in Central and South America. In the Department of Nariño, southwestern Colombia, reports show that Bothrops snakes (mainly Bothrops asper) have caused 43.6% of ophitic accidents 5. Bothrops spp. envenomation is known for its complex pathogenesis due to its local effects, such as edema, pain, redness, and necrosis, or its systemic manifestations characterized by coagulation disorders, thrombosis, kidney failure, and hemorrhages 6.
Although hemorrhagic stroke is a common neurologic complication of ophidic accidents 7-10, others such as muscle paralysis, neuromuscular junction disorders, 11 and ischemic stroke have been described (table 1). The toxic effects of the venom in cerebrovascular events have been described in hemorrhagic stroke; however, it has been argued that the secondary procoagulant effect of various components of the poison, the hypotensive shock, and the vasculitis mediated by the immune system are responsible for ischemic stroke leading to an unfavorable prognosis in most cases 1,7.
Table 1 Description of the reported cases of ischemic stroke and Bothrops snakebite
| No. case | Year and reference | Country | Snakebite | Vascular territory | Laboratory tests on admission | Deficit |
|---|---|---|---|---|---|---|
| 1 | Numeric, et al., Martinica (2002) 26 | Martinica | B. caribbaeus | Right ACA and multiple small foci in cerebellar cortex, right PCA, both MCA | CK: 1212 U/L (26-174); CRP: 147.7 mg/L (0-10); PTT: 29 s (control: 32); fibrinogen 6.33 g/L (2-4); D dimer: positive; PLT 201000 | Left hemiplegia and a partial Wernicke’s aphasia |
| 2 | Angarita,et al., Colombia (2003) 33 | Colombia | B. spp. | Right MCA, left MCA | PT> 2 min; PTT>2 min PLT : 23.500 | Ocular deviation to the right, dysarthria, left hemiparesis, bilateral Babinski |
| 3 | Merle, et al., Martinica (2005) 32 | Martinica | B. lanceolatus | Right PCA | PLT: 52,000 cells/mm3 PT: 14% (70-100%), aPTT: 51 s (normal, 32); fibrinogen <0.5 g/L (2-4 g/L); factor II level 66% (70- 120%); factor V level 17% (70-120%); FDP: 2560 pg/ml (normal <5 g/ml) | Left, lateral, homonymous quadranopsia with macular epargne |
| 4 | Thomas, et al., Martinica (2006) 34. | Martinica | B. lanceolatus. | Both PCA | PLT: 57,000; fibrinogen<0,5 (g/l); PT: 34 s; aPTT: 51 sec; FDP: 2560 pg/ml; CRP 1,9 mg/ml | Right hemiparesis and aphasia |
| 5 | Thomas, et al., Martinica (2006) 34. | Martinica | B. lanceolatus | Left MCA | PLT: 20000; fibrinogen 2,45 (g/l); PT: 15 s; aPTT: 35 s; FDP: 320 pg/ml; CRP: 25,3 mg/ml | Right hemiparesis and aphasia |
| 6 | Thomas, et al., Martinica (2006) 34. | Martinica | B. lanceolatus | Both PCA, left MCA | PLT: 260,000; fibrinogen 2,81 (g/l); PT: 13 s; aPTT: 28 s; CRP: 0 mg/ml | Left hemiparesis and left homonymous hemianopsia. |
| 7 | Cañas, et al. Colombia | Colombia | B. atrox | Basilar artery | PLT: 18,000/mm3, PT>1 min A PTT>1 min; fibrinogen was not detected | Comatose, miotic pupils without reaction to light and generalized hypotonia, lack of response of limbs to painful stimuli, bilateral Babinski |
| 8 | Martinez- Colombia Villota et al. | Colombia | Bothrops spp. | MCA | PT: 44 sec; blood test, normal; liver and renal function, normal; fibrinogen<50 mg/ml | Impaired level of consciousness, agitation, and left hemiparesis |
PT: Prothrombin time; PTT: Partial thromboplastin time; acPTT: Activated cephalin time or partial thromboplastin time; PLT: Platelet, CK: Creatine kinase; CRP: C-reactive protein; ACA: Anterior cerebral artery; MCA: Middle cerebral artery; PCA: Posterior cerebral arteries; FDP: Fibrin degradation product
We report here the case of a 50-year-old patient with a massive ischemic stroke caused by Bothrops spp. envenomation in a rural area in southwestern Colombia.
Case report
A 50-year-old male patient with no medical background was referred from a rural area in southwestern Colombia (department of Nariño) to the emergency room of our hospital. He had been bitten by a Bothrops spp. snake on the second finger of his left hand. At the primary care hospital, the physical examination showed the progression of the edema to the left arm but no evidence of ulceration, hemorrhage, necrosis, signs of infection, or other findings. The results from the laboratory tests were: Prothrombine time: 44 s, normal complete blood count, and normal kidney function.
The patient was treated with nine vials of polyvalent anti-venom serum four hours after his referral. He arrived at our hospital 13 hours after the snakebite; he was hemodynamically unstable, with a deterioration of his level of consciousness, agitation, and left hemiparesis which required management in the intensive care unit (ICU) and mechanical ventilation assistance. The blood test performed showed thrombocytopenia, dysfibrinogenemia with fibrinogen levels below 50 mg/ml, and clotting times without alterations. The patient was evaluated by a neurologist who ordered a brain MRI that revealed multiple ischemic areas in the bilateral frontal-temporal and bilateral occipital regions, as well as pons and cerebellum (figure 1).
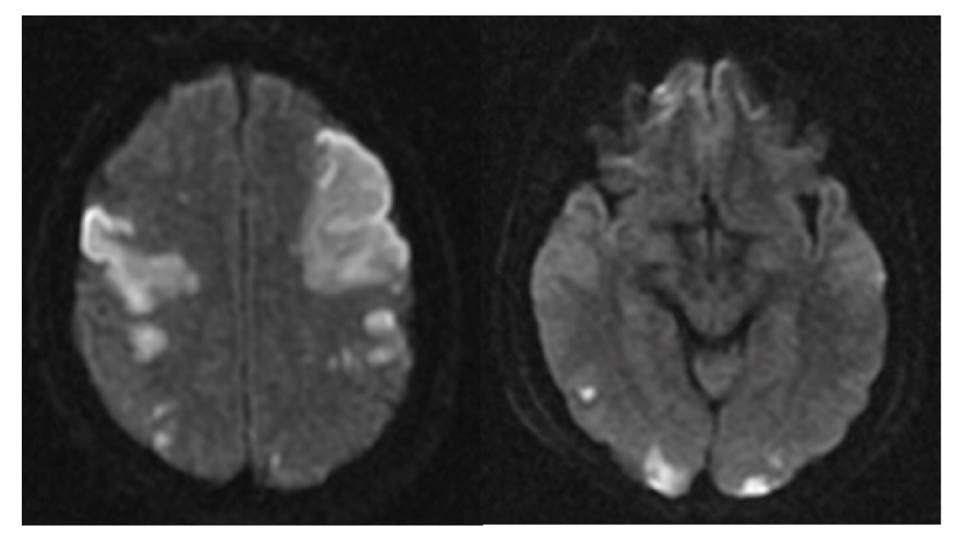
Source: Archive of the Department of Diagnostic Images, University Hospital of Nariño, Pasto, Colombia
Figure 1 MRI (DWI) showing multiple areas of bilateral fronto-temporal, and occipital ischemia.
Due to the severity of the stroke, he was given an additional anti-venom scheme (each vial contains 10 ml of lyophilized equine polyvalent anti-venom serum which neutralizes at least 25,10, and 5 mg of the venom of Bothrops asper/atrox, Crotalus, and Lachesis snakes, respectively). The patient did not receive plasma because this would have increased the procoagulant risk and fibrinogen levels gradually in the first 24 hours after the anti-venom treatment. The patient improved as recorded in the follow-up control two months later, but his bilateral paresis on the VI and III cranial nerves persisted with a strength of 2/5 on the left side, as well as left Babinski's sign with a Modified Rankin Scale (mRs) of 4.
We complied with all the ethical regulations for research in humans and no intervention was carried out. We received the informed consent of the patient authorizing the publication of the case and the images.
Discussion
Snake bites and strokes have been reported in detail in the medical literature. Mosquera, et al. 7, for example, found a 2.6% prevalence of cerebrovascular complications among 309 victims of snakebites 7. Ischemic stroke has been described in other snake species, mainly in Gloydius brevicaudus, Crotalus durissus terriftous, Hypnale, Echis carinatus, and Russell’s viper (Daboia russelii) 12-22.
A study conducted in Sri Lanka showed that 1.8% of 500 patients bitten by Russell’s viper had an ischemic stroke 23. Other studies In Martinique provided a closer panorama of ischemic stroke with pro-coagulant effects provoking up to 22% of thrombotic complications such as pulmonary thromboembolism and myocardial infarction, and 12% of stroke in the patients observed 24-26.
In Colombia, a study of 39 cases found that 12.8% of the patients bitten by a Bothrops snake had hemorrhages in the central nervous system, but no ischemic strokes were reported 27. In four additional Colombian studies involving around 698 patients, no ischemic strokes were registered 28-31. According to the literature review, and as far as we know, seven cases of ischemic stroke due to Bothrops spp. envenomation have been documented 26,32-35 (table 1) in the world. In our country, only some descriptions are found. Angarita, et al., reported a stroke associated with an ophidian accident 33, and Cañas, et al., recently reported an ischemic stroke in the brainstem caused by a Bothrops atrox snakebite 35. In this context, the present case is the third one reported in Colombia and the eighth in the world.
The etiopathogenesis of ischemic stroke still has to be fully elucidated 36,37. The most relevant hypothesis currently considered is thromboinflammation activated by the different toxins of the venom. The most representative enzymes in this process are metalloproteases and type-C lectins responsible for stimulating platelet function and pro-inflammatory activity 38 causing endothelial injury, stimulation of the immune system, immune-mediated vasculitis, hypercoagulable state, and systemic hypotension 1,38. Other toxins, such as digestive enzymes and complementary factors, produce local and systemic injury. The enzymes causing the most severe reactions are the zinc-dependent metalloproteinases called “hemorrhages” which cause hemorrhage due to hydrolysis of the basal lamina in capillaries 39. Similarly, phospholipase A2 is involved in the formation of edema and myotoxicity, and it has anticoagulant effects. Additional studies of snake venom including proteomics have revealed that some venom compounds are acid phospholipases A2, serine proteinases, acid 1-amino oxidases, zinc-dependent metalloproteinase, and specific C-type lectin binding molecules 40-42.
The molecules released in edema and hemorrhages are the metabolites of arachidonic acid (cyclooxygenase and lipoxygenase), bradykinin, histamine, and serotonin 43. It has been shown that Bothrops lanceolatus snakes are the most frequently associated with systemic thrombotic complications 26,44. It is understood, then, that toxins produce the thrombotic phenomenon through endothelial injury due to the direct action of the venom on the vessels 6. Endothelial damage is the result of the synergistic effect of metalloproteinase and phospholipase A2 45. The hyperviscosity produced by the hypovolemic state and hypoperfusion secondary to hypotension contributes to the occlusion of the vessels 46. Similarly, some toxins promote the activation of the complement system through the generation of anaphylatoxins hydrolyzing C3 and C5 and activating the three complement pathways, especially the lectin pathway and C1-INH inactivation, which is an important inhibitor of coagulation proteins contributing to inflammation and thrombosis 47 (figura 2).
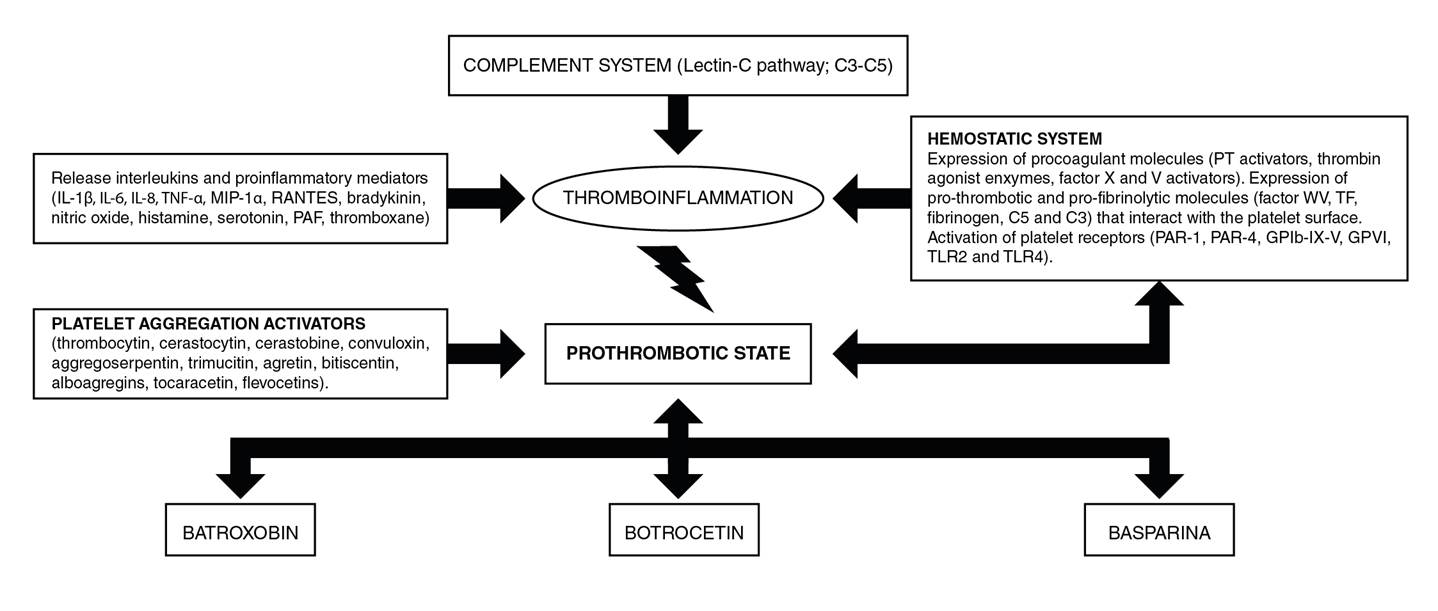
Figure 2 Flow-diagram of probable pathophysiology mechanisms of ischemic stroke in the Bothrops envenomation.
The factor V Leiden has also been considered since it plays a key role in the activation of the anticoagulant and procoagulant pathways and is activated by several toxins and proteases present in the venom of the vipers Vípera, Naja oxiana, and Bothrops atrox48. In a comparative analysis of the coagulant activity of the venoms of the different Bothrops spp. snakes, the venom of B. erythromelas showed high levels of factor X and prothrombin activators 49. Bothrops spp. venoms possess thrombin activating enzymes 50. The main molecules associated with the anticoagulant and procoagulant effect of the Bothrops snake venom are described in table 2. The cranial nerve involvement could be associated with cerebellar and pons infarction, as well as snakebite- associated ophthalmoplegia that has also been described 11.
Table 2 Main procoagulant and anticoagulant molecules of the hemostatic system of the Bothrops snake
| Anticoagulants factors | Procoagulants factors | |
|---|---|---|
| A. Thromboinflammation 38 | ||
| A. Aspercitin, hemorrhages, metalloproteinases: these substances can cause thrombocytopenia, prolongation PT and PTT, and disseminated intravascular coagulation. They produce hemorrhages of the cerebral parenchymal and subarachnoid 7. | Immune system: activation of the function and migration of the leucocytes release of pro-inflammatory mediators (IL-1β, IL-6, IL-8, TNF-α, MIP-1α, NO, histamine, serotonin, PAF, bradykinin, PGE2, TXA2, LTB4, and RANTES) generation of anaphylatoxins (C3 and C5) generation of DAMPS, TF, and vW factor | Hemostatic system: - expression of procoagulant molecules (PT activators, thrombin agonist enzymes, factor X and V activators) - expression of pro-thrombotic and pro-fibrinolytic molecules (vW factor, TF, fibrinogen, C5 and C3) that interact with the platelet surface - activation of platelet receptors (PAR-1, PAR-4, GPIb-IX-V, GPVI, TLR2, and TLR4) activation of platelets by SV-CLRPs of a non-enzymatic pathway by interaction of toxins with the CLEC-2 receptor |
| B. Activation of c-protein through the activation of the serine proteases | B. Batroxobin: It is a serine protease similar to the thrombin of Bothrops atrox moojeni with the ability to coagulate fibrinogen. Thrombin that releases fibrinopeptides a and b from the NH2 terminal domains of fibrinogen Aα and Bβ chains respectively. Batroxabine only releases fibrinopeptide A. Thus, batroxobin binds fibrin in a different way than thrombin, which may contribute to its higher affinity interaction, selective fibrinopeptide A release, and prothrombotic properties 51. | |
| C. Inhibition of factor IX and X | C. Botrocetin: It is a heterodimeric protein snake venom isolated in Bothrops jararaca that induces vwf and platelet glycoprotein gpib-dependent platelet agglutination 52. | |
| D. Inhibition of the trombin through the bothrojaracine | D. Basparin a: It was isolated from the venom of Bothrops asper; it does not induce local tissue alterations such as hemorrhage, myonecrosis, and edema. It does not induce systemic hemorrhage, thrombocytopenia, or prolongation of the bleeding time following intravenous administration. At low doses, the only observed effect induced by basparin a when injected intravenously or intramuscularly into mice is defibrinogenation. At higher doses, intravenous administration resulted in sudden death due to numerous occlusive thrombi in the pulmonary vessels 53. | |
| E. Inhibition of the complexprothrombinase: action through the phospholipase A2 | E. Aspercitin, hemorrhages, and metalloproteinases: The cause of injury to the blood vessels wall produce cerebral infarct 7. | |
| F. Fibrinolytic activity: adamalysin, fibrolase, atroxase, lebetase | F. Complement system: Activation of the 3 complement pathways, especially the lectin-C pathway through the hydrolysis of peptides C3, C4, and C5. The venom also acts directly on the C5a fragment for the generation of C5a convertase. The C1 inhibitor is a serine protease that regulates the complement cascade (inhibits C1r, C1s, and MASPs), and the coagulation cascade acting on fibrinolytic proteins (kallikrein, FXIIa, FXIa, and plasmin) 46 | |
| G. Platelet aggregation inhibitors: α-fibrinogenase, phospholipase A2, 5'-nucleotidases, jarahagin, catrocollastatin, crovidisin, disintegrins, cerastatin, barbourin, albolabrin, cistrin, flavoviridine, elegantin, rhodostatin, tigramine 53 | G. Platelet aggregation activators: Thrombocytin, cerastocytin, cerastobine, convuloxin, aggregoserpentin, trimucitin, agretin, bitiscentin, alboagregins, tocaracetin, flavocetins 54 | |
PGE2: Prostaglandins; TXA2: Thromboxane; LTB4: Leukotrienes; C3 and C5: Complement fragments; DAMPs: Molecular patterns associated with damage; TF: Tissue factor; vW: von Willebrand factor; PAR-1, PAR-4: Protease activating receptors; GPIb-IX-V: GPIb-IX-V glycoprotein complex; GPIV: Glycoprotein VI;TLR2 and TLR4:Tolllike receptors; CLEC-2: C-type lectin receptor
Conclusion
The pathogenesis of the Bothrops envenomation and the thrombotic phenomenon is complex. There are numerous theories that attempt to explain their mechanism but further research is required for their full understanding. The thromboinflammation theory explains some of the local and systemic effects, such as thrombocytopenia, prolonged clotting times, disseminated intravascular coagulation, and thrombotic events. Procoagulant activity, hypovolemic shock, and endothelial injury have been considered as factors that promote ischemic stroke. In the case we report, despite an early initiation of the treatment, a catastrophic ischemic stroke occurred resulting in considerable disability of the patient. It is imperative to understand this phenomenon as a severe neurological complication in American countries as Colombia where Bothrops snakes are widely distributed and ophidian accidents are a frequent reason for consultation in the emergency room.














