The coronavirus disease 2019 (COVID-19) caused by the novel coronavirus 2019 (2019-nCoV) 1, also called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) 2, has resulted in more than 160 million confirmed cases and 5 million deaths worldwide (November 29, 2021) 3. COVID-19 pandemic endangered global public health and economies and necessitated the development of effective vaccines to protect at-risk populations.
Containment measures failed to stop the spread of the virus, and there are no specific treatments against COVID-19. Vaccines prepare the immune system to detect and counteract viruses with greater success than other interventions. Successful cases of mass vaccination have controlled or eliminated smallpox, poliomyelitis, measles and rubella in America, which avoided illness, premature death and health costs 4.
Efforts to develop SARS-CoV-2 vaccines to control the pandemic have been underway since the virus emerged, with more than 500 candidate vaccines in clinical trials 5. Some vaccine development are more advanced than others. More than 80 candidate vaccines are being tested in humans 6. The impact of COVID-19 vaccines on the pandemic depends on several factors: the effectiveness of the vaccines; the speed of vaccine approval, manufacture, and delivery; the possible development of other variants; individual factors; and the number of people vaccinated 7.
The BNT162b2 (Pfizer BioNTech), mRNA-1273 (Moderna), ChAdOx1-S (Oxford University/AstraZeneca), and rAd26-S/rAd5-S (Gam-COVID-Vac) vaccines received emergency use authorization in some countries 8-10. The main countries where clinical trials of BNT162b2 vaccine were performed are the United States, Argentina, Brazil, Germany, South Africa, and Turkey. For ChAdOx1-S, the United Kingdom, South Africa and Brazil. The United States for mRNA-1273, and the Russian Federation for Gam-COVID-Vac rAd26-S/ rAd5-S. The World Health Organization (WHO) has also granted EUA to BNT162b2, mRNA-1273 and ChAdOx1-S vaccines 11.
Due to these public policy decisions on health, besides the promising preliminary results of these vaccines against the virus SARS-CoV-2 and the initiation of mass vaccination campaigns, it is necessary to evaluate the available evidence on its efficacy and safety.
Materials and methods
We adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines throughout the manuscript 12. The results were submitted to the International Register of Systematic Perspective Reviews (PROSPERO) and approved with registration number CRD42021229802. Two researchers performed the screening process independently and applied pre-established inclusion and exclusion criteria to select studies for a complete reading.
Search strategy
On March15, 2021, we searched in PubMed/MEDLINE, Google Scholar, Cochrane, and the WHO International Clinical Trials Registry Platform. The search terms used were: "vaccine" OR "vaccination" AND "covid19" OR "coronavirus" OR "sarscov2" AND "bnt162b2" OR "chadox1-S" OR "azd1222" OR "sputnik" OR "Gam-COVID-Vac" OR "mrna" OR "mRNA-1273" These terms could be found anywhere in the article, title, or abstract. The search equations used for each of the databases are provided in online supplement 1. The Rayyan® web application was used to organise the list of references, remove duplicates, and obtain the full document for review.
Inclusion criteria
According to the PICOT question-model, population, intervention, comparison, and outcomes are shown in Table 1.
Table 1 Research questions using the PICOT framework
Studies: Randomized clinical trials (RCT) phases II/III and III in humans.
Results report: Studies that reported individual effect estimates for each primary research study that was attributable to the comparison of interest and at least one outcome.
- No language or country restrictions were applied.
Exclusion criteria
We did not include studies that were only available on abstract format, because the information reported is insufficient to evaluate methodological quality.
Data collection and analysis
Screening and selection of studies
Two reviewers screened the total number of references identified in the search by examining the titles and abstracts against independently predefined eligibility criteria. From the group of pre-selected references, a smaller number of studies were selected. The reviewers verified that each study met the eligibility criteria by reading the full text on each publication. Disagreements were resolved via consensus. To extract the information, a standardized Excel tool was used, which was tested by the reviewers before use. The structure was based on collecting information on the basic characteristics of each study, such as participants, intervention (vaccine), comparators and outcomes. The data extraction was performed in duplicate and subsequently verified by the researchers involved, who compared the extracted data with the studies.
Reviewers selected the effect estimates of for the comparison and critical outcomes from reported values in the studies.
Assessments of risk of bias and certainty of the evidence
The Cochrane risk of bias tool was used to assess the following domains: randomization, deviations from intended intervention, missing outcome data, measurement of the outcome, selection of the reported results, and overall risk of bias 13. Studies were categorized as: "high risk", "low risk" or "some concerns" The certainty of the evidence was measured using GRADE profiles 14. A single-first reviewer author rated the risk of bias and certainty of the evidence for each study, and a second reviewer author checked the ratings.
Statistical analysis
Abstracted data were aggregated in tables. Risk ratios (RR), risk differences and corresponding 95% confidence intervals (CI95%) were calculated or extracted from the selected publications. We computed the RR for the outcomes of the adverse events and serious adverse events. Data on the vaccine efficacy were extracted from the publications and calculated ([1-(risk ratio or rate ratio comparing vaccine and placebo recipients)] x 100) for the outcome asymptomatic or unknown infection of the vaccine ChAdOx1-S.
We performed inverse variance-weighted random-effects meta-analyses using the Paule and Mandel t 2 estimator for heterogeneity 15. Heterogeneity across the RCTs was described using the I 2 and t 2 metrics 16. Data were analysed using the statistical package Stata™, version 15 (Stata Corporation, College Station, Texas, United States).
Results
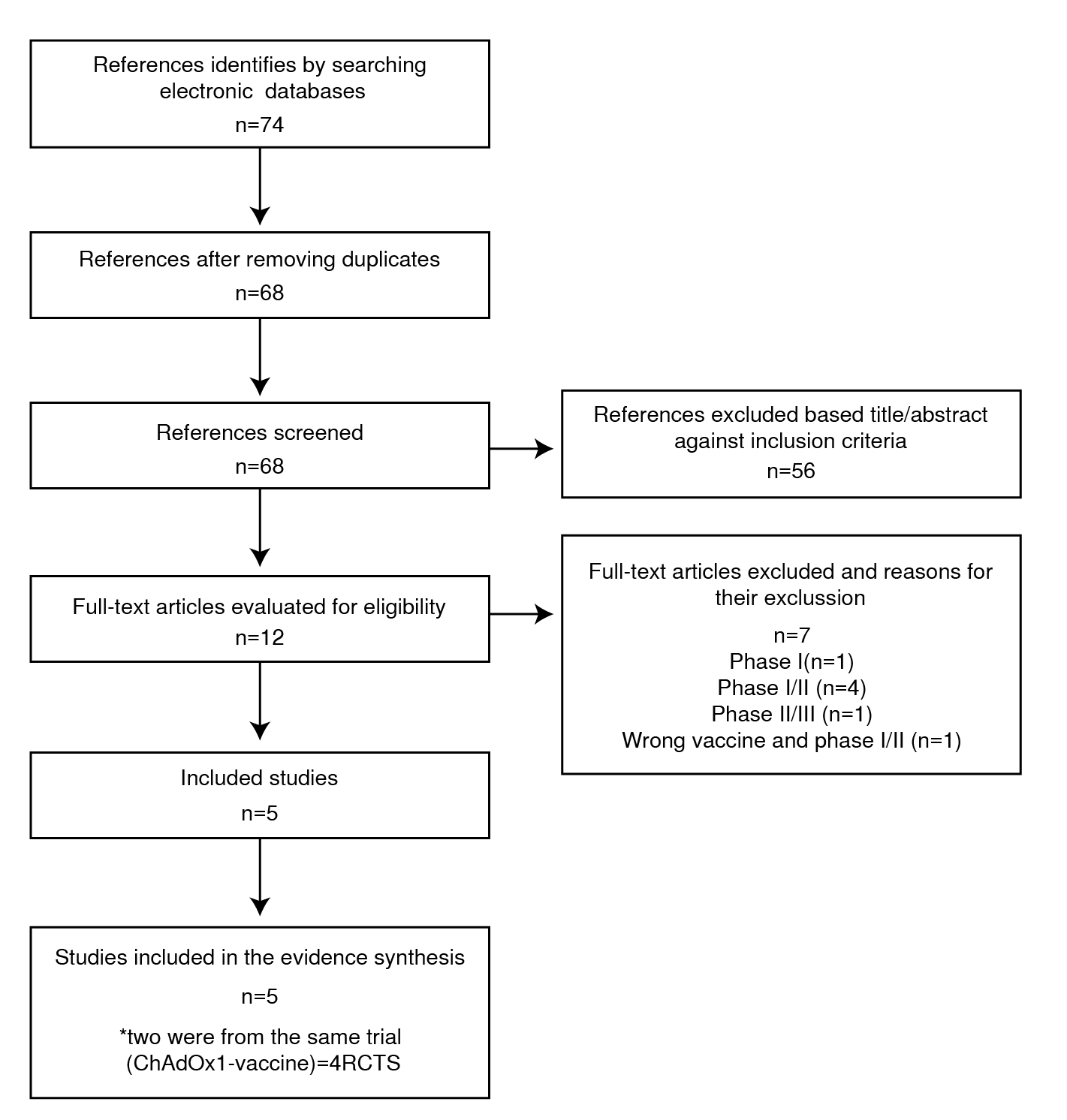
Figure 1 shows the search results, screening, and selection of evidence for this rapid review. The search of the identified databases detected 74 documents. After exclusion of duplicates, 68 articles remained for title and abstract screening. Fifty-six of these papers were deemed irrelevant according to the titles and abstracts and were later excluded. We assessed the full text of the remaining 12 articles: seven of these articles were excluded due to RCT phase I (n=1), phase I/II (n=4), phase II/III (n=1, no outcomes of interest), and wrong vaccine and phase I/II (n=1). Bibliographic data of these 7 studies were excluded after full-text assessment 17-23. Of the 5 included articles, two reports were from the same trial (ChAdOx1-S vaccine). When outcomes were reported in both publications, the updated version was used. A total of 4 RCTs were included, and all studies were performed in high- or middle-income countries, such as the United States, Argentina, Brazil, Germany, South Africa, Turkey, United Kingdom and the Russian Federation. There were 105,118 participants (57,591 randomized to the vaccine against COVID-19 and 47,527 to placebo) 24-28. The age of the participants at study entry ranged from 16 to 85 years. We summarized the characteristics of the studies included in Table 2.
Table 2 Characteristics of the included studies
SD: Standard deviation
Risk of bias
The overall risk of bias was classified as having some concerns in all 4 RCTs due to deviations in the interventions. In general, all trials assessed the main efficacy outcomes using per-protocol and not intention-to-treat analyses. Details of the risk of bias assessment are provided in online supplement 2.
Data availability
Symptomatic SARS-CoV-2 infection confirmed by laboratory, severe or critical disease due to COVID-19, serious adverse events and all-cause mortality were assessed in all 4 RCTs 24-28 (Tables 3 and 4). The outcomes of hospitalization for COVID-19 and asymptomatic SARS-CoV-2 infection were reported in one RCT (Tables 3 and 4) 26,27.
Table 3 Efficacy of vaccination against SARS-CoV-2 infection (COVID-19)
| Symptomatic SARS-CoV-2 infection confirmed by laboratory | Severe disease due to COVID-19 | Asymptomatic or unknown infection | Hospitalization due to COVID-19 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | n of events/n of participants | VE (CI95%) | n of events/n of participants | VE (CI95%) | n of events/n of participants | VE (CI95%) | n of events/n of participants | VE (CI95%) | n of events/n of participants | VE (CI95%) | n of events/n of participants | VE (CI95%) | n of events/n of participants | VE (CI95%) | |||||||
| Vaccine | Placebo | After dose 2 | Vaccine | Placebo | After dose 1 | Vaccine | Placebo | From 14 days after dose 1 | Vaccine | Placebo | From day 22 to day 90 after dose 1 | Vaccine | Placebo | Vaccine | Placebo | Vaccine | Placebo | ||||
| Polack, 2020 [24] | 8/17,411 | 162/17,511 | 95.0% (90.3 to 97.6)* | 50/21,669 | 275/21,686 | 82.0% (75.6 to 86.9) | 2/21,669 | 27/21,686 | 92.6% (69.0 to 98.3) | NA | NA | NA | 1/21,314 | 9/21,259 | 88.9% (12.5 to 98.6) | NA | NA | NA | NA | NA | NA |
| Baden, 2020 [25] | 11/14,134 | 185/14,073 | 94.1% (89.3 to 96.8)** | NA | NA | NA | 2/14,550 | 35/14,598 | 94.3% (76.2 to 98.6) | NA | NA | NA | 0/14,134 | 30/14,073 | 100% | NA | NA | NA | NA | NA | NA |
| Voysey, 2021 [26] [27] | 84/8,597 | 248/8,580 | 66·7% (57.4 to 74.0)** | NA | NA | NA | NA | NA | NA | 17/9,257 | 71/9,237 | 76.0% (59.3- 85.9) | 0/12,021 | 1/11,724 | 100% | 41/2,692 | 42/2,751 | 2·0% *** (−50·7 to 36·2) | 0/11,794 | 15/11,776 | 100% |
| 16/1,379 | 31/1385, | 49·3%**** (7·4 to 72·2) 22.2% (-9.9 to 45.0)**** | |||||||||||||||||||
| 57/4,071 | 73/4,136 | ||||||||||||||||||||
| Logunov, 2021 [28] | 13/14,094 | 47/4,601 | 91.1% (83.8 to 95.1)* | 16/14,964 | 62/4,902 | 91.6% (85.6 to 95.2) | 30/14,999 | 79/4,950 | 87·6% (81.1 to 91.8) | NA | NA | NA | 0/14,964 | 20/4,902 | 100% | NA | NA | NA | NA | NA | NA |
*7 days after the second dose; ** 14 days after the second dose; VE: vaccine efficacy; NA: not applicable; ***Two standard doses; ****Low dose plus standard dose; ***** All doses
Table 4 Vaccine efficacy according to age, sex, and race subgroup
VE: vaccine efficacy; NR: Not reported; Yr: years old
Efficacy outcomes
The efficacies of the vaccines BNT162b2, mRNA-1273, ChAdOx1-S and Gam-COVID-VacrAd26-S/rAd5-S against SARS-CoV-2 infection (COVID-19) via assessment of symptomatic SARS-CoV-2 infection confirmed by a laboratory after the second dose were 95.0% (CI95% 90.3-to 97.6), 94.1% (CI95% 89.3-96.8), 66.7% (CI95% 57.4-74.0) and 91.1% (CI95%, 83.8-95.1), respectively ° (24-28). The efficacy results after the first doses were also reported (Table 3). The efficacy of vaccine BNT162b2 against severe disease due to COVID-19 was 88.9% (CI95%, 12.5 to 98.6), and 100% for the other vaccines (mRNA-1273, ChAdOx1-S and Gam-COVID-VacrAd26-S/rAd5-S) 24-28. The efficacy of the vaccine ChAdOx1 in asymptomatic or unknown SARS-CoV-2 infection participants was 22.2% (CI95% -9.9 to 45.0), and 100% in hospitalized patients with COVID-19 (Table 3) 26,27. Vaccine efficacy in subgroups defined by age, sex, and race was generally consistent with the overall population (Table 4). Efficacy meta-analysis was not performed because RCTs assessed this outcome differently.
Adverse events
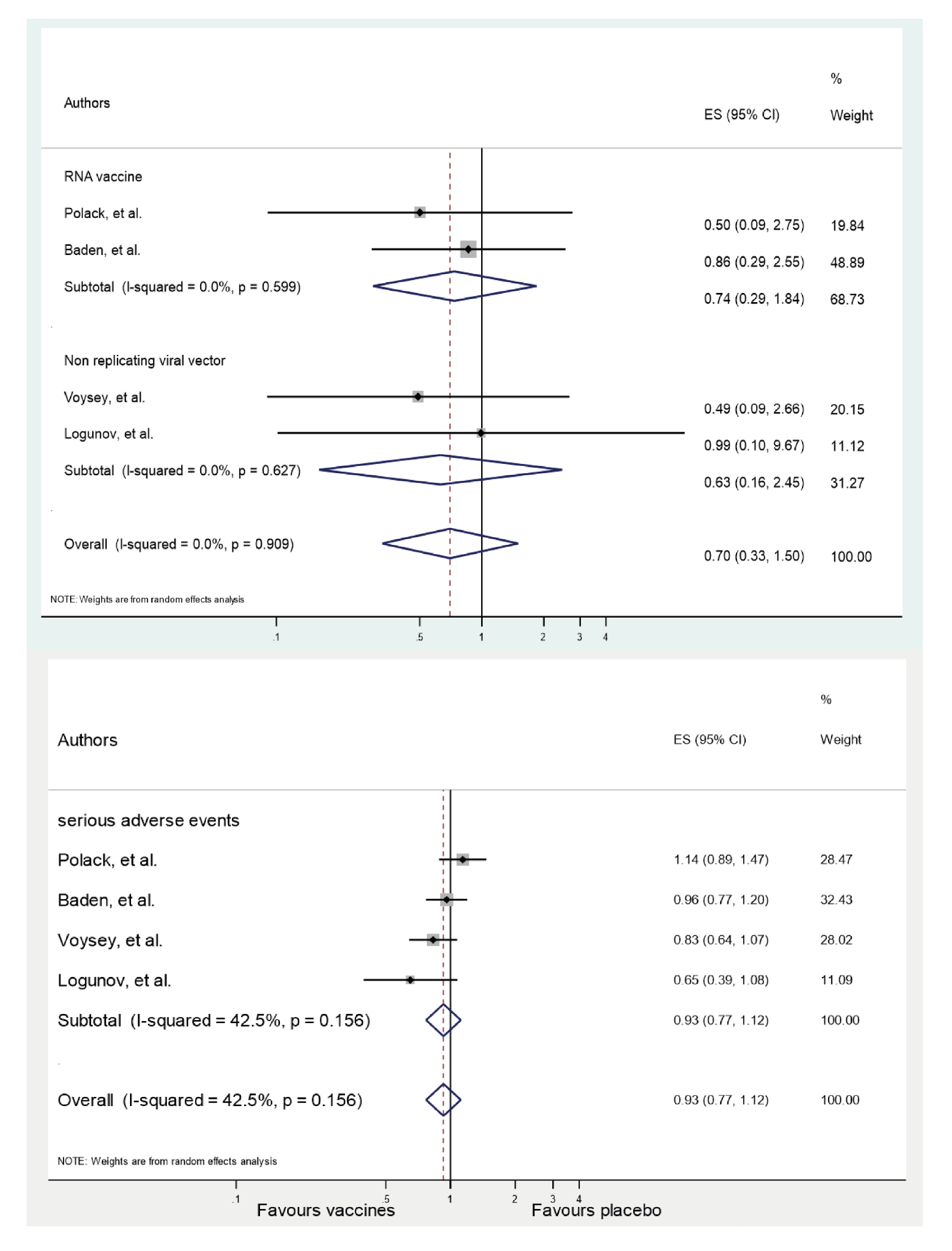
More vaccine recipients than placebo recipients reported any serious moderate or mild adverse events (Table 5) 24-28. Serious adverse events did not show statistically significant differences between the analysis groups. Across the 4 RCTs, the summary RR for serious adverse events was 0.93 (CI95% 0.77-1.12; p=0.16). The heterogeneity between trials was (I2=42.5%; t2=0.02; p=0.424). Reports of deaths from all causes were rare. Across the 4 RCTs, the summary RR for all-cause mortality with vaccines against SARS-CoV-2 infection was 0.70 (CI95% 0.33-1.50; p=0.90). There was no significant difference between-trial heterogeneity (I2=0%; t2=0; p=0.358) (Figures 1 s and 2 s in online Supplement 3). No participant who received any vaccine died of COVID-19 24-28. There was one death from COVID-19 in the placebo group in the RCT of the mRNA-1273 vaccine 25.
Table 5. Number of participants reporting any event and serious adverse events
RR: Risk ratios
Certainty of the evidence
For the efficacy outcome evaluated via symptomatic SARS-CoV-2 infection confirmed by a laboratory, the certainty of the evidence (using GRADE) was moderate due to serious indirectness (data from an interim analysis of the trial, with a short follow-up duration; estimates may change during longer follow-up; population included in RCTs may not represent all persons aged 16 years in BNT162b2; and 18 years in mRNA-1273, ChAdOx1-S, Gam-COVID-VacrAd26-S/rAd5-S). For the severe or critical disease due to COVID-19 in the RCTs regarding BNT162b2 and ChAdOx1 vaccines, the certainty of the evidence was very low, and moderate for mRNA-1273 and Gam-COVID-VacrAd26-S/rAd5-S vaccines. In the four RCTs, the certainty of the evidence for the outcomes of all-cause mortality and serious adverse events was very low due to serious indirectness and very serious imprecision based on the wide CI95% for RR, which is consistent with substantial benefit or harm (see GRADE tables for more details in online supplement 4).
Discussion
Available data indicated that the vaccines evaluated in this systematic review effectively prevented symptomatic laboratory-confirmed COVID-19 (range 69.7-95%) 24-28, with moderate certainty of evidence. For severe cases of the disease, the four evaluated vaccines were between 90and 100% effective with moderate to very low certainty of evidence. The subgroup analysis in each RCT revealed that the global efficacy of the vaccine was similar to the efficacy showed in different populations according to age, sex, comorbidities, and race/ethnicity 24-28.
The current concern related to asymptomatic infections is based on people who is able to continue transmitting the virus to others despite being vaccinated. Although asymptomatic infections are not a direct measure of disease transmission, researchers have viewed the information on this result as an indicator of how vaccines would help reducing the risk of the spread of SARS-CoV-2. The reports included in this review revealed information on this outcome from the ChAdOx1-S vaccine, which provided preliminary data on the prevention of asymptomatic SARS-CoV-2 infection. Showing an efficacy of 49.3% (CI95% 7.4-72.2) in the group that received a half dose followed by a standard dose, compared to only 2% (CI95% -50.7-36.2) in the group that received two standard doses 26,27. These results suggest that COVID-19 vaccines also reduce asymptomatic infection and potentially transmission.
The evidence shows that vaccination prevents a person from getting COVID-19 in its severe form and reduces the possibility of transmission to others 29. Substantial reductions in SARS-CoV-2 infections (symptomatic and asymptomatic) will help to reduce overall levels of disease and the transmission of the virus worldwide.
For adverse events and death, the analysed vaccines showed an adequate safety profile. However, the level of certainty of evidence was very low. Therefore, surveillance and follow-up of vaccinated cohorts is very important for the identification of possible adverse effects, especially rare events of death or disability.
Our review synthesized the evidence in a simple and methodical way to inform health providers and readers about the general efficacy and safety of four vaccines against COVID-19. These vaccines exhibited different degrees of effectiveness in the prevention of SARS-CoV-2 infection. However, all were very effective in preventing serious forms of illness and secondary death from COVID-19. The BNT162b2, mRNA-1273, ChAdOx1-S, and Gam-COVID-VacrAd26-S/rAd5-S vaccines showed high efficacy based on the minimum criteria established by the WHO to recommend a vaccine against COVID-19, which consisted of an estimate of 50% and with a clear demonstration of efficacy in the base population 30.
Global, national and regional regulatory procedures evaluating the suitability of new medical devices for public health emergencies, are responsible for performing a rigorous process to decide the administration of a vaccine with specified prioritisation for the earliest use 31. Other vaccines that were not the subject of this review have also received approval for emergency use by health regulatory agencies in different countries, such as CanSino, Sinopharm, Sinovac and Johnson & Johnson's vaccines 32-35. This information is encouraging due to the main challenge producing safe and effective vaccines in sufficient quantity for equitable distribution worldwide. More vaccines provide greater hope of ending the pandemic.
Strengths and limitations
Rapid review is a methodology that may be particularly important in the COVID-19 pandemic because the evidence is rapidly emerging and verified information is needed to make policy or practical decisions. However, there are some limitations to our study, which primarily relate to the performance of this review in a limited time frame. First, while a comprehensive search was performed in three databases, there was insufficient time to search other sources (grey literature) to ensure comprehensiveness. However, making these methodological trade-offs is consistent with the time-limited approach taken in other rapid review methodologies 36,37.
Second, this review included only 4 phase II/III and III clinical trials with preliminary published data because they involve ongoing research. We did not include other study designs that analysed real-life data, which provide more reliable and representative conclusions for the population. Third, there is no evidence of the long-term effectiveness and safety of the vaccine.
These trials had a short follow-up of up to 28 days after vaccination and unsolicited serious adverse events through 6 months after the second dose. It is necessary to highlight that the level of evidence of the outcomes evaluated in this review ranged between moderate and very low. This is primarily because the analysis was performed per-protocol, as planned for the interim analysis, which generates uncertainty in the results because the estimates may change during longer follow-up. The results of an RCT acquire greater validity when the patients are analysed according to the group in which they were assigned, i.e., application of the intention-to-treat principle. This application makes it possible to maintain the advantages of randomization, avoiding overestimates of the effects of the therapy under study, and admitting the non-adherence of some patients, which is a situation that is closer to reality. Finally, we found no major limitations in study designs in relation to randomization sequence, blinding of investigators, personnel involved in the study, or significant losses to follow-up. However, the sample sizes of the intervention group (n=14,964) and the control group (n=4,902) (Table 3) in the RCT of the Gam-COVID-VacrAd26-S/rAd5-S vaccine were striking, but the distributions observed of the baseline characteristics between the study groups 28 were compatible with chance.
Implications for practice
Health care providers and professionals should communicate consistent, complete, and clear information on the benefits, risks, contraindications, safety issues, warnings and follow-up recommendations to persons receiving COVID-19 vaccines and their caregivers. It should be explained that there is limited evidence on how much COVID-19 vaccines reduce transmission ingeneral population and for how long protection lasts. Also, prevention and biosecurity guidelines should be strictly followed. This includes the use of masks, hand washing, social distance greater than 2 meters, avoiding crowdsand enclosed unventilated areas 38,39.
We should generate pharmacovigilance systems to identify and respond quickly to any adverse events in a recipient after vaccination, including vaccine administration errors, serious adverse events, multisystemic inflammatory syndrome cases, and COVID-19 cases resulting in hospitalization and death. It is recommended that any other clinically significant adverse event should be reported, even if its association with the administration of the vaccine is not clear.
Implications in research
It is necessary to keep track of the studies and reports that evaluate the beneficial and harmful effects of the different vaccines, which may be possible using living systematic reviews periodically informing the best practices in vaccine prevention and clinical research of this highly prevalent disease.
The results of this review support the level of evidence for the efficacy and safety of the COVID-19 vaccines that were analysed. The information presented in this manuscript has not been presented elsewhere.
Supplementary material
Supplement 1 Evidence search reports in electronic databases
| Data source | Search equation |
|---|---|
| PubMed/MEDLINE | ((((("vaccine"[All Fields] OR "vaccination"[All Fields]) AND "covid19"[All Fields]) OR "coronavirus"[All Fields] OR "sarscov2"[All Fields]) AND "bnt162b2"[All Fields]) OR "chadox1"[All Fields] OR "azd1222"[All Fields] OR "sputnik"[All Fields] OR "mrna"[All Fields]) AND ((randomizedcontrolled trial[Filter]) AND (2020:2021[pdat])) |
| Cochrane | #1 Covid19 |
| Embase |
|
| Google Scholar | “vaccine" OR "vaccination" AND “Covid19” |
Supplement 2 Risk of bias in randomized controlled trials
| Study | Randomisation | Deviations from intervention | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall risk of bias | Outcome | Details |
|---|---|---|---|---|---|---|---|---|
| Polack, 2020 24 BNT162b2 | + | ± | + | + | + | ± | Low risk of bias: Adverse events; death from all causes Some concerns risk: Symptomatic SARS-CoV-2 infection confirmed by laboratory | Analysed by perprotocol and not by intention to treat; losses to follow-up were not described. |
| Baden, 2020 25 mRNA-1273 | + | ± | + | + | + | ± | Low risk of bias: Adverse events; death from all causes Some concerns risk: Symptomatic SARS-CoV-2 infection confirmed by laboratory and severe case of COVID-19 | Analysed by perprotocol and not by intention to treat |
| Voysey, 2020 26 ChAdOx1 | + | ± | + | + | + | ± | Low risk of bias: Adverse events; death from all causes Some concerns risk: Symptomatic SARS-CoV-2 infection confirmed by laboratory | Analysed by per- protocol and not by intention to treat; Vaccine administration errors |
| Logunov, 2021 28) Gam-COVIDVac rAd26-S/ rAd5-S | + | ± | + | + | + | ± | Some concerns: Symptomatic SARS-CoV-2 infection confirmed by laboratory; severe case of COVID-19; Adverse events; death from all causes | Analysed by per- protocol and not by intention to treat; Vaccine administration errors |
Supplement 3
Supplement 4s GRADE assessment
| Certainty assessment | No. of patients | Effect | Certainty | Importance | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Vaccine | Placebo | Relative (95% CI) | Absolute (95% CI) | |||
| Polack, 2020 15 Vaccine: BNT162b2 | 1 | Randomized trials | Not serious | Not serious | Seriousa b | Not serious | None | 8/17,411 | 162/17,511 | RR=0.05 (0.02 to 0.10) | ⨁⨁⨁◯ Moderate | Critical | |
| Baden, 2020 16) Vaccine: mRNA-1273 | 1 | Randomized trials | Not serious | Not serious | Seriousa b | Not serious | None | 11/14,134 | 185/14,073 | RR=0.06 | ⨁⨁⨁◯ Moderate | Critical | |
| Voysey, 2020 17,18 Vaccine: ChAdOx1 | 1 | Randomized trials | Not serious | Not serious | Seriousa b | Not serious | None | 84/8,597 | 248/8,580 | RR=0.33 (0.26 to 0.43) | ⨁⨁⨁◯ Moderate | Critical | |
| Logunov, 2021 19 Vaccine: Gam-COVIDVac rAd26-S/ rAd5-S | 1 | Randomized trials | Not serious | Not serious | Seriousa b | Not serious | None | 13/14,094 | 47/4,601 | RR=0.09 (0.05 to 0.16) | ⨁⨁⨁◯ Moderate | Critical | |
CI: Confidence interval; RR: Risk ratio Explanations
a. Data are from an interim analysis of the trial, with a short duration of follow-up. Estimates may change over a longer duration of follow-up.
b. The population included in the RCT may not represent all persons aged ≥16 years (BNT162b2) or ≥18 years (mRNA-1273; ChAdOxl; Gam-COVID-Vac rAd26-S/rAd5-S)
Severe or critical disease due to COVID-19
| Certainty assessment | No. of patients | Effect | Certainty | Importance | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Vaccine | Placebo | Relative (95% CI) | Absolute (95% CI) | |||
| Polack, 2020 24 Vaccine: BNT162b2 | 1 | Randomized trials | Not serious | Not serious | Seriousa b | Very serious c | None | 1/21,314 | 9/21,259 | RR=0.11 (0.01 to 1.81) | ⨁◯◯◯ Very low | Critical | |
| Baden, 2020 25 Vaccine: mRNA-1273 | 1 | Randomized trials | Not serious | Not serious | Seriousa b | Not serious | None | 0/14,134 | 30/14,073 | RR=0.02 (0.00 to 0.27) | ⨁⨁⨁◯ Moderate | Critical | |
| Voysey, 2020 26,27 Vaccine: ChAdOx1 | 1 | Randomized trials | Not serious | Not serious | Seriousa b | Very serious c | None | 0/12,021 | 1/11,724 | RR=0.33 (0.01 to 7.98) | ⨁◯◯◯ Very low | Critical | |
| Logunov, 2021 28 Vaccine: Gam-COVID- Vac rAd26-S/ rAd5-S | 1 | Randomized trials | Not serious | Not serious | Seriousa b | Very serious c | None | 0/14,964 | 20/4,902 | RR=0.00 (0.00 to 0.06) | ⨁◯◯◯ Very low | Critical | |
CI: Confidence interval; RR: Risk ratio Explanations
Data are from an interim analysis of the trial, with a short duration of follow-up. Estimates may change over a longer duration of follow-up.
The population included in the RCT may not represent all persons aged ≥16 years (BNT162b2) or ≥18 years (mRNA-1273; ChAdOxl; Gam-COVID-Vac rAd26-S/rAd5-S) Imprecision was downgraded by 2 levels because the 95% of the relative risk (RR) was sufficiently wide that the estimate could include appreciable harm or benefit of the intervention. This outcome may be imprecise due to the small number of events reported during the observation period.
Any adverse event
| Certainty assessment | No. of patients | Effect | Certainty | Importance | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Vaccine | Placebo | Relative (95% CI) | Absolute (95% CI) | |||
| Polack, 2020 15 Vaccine: BNT162b2 | 1 | Randomized trials | Not serious | Not serious | Seriousa b | Not serious | None | 5,770/21,621 | 2,638/21,631 | RR=2.19 (2.10 to 2.28) | ⨁⨁⨁◯ Moderate | Critical | |
| Baden, 2020 16 Vaccine: mRNA-1273 | 1 | Randomized trials | Not serious | Not serious | Seriousa b | Not serious | None | 3,632/15,185 | 3,277/15,166 | RR=1.11 (1.06 to 1.15) | ⨁⨁⨁◯ Moderate | Critical | |
| Voysey, 2020 17,18 Vaccine: ChAdOx1 | 1 | Randomized trials | Not serious | Not serious | Seriousa b | Not serious | None | 95/12,021 | 126/11,724 | RR=0.74 (0.56 to 0.96) | ⨁⨁⨁◯ Moderate | Critical | |
| Logunov, 2021 19 Vaccine: Gam-COVID- Vac rAd26-S/ rAd5-S | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | |
CI: Confidence interval; RR: Risk ratio Explanations
a. Data are from an interim analysis of the trial, with a short duration of follow-up. Estimates may change over a longer duration of follow-up.
b. The population included in the RCT may not represent all persons aged ≥16 years (BNT162b2) or ≥18 years (mRNA-1273; ChAdOx1; Gam-COVID-Vac rAd26-S/rAd6-S)
Serious adverse event
| Certainty assessment | No. of patients | Effect | Certainty | Importance | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Vaccine | Placebo | Relative (95% CI) | Absolute (95% CI) | ||||
| Polack, 2020 15 Vaccine: BNT162b2 | 1 | Randomized trials | Not serious | Not serious | Seriousa b | Very serious c | None | 126/21,621 | 111/21,631 | RR=1.14 (0.88 to 1.46) | ⨁◯◯◯ Very low | Critical | ||
| Baden, 2020 16 Vaccine: mRNA-1273 | 1 | Randomized trials | Not serious | Not serious | Seriousa b | Very serious c | None | 147/15,185 | 153/15,166 | RR=0.96 (0.77 to 1.20) | ⨁◯◯◯ Very low | Critical | ||
| Voysey, 2020 17,18 Vaccine: ChAdOx1 | 1 | Randomized trials | Not serious | Not serious | Seriousa b | Very serious c | None | 108/12,284 | 127/11,962 | RR=0.83 (0. 64 to 1.07) | ⨁◯◯◯ Very low | Critical | ||
| Logunov, 2021 19 Vaccine: Gam-COVID- Vac rAd26-S/ rAd5-S | 1 | Randomized trials | Not serious | Not serious | Seriousa b | Very serious c | None | 45/16,427 | 23/5,435 | RR=0.65 (0. 39 to 1.07) | ⨁◯◯◯ Very low | Critical | ||
CI: Confidence interval; RR: Risk ratio Explanations
a. Data are from an interim analysis of the trial, with a short duration of follow-up. Estimates may change over a longer duration of follow-up.
b. The population included in the RCT may not represent all persons aged ≥16 years (BNT162b2) or ≥18 years (mRNA-1273; ChAdOx1; Gam-COVID-Vac rAd26-S/rAd5-S)
c. Imprecision was downgraded by 2 levels because the 95% of the relative risk (RR) was sufficiently wide that the estimate could include appreciable harm or benefit of the intervention. This outcome may be imprecise due to the small number of events reported during the observation period.
All-cause mortality
| Certainty assessment | No. of patients | Effect | Certainty | Importance | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Vaccine | Placebo | Relative (95% CI) | Absolute (95% CI) | |||
| Polack, 2020 15 Vaccine: BNT162b2 | 1 | Randomized trials | Not serious | Not serious | Seriousa b | Very serious c | None | 2/21,621 | 4/21,631 | RR=0.50 (0.09 to 2.73) | ⨁◯◯◯ Very low | Critical | |
| Baden, 2020 16 Vaccine: mRNA-1273 | 1 | Randomized trials | Not serious | Not serious | Seriousa b | Very serious c | None | 6/15,185 | 7/15,166 | RR=0.86 (0.29 to 2.55) | ⨁◯◯◯ Very low | Critical | |
| Voysey, 2020 17,18 Vaccine: ChAdOx1 | 1 | Randomized trials | Not serious | Not serious | Seriousa b | Very serious c | None | 1/12,282 | 4/11,962 | RR=0.49 (0.09 to 2.66) | ⨁◯◯◯ Very low | Critical | |
| Logunov, 2021 19 Vaccine: Gam-COVID- Vac rAd26-S/ rAd5-S | 1 | Randomized trials | Not serious | Not serious | Seriousa b | Very serious c | None | 3/16,427 | 1/5,435 | RR=0.99 (0.10 to 9.54) | ⨁◯◯◯ Very low | Critical | |
CI: Confidence interval; RR: Risk ratio Explanations
a. Data are from an interim analysis of the trial, with a short duration of follow-up. Estimates may change over a longer duration of follow-up.
b. The population included in the RCT may not represent all persons aged ≥16 years (BNT162b2); ≥18 years (mRNA-1273; ChAdOx1; Gam-COVID-Vac rAd26-S/rAd5-S)Imprecision was downgraded by 2 levels because the 95% of the relative risk (RR) was sufficiently wide that the estimate could include appreciable harm or benefit of the intervention. This outcome may be imprecise due to the small number of events reported during the observation period. CI: Confidence interval; RR: Risk ratio