Monkeypox virus (MPXV) is a zoonotic pathogen associated with a febrile rash disease in humans. It has caused multiple outbreaks in the Africa 1 and since May 13, 2022, human cases of monkeypox were identified in 12 nonendemic African countries in Europe, Australia and North America. Individuals were infected with the West African clade and cases were mainly but not exclusively reported amongst men who have sex with men (MSM) 2.
Monkeypox virus (MPXV) is composed of a double-stranded DNA genome of approximately 197.209 bp. Two genetic clades have been characterized: West African and Central African. However, a new classification has been implemented by the WHO: clades I, IIa, and IIb 3,4. The current international 2022 clade is named B.1. On July 1st., 2022, 5,783 cases were reported in 52 countries 5. In Colombia there were 5 imported cases from Europe until July 5. Here we report the complete genome and phylogenetic analysis of a human monkeypox case detected in Colombia.
Materials and methods
Direct sequencing
Exudate from vesicular lesions was received on June 23, 2022, from a male patient with recent travel history to Spain. This was a complex sample that contained genetic material from the host and microbiome, and other coinfections.
Total DNA purification was performed using 200 μl of sample and the PureLink Viral RNA/DNA Mini Kit™ (Life Technologies, USA), according to the manufacturer’s instructions. DNA was quantified by fluorimetry with the Qubit dsDNA High Sensitivity Assay™ (Life Technologies, USA) on the Qubit 4.0™ instrument (Life Technologies, USA). Sequencing was performed using 400 ng of DNA using the native barcode kit EXP-NBD196™ (Oxford Nanopore Technologies ONT, UK) and a 1:1 ratio of AMPure XP™ beads (Beckman Coulter, UK). The library was loaded onto FLO-MIN106™ flow cells on the GridION™ sequencer (Oxford Nanopore Technologies ONT, UK).
Basecalling and demultiplexing were performed on nanopore sequence reads using Guppy™, v.6.1.7 (Oxford Nanopore Technologies), and adapters were trimmed by using Porechop™, version 0.2.4. Processed reads were aligned against the MPXV reference genome (GenBank reference No. NC063383.1) using minimap2, v.2.24 6. Variant calling for single-nucleotide variants was performed with Medaka, v.1.15.0. Sites with depth less than 10x were masked with Ns. maximum likelihood phylogenetic reconstruction was performed on the alignment with 22 genomes using IQ-TREE software 7, K3Pu+F+l nucleotide substitution model, and bootstrap for branch support (UFBoot) with 1000 replicates. Variant calling as single nucleotide polymorphism (SNP) and multiple nucleotide polymorphism (MNP) were cross-checked by two methods through manual curation, using Snippy, v.4.6.0, and samtools pileup, v.1.15 8.
Ethics
According to the national law 9/1979, decrees 786/1990 and 2323/2006, the Instituto Nacional de Salud is the reference lab and health authority of the national network of laboratories and in cases of public health emergency or those in which scientific research for public health purposes as required, the Instituto Nacional de Salud may use the biological material for research purposes, without informed consent, which includes the anonymous disclosure of results.
This study was performed following the ethical standards of the Declaration of Helsinki 1964 and its later amendments. The information used in this study comes from secondary sources of data that were previously anonymized and do not represent a risk to the community.
Results
A total of 11.951 reads mapped directly to a reference genome with 96.8% of coverage (190.898 bp), and the consensus sequence was submitted to the GISAID database. The sequence is available under the GISAID accession ID EPI_ISL_13511312.
Analysis by BLASTn shows a 98.77% identity to MPXV Clade l (Accession NC003310.1) and 99.42% identity to MPXV Clade IIb (Accession ON568298.1).
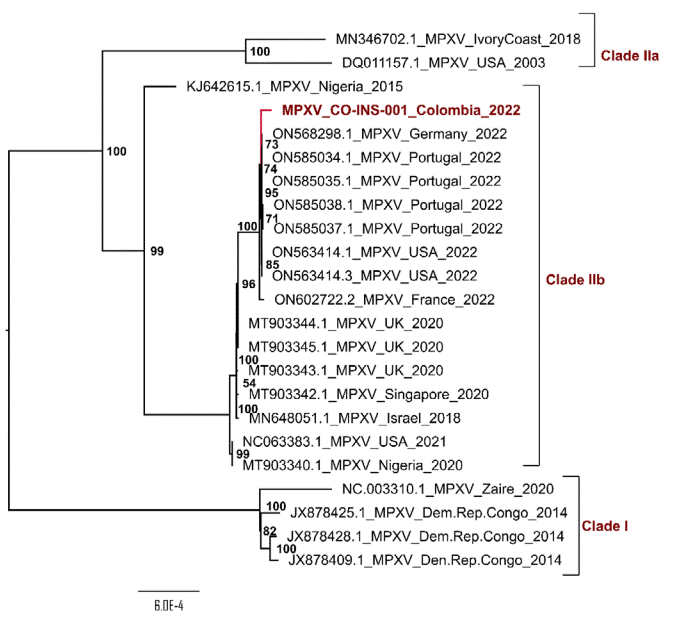
Phylogenetic analysis of the MPXV genome circulating in Colombia with genome sequences from NCBI (table 1) demonstrated its close relationship to Clade IIb (previously known as West African clade) and to genomes described during the multi-country outbreak in 2022 (figure 1).
Table 1 MPXV genomes
| Accession | Country | Year | Reference |
|---|---|---|---|
| JX878425 | USA | 2014 | (Kugelman, et al., 2014) 9 |
| JX878428 | USA | 2014 | (Kugelman, et al., 2014) 9 |
| JX878409 | USA | 2014 | (Kugelman, et al., 2014) 9 |
| MN346702 | Berlin | 2018 | (Patrono, et al., 2020) 10 |
| DQ011157 | USA | 2003 | (Likos, et al., 2005) 11 |
| KJ642615 | Nigeria | 2015 | (Nakazawa, et al., 2015) 12 |
| CO-001 | Colombia | 2022 | This work |
| ON568298 | Germany | 2022 | (Antwerpen, et al., 2022) 13 |
| ON585034 | Portugal | 2022 | (Isidro, et al., 2022) 14 |
| ON585035 | Portugal | 2022 | (Isidro, et al., 2022) 14 |
| ON585038 | Portugal | 2022 | (Isidro, et al., 2022) 14 |
| ON585037 | Portugal | 2022 | (Isidro, et al., 2022) 14 |
| ON563414 | USA | 2022 | (Gigante, et al., 2022) 15 |
| ON602722 | France | 2022 | (Croville, et al., 2022) 16 |
| MT903344 | UK | 2018 | (Mauldin, et al., 2022) 17 |
| MT903345 | UK | 2018 | (Mauldin, et al., 2022) 17 |
| MT903343 | UK | 2018 | (Mauldin, et al., 2022) 17 |
| MT903342 | Singapore | 2019 | (Mauldin, et al., 2022) 17 |
| MN648051 | Israel | 2018 | (Cohen-Gihon, et al., 2020) 18 |
| NC_063383 | Nigeria | 2022 | (Mauldin, et al., 2022) 17 |
| MT903340 | Nigeria | 2018 | (Mauldin, et al., 2022) 17 |
| NC_003310 | Russia | 2020 | (Shchelkunov, et al., 2021) 19 |
Discussion
A complete genome sequence was successfully obtained with the described approach. This strategy allows the assembly of a full genome without viral culture and without amplification of viral DNA. The assembled preserves a close relation to samples from the 2022 MPXV outbreak. However, due to the possibility of artifacts proper of the sequencing technology used, manual curation of called variants is necessary as implemented in this work.
Microevolution of MPXV has been observed worldwide in the sequences of the 2022 outbreak 20,21. It is necessary to continue the genomic surveillance of MPXV in order to detect possible changes in transmission.
In Colombia, real-time genomic surveillance and the implementation of NGS sequencing methods allowed the early detection of the introduction of MPXV in the country. This strategy will be established to monitor secondary autochthonous cases to describe local viral evolution during the transmission, characterize local transmission dynamics, study the impact of imported cases, and track viral diversity.