Strongyloidiasis, a neglected tropical disease that affects around 370 million people worldwide, is caused by soil-transmitted helminths of the genus Strongyloides mostly distributed throughout tropical and subtropical regions 1-3. Strongyloides stercoralis, the most common agent of this disease, is classified according to its prevalence: sporadic (<1%), endemic (1-5%), or hyperendemic (> 5%) (Pires, Dreyer, 1993). Hyperendemic areas are located mainly in the tropics, especially in the developing countries of Asia, Sub-Saharan Africa, and Latin America (notably Brazil and Colombia) 4,5. In Brazil, the average rate of S. stercoralis infection between 1990 and 2009 was approximately 5.5%, which means the country is hyperendemic 6. In Salvador, the capital city of the state of Bahia, Brazilian Northeast, the prevalence of infection ranges from 4.6% to 6.6% 7,8.
In the general population, S. stercoralis infection can be characterized as chronic or asymptomatic. However, immunosuppressed individuals, such as those infected with human T-lymphotropic virus type 1 (HTLV-1), have a greater susceptibility to infection, which can progress to life-threatening forms of strongyloidiasis 9-11 and poor therapeutic response 12,13.
Five to ten million people are infected with HTLV-1 worldwide 14. Brazil is the country with the highest absolute number of HTLV-1 cases, about 800,000 15. In a study conducted in Salvador, the overall prevalence of HTLV-1 infection was 1.74%, which increases significantly in females over 51 years of age reaching up to 9% 16,17. Previous studies have reported elevated frequencies of S. stercoralis in patients infected with HTLV-1 from Japan 12,18. In Brazil, with the highest number of HTLV-1 carriers, the frequency of S. stercoralis infection varies according to the geographic region with greater occurrence in the North and Northeast regions 19 where it ranges from 12 to 15.7% 9,20,21.
The HTLV-1 transmission may occur in three ways: a) through sexual contact with a 60% efficiency when transmitted from man to woman and 4% from female to male; b) via the blood when sharing syringes or contaminated needles, or by blood transfusion, and c) vertically from mother to child, especially through breastfeeding 17,22. HTLV-1 transmission via organ transplant has also been described and is associated with the development of myelopathy/tropical spastic paraparesis (HAM/TSP) possibly due to the immunosuppression to which these individuals are subjected 23,24.
The present study aimed to investigate the prevalence of S. stercoralis infection and the seroprevalence of IgG anti-S. stercoralis antibodies in individuals infected with HTLV-1 attending the Reference Center for HTLV-1 (CHTLV) in Salvador, Bahia, Brazil.
Materials and methods
Study description
The present cross-sectional study was conducted in 178 HTLV-1-infected individuals seen and treated at the Integrated Multidisciplinary Center for HTLV (CHTLV) of the Bahiana School of Medicine and Public Health (EBMSP), in Salvador, Bahia, from January 2014 to December 2018.
CHTLV is a public outpatient clinical center that provides interdisciplinary care and services including general medical treatment, laboratory diagnosis, psychological counseling, and physical therapy. All individuals with associated comorbidities, such as immunosuppression due to the chronic use of glucocorticosteroids, HIV infection, or chronic alcohol abuse, were excluded from the study. The parasitological and immunological diagnoses of S. stercoralis were performed at the Faculty of Pharmacy of the Federal University of Bahia, Salvador, Brazil.
Data and sample collection
A questionnaire was drawn up to collect socio-demographic data and information on individuals’ residential sanitary conditions. Fresh stool samples were obtained from all enrolled subjects and submitted to parasitological examination as described below. Blood samples were collected in tubes containing polymer gel for serum separation and then centrifuged for 10 minutes at 1,620g. Sera were frozen at −20°C until use.
HTLV-1 diagnosis
We screened serum samples for HTLV-1 antibodies at the CHTLV by microparticle CLIA chemiluminescence (Architect rHTLV-1/2, Abbott Diagnostics Division, Wiesbaden, Germany) and confirmed by Western blot following the manufacturer’s instructions (HTLV Blot 2.4, Genelabs Diagnostics, Singapore).
Strongyloides stercoralis and other intestinal parasites diagnosis
Fresh stool samples from each subject were examined by three different parasitological methods: Hoffman, Pons and Janer 25, Baermann-Moraes modified by Rugai 26,27, and agar plate culture (APC) 28. The detection of anti-S. stercoralis IgG was performed by ELISA 29 as described below.
Larvae quantification
The parasite load was quantified by counting the number of larvae under microscopy (10 x objective lens) found in approximately 1 g of feces using the Baermann-Moraes method. The number of larvae was categorized as “non-quantified” when the parasite was not detected using the Baermann-Moraes method, 1-10, 11-50, 51-100, 101-500, and higher than 500 larvae/g of feces.
Strongyloides stercoralis antigens for ELISA
Strongyloides stercoralis third-stage infective larvae (L3) were obtained from the stool of hyperinfested patients. The larvae were cultured in animal charcoal at 28° C for five days and recovered using Rugai’s method 27 and then washed 5 times in 0.15 mol/L of phosphate buffered saline (PBS), pH 7.2. Next, parasites were suspended for 5 min in 0.25% sodium hypochlorite and rewashed 5 times in PBS. Larvae were then re-suspended in PBS with protease inhibitors (5 µmol/L EDTA, 1 µmol/L phenyl-methyl sulfonyl fluoride [Sigma], 0.05 µmol/L TPCK/TLCK, 1 µg/ml leupeptin) and sonicated in an ice bath for nine cycles lasting 80s each at 40 kHz (Branson Sonifier Cell Disruptor™, Branson Instruments, Danbury, CT, USA). The larvae homogenate was then centrifuged at 11,000g for 30 min at 4 °C, after which the supernatant was collected and analyzed for protein content by the Lowry, et al. 30 method, divided into aliquots, and stored at −70 °C until use.
Strongyloides stercoralis IgG-ELISA
The wells of microtiter plates (Corning Inc. Costar polystyrene EIA/RIA plates) were coated with 100 μL of 10 μg/mL S. stercoralis antigen in 0.06 mol/L carbonate-bicarbonate buffer, pH 9.6, then incubated overnight at 4 °C and washed 3 times in PBS containing 0.05% Tween-20 (PBS-T). All plates were then blocked with 100 μL PBS-T containing 8% w/v skim milk (PBS-T-Milk) for 1 hour at 37 °C. After the blocking, the wells were washed as described previously. Serum samples diluted at 1:100 in PBS-T-Milk were incubated at 37°C in duplicate in a volume of 100 μL per well for 1 hour. After washing, 100 μL of 1:4000 anti-human IgG conjugated to horseradish peroxidase (Sigma-Aldrich, St. Louis, MO, USA) was added to each plate and incubated under identical conditions. Reactions were visualized by adding substrate, 100 μL of 0.051 mol/L citrate-phosphate buffer (pH 5.0) containing 0.0037 mol/L p-phenylenediamine, and 0.04% hydrogen peroxide followed by a 20 minute incubation period in the absence of light after which 20 μL of 8N sulfuric acid were added to stop the reaction. Absorbance was measured at 450-630 nm on a microplate reader (Awareness Technology, USA).
Statistical analysis
As the sampling plan was not probabilistic, inferential statistics (hypothesis test and confidence interval) were not used due to the skewed estimate of the standard error 31,32. Data were analyzed using the statistical program IBM SPSS (19.0 for Windows) with quantitative variables being presented in measures of central tendency and dispersion and categorical variables in absolute and relative frequencies.
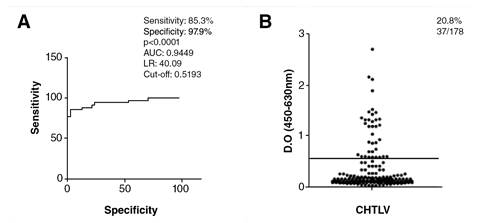
The cut-off, sensitivity, and specificity for the IgG- anti-S.stercoralis ELISA were determined by the receiver operating characteristic curve (ROC) using a total of 81 HTLV-negative sera from 34 samples of individuals infected with S. stercoralis (positive controls), 24 without parasitic infections, and 23 who had intestinal parasites other than S. stercoralis (negative controls).
Ethical aspects
This project was approved by the Research Ethics Committee, Faculty of Pharmacy, Federal University of Bahia, under number 2616338. All individuals who agreed to participate in the study signed the Informed Consent Form. Patients diagnosed with S. stercoralis and other parasites received prompt treatment.
Results
Demographic and socioeconomic characteristics
The mean age of HTLV-1 individuals was 45.60±17.26 years. The majority (65.7%; 117/178) were females from Salvador and the outlying metropolitan area (69.7%; 124/178). Most people (113/178; 63.5%) had a low level of formal education varying from no formal education to incomplete high school and came from low-income families (55.6%; 99/178) receiving between half and a full monthly minimum wage. Regarding their residential sanitary conditions and hygiene habits, most had access to piped water (83.7%; 149/178), sewage system and/or septic tank (88.2%; 157/178) and lived in areas with paved streets (78.1%; 139/178); 16.3% (29/178) of individuals had a habit of walking barefoot (table 1).
Table 1 Demographic and socioeconomic characteristics of individuals infected with HTLV-1 (n=178) seen at the Integrated Multidisciplinary Center for HTLV, Salvador, Bahia, Brazil
| Variable | Strongyloides stercoralis | Total | ||
|---|---|---|---|---|
| Positive n (%) | Negative n (%) | n (%) | ||
| Sex | ||||
| Male | 5 (83.3) | 56 (32.6) | 61 (34.4) | |
| Female | 1 (16.7) | 116 (67.4) | 117 (65.7) | |
| Age (years) | ||||
| < 20 | 2 (33.3) | 10 (5.8) | 12 (6.8) | |
| 20-60 | 3 (50.0) | 122 (70.9) | 125 (70.2) | |
| > 60 | 1 (16.7) | 40 (23.3) | 41 (23.0) | |
| Mean age | 29.17±19.36 | 45.91±16.97 | 45.60±17.26 | |
| Residence | ||||
| Salvador and Metropolitan area | 3 (50.0) | 121 (70.3) | 124 (69.7) | |
| Other urban cities of the state of Bahia | 0 | 37 (21.5) | 37 (20.8) | |
| Rural area | 3 (50.0) | 14 (8.1) | 17 (9.6) | |
| Education level | ||||
| No formal education | 0 | 14 (8.1) | 14 (7.9) | |
| 1st to 4th grade | 3 (50.0) | 32 (18.6) | 35 (19.7) | |
| 5th to 8th grade | 3 (50.0) | 33 (19.2) | 36 (20.2) | |
| Incomplete high school degree | 0 | 28 (16.3) | 28 (15.7) | |
| High School degree | 0 | 51 (29.7) | 51 (28.7) | |
| University level education | 0 | 14 (8.1) | 14 (7.9) | |
| Monthly income | ||||
| Up to ½ MW* | 3 (50.0) | 12 (7.0) | 15 (8.4) | |
| ½ MW to <1 MW | 1 (16.7) | 98 (57.0) | 99 (55.6) | |
| ≥ 1 MW up to 2 MW | 2 (33.3) | 62 (36.0) | 64 (36.0) | |
| Sanitation conditions (yes) | ||||
| Piped water | 2 (33.3) | 147 (85.5) | 149 (83.7) | |
| Residential water filter | 2 (33.3) | 135 (78.5) | 137 (77.0) | |
| Sewage system and/or septic tank | 2 (33.3) | 155 (90.1) | 157 (88.2) | |
| Paved streets | 2 (33.3) | 137 (79.7) | 139 (78.1) | |
| Bathroom inside the residence | 2 (33.3) | 156 (90.7) | 158 (88.8) | |
| Sink in bathroom | 2 (33.7) | 146 (84.9) | 148 (83.1) | |
| Garbage collection service | 2 (33.7) | 143 (83.1) | 145 (81.5) | |
| Habit of walking barefoot | 4 (66.7) | 25 (15.5) | 29 (16.3) | |
*MW: minimum wage in Brazilian Real, BRL = 954.00 or United State Dollar, USD = 247.00 for December, 2018
Among the HTLV-1 individuals, six were coinfected with S. stercoralis. From these, 83.3% (5/6) were males, 50% (3/6) lived in rural areas of the state of Bahia, and 66.7% (4/6) reported walking barefoot regularly (table 1). All HTLV- 1 individuals from the rural area had low socioeconomic conditions and lived in poor sanitary conditions with no access to the sewage system or potable water (data not shown).
Parasitological diagnosis
The overall frequency of infection by enteroparasites was 23% (41/178) with 15.2% (27/178) of monoparasitism and 7.9% (14/178) of polyparasitism. The most frequent helminths were A. lumbricoides, T. trichiura, hookworm, and S. stercoralis (6.7%, 12/178; 5.1%, 9/178; 3.9%, 7/178, and 3.4%, 6/178, respectively). The pathogenic protozoa Giardia duodenalis was found in 2.8% (5/178) of individuals. Other non-pathogenic protozoa were more prevalent, such as Endolimax nana (10.1%; 18/178) and Entamoeba coli (6.2%; 11/178) (table 2).
Table 2 Strongyloides stercoralis and other intestinal parasitic infections in HTLV-1 patients (n=178)
| Parasite infection | Group 1 - urban areas n=161 n (%) | Group 1 - urban areas n=161 n (%) | Total n=178 n (%) | |
|---|---|---|---|---|
| Positive | 26 (16.1) | 15 (82.2) | 41 (23.0) | |
| Monoparasitism | 21 (13.0) | 6 (35.3) | 27 (15.2) | |
| Polyparasitism | 5 (3.1) | 9 (52.9) | 14 (7.9) | |
| Negative | 135 (83.9) | 2 (11.8) | 137 (77.0) | |
| Helminths | ||||
| Ascaris lumbricoides | 3 (1.9) | 9 (52.9) | 12 (6.7) | |
| Trichuris trichiura | 1 (0.6) | 8 (47.1) | 9 (5.1) | |
| Hookworm | 3 (1.9) | 4 (23.5) | 7 (3.9) | |
| Strongyloides stercoralis* | 3 (1.9) | 3 (17.6) | 6 (3.4) | |
| Enterobius vermicularis | 0 | 5 (29.4) | 5 (2.8) | |
| Schistosoma mansoni | 1 (0.6) | 0 | 1 (0,6) | |
| Protozoa | ||||
| Endolimax nana | 14 (8.7) | 4 (23.5) | 18 (10.1) | |
| Entamoeba coli | 5 (3.1) | 6 (35.3) | 11 (6.2) | |
| Giardia duodenalis | 0 | 5 (29.4) | 5 (2.8) | |
| Iodamoeba butschlli | 2 (1.2) | 0 | 2 (1.1) | |
| Chilomastix mesnili | 0 | 1 (5.6) | 1 (0.6) | |
| Entamoeba histolytica/díspar | 1 (0.6) | 0 | 1 (0.6) |
*Three HTLV-1 and S. stercoralis coinfected patients belonged to the same family and had S. stercoralis hyperinfection (>3.000 larvae/gram of fecal sample). The other three infected individuals had low parasite load (<5 larvae/gram of stool).
S. stercoralis was diagnosed in 3.4% (6/178) of the study population. When participants were separated into groups 1 (urban areas) and 2 (rural areas), the infection rate was 1.9% (3/161) and 17.6% (3/17), respectively. The total frequency of other parasites in individuals in the rural areas was 88.2% (15/17) while in individuals from urban areas, the parasitic frequency was 16.1% (26/161). The most frequent pathogenic helminths in individuals from rural areas were Ascaris lumbricoides 52.9% (9/17), Trichuris trichiura 47.1% (8/17), Enterobius vermicularis 29.4% (5/17) while in urban areas S. stercoralis, hookworms, and Ascaris lumbricoides had a low frequency of 1.9% (3/161 each). Helminth eggs including E. vermicularis were diagnosed using the Hoffman and Pons and Janer parasitological methods. The pathogenic protozoa G. duodenalis was found in 29.4% (5/17) of individuals from rural areas and none in urban areas (table 2).
Three HTLV-1 individuals with S. stercoralis were from the same family and lived in a rural area located on the Southern Coast of Bahia. They presented a parasitic hyperinfection as evidenced by the presence of more than 3,000 larvae/gram of stool quantified by the Baermann-Moraes method with both rhabditiform and filariform larval stages in feces. Additionally, one of these three individuals had free-living males and females, as well as Strongyloides eggs released into the stool. The other three infected individuals had low parasite load and discharged <5 larvae/gram of stool (table 2).
Detection of anti-Strongyloides stercoralis IgG antibodies
To evaluate the exposure of HTLV-1 patients to S. stercoralis infection, specific IgG antibodies were analyzed in sera. The IgG-ELISA showed 85.29% (42/47) sensitivity and 97.87% (23/24) specificity. S. stercoralis IgG antibodies were detected in 20.8% (37/178) of HTLV-1 individuals (figure 1). All six patients with the presence of larvae in their feces were also positive for the S. stercoralis IgG-ELISA. All of the 17 subjects who lived in rural areas had specific IgG detected by ELISA.
Discussion
We found a frequency of 3.4% S. stercoralis infection in HTLV-1 individuals from Bahia treated at a specialized medical center. The association between S. stercoralis and HTLV-1 was first reported in Okinawa, Japan 33. Since then, S. stercoralis infection prevalence has been found to be at least 2.4 times higher in individuals infected with the virus than in uninfected individuals 12,18,34,35. Moreover, it has been demonstrated that strongyloidiasis increases the risk of the development of HTLV-1-associated diseases, for example, adult T-cell leukemia/lymphoma 36. Studies conducted in Brazil have also demonstrated high rates of S. stercoralis infection (around 12 to 14%) in association with HTLV-1 20,21.
Although they came from low-income families, most HTLV-1 subjects lived in the city of Salvador or in other urban areas of cities in the state of Bahia where basic urban amenities are available: treated potable water supply, sewage system connection, regular rubbish disposal, and paved streets and sidewalks. In contrast, the individuals living in rural areas were from poor families with very low incomes.
Besides S. stercoralis, other geohelminths such as A. lumbricoides, T. trichiura, and hookworm were found in the parasitological examination of HTLV-1 patients. These geohelminths were more frequently found in individuals living in rural areas. Several studies have shown that precarious living conditions such as lack of access to basic sanitation, health services, and schooling are the main determinants in the acquisition of intestinal parasitic infections, which continue to represent a major threat to public health in rural areas, as well as in peripheral regions next to urban zones 37,38. In this sense, the epidemiological triangle for the development of parasitic diseases involves the host health status, the parasite, and the environmental conditions 39,40.
The frequency of specific anti-S. stercoralis antibodies was 20.8%, which was much higher than the prevalence of larvae in feces. Souza, et al. 41 demonstrated a seroprevalence of 16.0% of S. stercoralis antibodies contrasting with 1.3% of positive parasitological diagnosis in individuals with lupus erythematosus. Conversely, frequencies of specific antibodies and S. stercoralis larvae in feces were very similar in alcoholic individuals, 22.0% and 23.5%, respectively, with a high agreement between the diagnostic methods 42. These divergent results could be explained by the continuous exposure to S. stercoralis infections by individuals living in endemic areas due to precarious hygiene habits and/or sanitary conditions.
Three individuals with a parasitological diagnosis of S. stercoralis were considered hyperinfected with one presenting all parasite evolutionary forms in feces. Factors linked to genetics and host immune response can trigger the infection and determine the severity of strongyloidiasis in individuals with HTLV. HTLV-1 coinfection induces a strong activation of the immune system. The exacerbated production of IFN-γ and TNF-α induced by HTLV-1 infection may negatively modulate the Th2-type cellular response and, consequently, decrease the levels of the main immune mediators involved in the defense against S. stercoralis such as IL-4, IL-5, and IL-13, and IgE 43-46. The analysis of serum cytokines in one child with HTLV-1 and S. stercoralis hyperinfection showed no alterations except for a significant increase in IL-17 levels following strongyloidiasis treatment 13. This could reflect inhibition of HTLV-1 inflammation response by Strongyloides in coinfected patients, although a larger number of individuals should be studied to evaluate the immunomodulation in HTLV-1 and S. stercoralis coinfection by IL-17.
In conclusion, our study suggests that HTLV-1-infected people living in poverty with precarious sanitary conditions are more predisposed to develop severe forms of S. stercoralis infection. Considering the high susceptibility and unfavorable outcome of the infection in these individuals, early diagnosis using parasitological and immunological methods and prompt treatment are critical for the successful management of strongyloidiasis in HTLV-1 carriers, especially those living in rural areas. Additionally, public policies are necessary to improve access to health services and basic sanitation for individuals at high risk of developing severe strongyloidiasis, such as HTLV-1-patients.