Inflammatory bowel diseases are characterized by chronic inflammation. Crohn’s disease and ulcerative colitis are the most prevalent forms of this group of diseases. The etiology of inflammatory bowel diseases is not well understood, but it is known that environmental, genetic and immune response factors are involved in the development of the inflammatory response 1-3.
In one of the hypotheses raised, the dysregulated mucosal barrier would facilitate the access of luminal antigens through the epithelium. This would induce the recruitment of lymphocytes and macrophages with the release of cytokines and several inflammatory mediators causing intestinal damage 4. To promote symptomatic remission, antiinflammatories, immunosuppressants, and immunobiological are commonly used in the treatment of inflammatory bowel disease 5. However, some patients do not present an adequate response or loss of effective response during the treatment 5-7. For this reason, the interest in plants with medicinal properties is extensively investigated.
Mimosa caesalpiniifolia (Fabaceae: Mimosoideae) is a medicinal plant popularly known as “sansão-do-campo” or “sabiá” 8 in the northeastern region of Brazil. The leaves and the bark were prepared as an infusion, decoction or “bottled” against hypertension, inflammatory diseases and infections 9,10. Mimosa caesalpiniifolia hydroalcoholic extract also exhibited antimicrobial, antioxidant 11 and cytotoxic activity against the human breast cancer cell line MCF7 12 and reduced DNA damage in blood cells from cadmium-affected mice 13.
A phytochemical study of a M. caesalpiniifolia leaf extract with antifungal activity allowed the identification of 28 compounds. Apigenin 6-C-e- boivinopyranoside, apigenin 8Ceoliopyranoside, (E)-6-(2-carboxyethenyl) apigenin, (E)8(2carboxyethenyl) apigenin and 7,5”-anhydroapigenin 6<-α- (2,6-dideoxy-5-hydroxy-arabinohexopyranoside had not been previously described 14,15.
In a recent study, we found a potential anti-inflammatory effect of the hydroalcoholic extract of M. caesalpiniifolia on trinitrobenzene sulfonic acid (TNBS)-induced colitis in Wistar rats (16). Since the incorporation of plant extracts into pharmaceutical forms can improve their solubility and bioavailability, the present study proposes to evaluate the effects of M. caesalpiniifolia pre-formulation on the intestinal barrier during dextran sulfate sodium (DSS)induced colitis.
In the study of Silva et al., members of the Mimosoideae family were well- characterized by phytochemical diversity. Polyphenols including flavonoids and tannins are the most commonly found bioactive compounds in M. caesalpiniifolia. Catechins were present both in the ethyl acetate fraction and in the alcoholic extract. The hydroalcoholic extract and the ethyl acetate fraction were effective for the treatment of induced colitis 16.
It has been suggested that M. caesalpiniifolia is able to contain TNBS- induced tissue damage as a result of down-regulation of the tumor necrosis factor alpha (TNF-α) expression when using low doses of Mimosa16.
In that same study, M. caesalpiniifolia demonstrated the ability to inhibit cyclooxygenase-2 (COX-2) expression when administered at a dose of 250 mg after colitis induction and 50 mg of the ethyl acetate fraction. Therefore, COX-2 inhibition was only achieved when M. caesalpiniifolia was administered at higher doses. It appears that TNF-α and COX-2 play a key role during chronic colitis caused by TNBS 16.
In summary, the results suggest that M. caesalpiniifolia attenuated colonic lesions as a result of reduced inflammation 16. The main difference between the previous study and the present one, in addition to the model, is that in this case a pre-formulation was made with the vegetal extract of the plant.
Materials and methods
Ethics approval
The study was approved by the Commission for the Use of Animals in Research (CEUA 4362180119) of UNIFESP-EPM.
Plant material
To prepare the preformulation, the leaves of M. caesalpiniifolia were collected in January 2012, in the city of Alfenas, Minas Gerais, Brazil. The geographic coordinates of the collection site are 21° 24' 44.1" S, 45° 55' 19.9" W. The plant was authenticated by Dr. Geraldo Alves da Silva at the Federal University of Alfenas (UNIFAL-mG). A voucher sample (695) was deposited in the Herbarium of UNIFAL-MG.
Pre-formulation of Mimosa caesalpiniifolia plant extract
To create the pre-formulation for rat colitis treatment, dried and powdered leaves (256 g) of M. caesalpiniifolia were extracted individually by simple percolation. The ethanolic extract (4 g) was dissolved in distilled water (300 ml) and then, subjected to the liquid-liquid extraction process (partition) for seven days. After extraction, the solvent was removed by a rotary evaporator and lyophilized.
Mimosa caesalpiniifolia lyophilized extracts were prepared in a solution with 70% ethanol and dried with a Buchi B19 Mini-spray dryer with a 20% Aerosil® solution. The operational conditions used were followed by the Medicinal Plants and Herbal Medicines Laboratory of UNIFAL-MG 16.
The pre-formulation was diluted in 1.0 ml of water and given orally (gavage) to the rats, according to the experimental groups, at a dose of 125 mg/kg/day.
Experimental design
We used 32 male Wistar rats (Rattus norvegicus albinus) from the Center for the Development of Experimental Models for Medicine and Biology (CEDEME) of UNIFESP-EPM. These animals weighted between 200 and 250 g and were kept in rooms with restricted access, controlled temperature (23°C) and a 12 hours-light period, 12 hours-dark cycle. Standard laboratory chow and drinking water were provided ad libitum. The rats were randomized into four groups: basal control (BC), 5% untreated DSS-induced colitis (DSS), MC pre-formulation control (125 mg/kg/day) (MCC), and 5% DSS-induced colitis treated with MC pre-formulation (125 mg/kg/day) (DSSMC).
Induction of colitis and euthanasia
A 5% DSS solution (MP Biomedicals) was administered to induce colitis, diluted in water and offered ad libitum for five days. Vials containing the DSS solution were changed to maintain drug efficacy on the third day. The DSS solution was changed back to drinking water after five days. The M. caesalpiniifolia pre-formulation was diluted in 1.0 ml of water and administered by oral gavage per six days. The clinical activity index (CAI) -percentage of weight loss, stool consistency and rectal bleeding- was recorded daily using the score 17.
All rats were euthanized on the ninth day with a lethal dose of halothane (Tanohalo®). Cardiac puncture was performed to confirm death ten minutes after the total loss of reflexes. Abdominal cavity was open to extract the entire colon, and then we washed it with 0.9% saline solution to remove fecal residues.
Histopathological analysis
After dissection, the distal colon was fixed in 10% buffered formalin and soaked in paraffin. Tissue sections of 3 µm were stained with hematoxylin and eosin for histopathological analysis of colon damage by light microscopy. The images were captured at 250X magnification with an Axio cam MRc5 camera attached to an Axioskop 40 Zeiss™ optical microscope. Setting and image acquisition were performed using Axio Vision software. The scale bar was inserted with the same software 18.
Transmission electron microscopy
To analyze colon damage, samples from rat’s colon (two from each group) measuring 1 mm χ 1 mm χ 2 mm were fixed in 2% formaldehyde plus 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.2) for two hours at 4°C. Subsequently, samples were fixed in 2% osmium tetroxide in 0.1 M sodium cacodylate buffer for the same period. The samples were washed in phosphate buffered saline solution (PBS), dehydrated with ethanol, soaked in mounting medium at 37 °C for three hours and finally polymerized with EPON epoxy resin at 60 °C for 36 hours.
We used semithin sections (300-500 µm) for area delimitation. The slides were subjected to light microscopy and hot stained with 1% toluidine blue solution. Subsequent analyzes were carried out using a transmission electron microscope, model EX II 1200 (JEOL, Japan).
Goblet cells index
For mucin analysis of the goblet cells, the material was stained using alcian blue histochemical technique and counterstained with hematoxylin for identification and quantification of goblet cells.
The alcian blue dye (Ventana) was added to the sections for 20 minutes and rinsed in distilled water. Hematoxylin was applied to the sections for 30 seconds, followed by rinsing in running water, alcohol and xylene, and mounted in Entellan resin (Merck).
About 15 fields were captured in five different sections for each animal, containing approximately 1,000 consecutive cells. Indistinctly of alcian blue positivity, we quantified the cells with an Olympus BX 40™ optical microscope at 200X magnification. The ratio between the number of goblet cells and the number of enterocytes in the mucosa was defined as the goblet cell index (CI), as follows: CI = (Number of goblet cells / total number of mucous cells) x 100.
Short-chain fatty acids measurement
To indicate the level of intestinal epithelial barrier protection we measured short-chain fatty acids according to the protocol of Ribeiro et al.19 with adaptations.
To quantify short-chain fatty acids, we generate a standard curve for the concentration range of 0.15610 mmol/L using rat feces as a matrix.
Fecal samples were weighed into microtubes (approximately 30 mg per sample) and homogenized with 100 µl of distilled water. Then, we added 40 mg of sodium chloride, 50 µl of 1M hydrochloric acid and 300 µl of n-butanol. The microtubes were vortexed for two minutes and centrifuged at 18,000g for 15 minutes. The organic phase was transferred to chromatographic vials and analyzed using a GC-FID 2010 (Shimadzu) equipped with an AOC20i Autosampler and fused silica capillary wax columns (Rtx) of 30 mm x 0.25 mm (Restek Corp.) coated with 0.25 µm polyethylene glycol. Samples (1 µl) were injected at 260 °C using a 20:1 split ratio. High-grade pure nitrogen was used as carried gas at 1 ml/minute constant flow and the results were expressed in terms of concentration (μΜ).
Results
Effects of Mimosa caesalpiniifolia pre-formulation on the clinical activity index
As expected, the animals in the basal control group remained healthy from the beginning to the end of the experiment, showing gain weight. The only administration of M. caesalpiniifolia preformulation in the control group did not cause diarrhea or weight loss, demonstrating therapeutic safety. On the other hand, the animals in the untreated DSS-induced colitis group showed all the clinical signs such as weight loss, diarrhea, and rectal bleeding. Treatment with the M. caesalpiniifolia pre-formulation attenuated colitis symptoms (p=0.0003). The results of this analysis are summarized in figure 1.
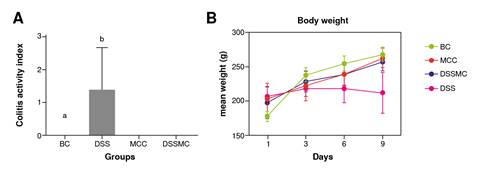
BC: basal control; DSS: dextran sulphate sodium; MCC: Mimosa caesalpiniifolia pre formulation control; DSSMC: DSS-induced colitis treated with Mimosa caesalpiniifolia pre-formulation
Figure 1 Effects of Mimosa caesalpiniifolia pre-formulation on the clinical activity index. A) Total clinical score activity index: a basal control vs 5% untreated dextran sulphate sodium (DSS) induced colitis - p=0.0003; b 5% untreated DSS-induced colitis (DSS) vs Mimosa caesalpiniifolia pre formulation control and 5% DSS-induced colitis treated with Mimosa caesalpiniifolia preformulation (DSSMC) - p=0.0003; B) Body weight. There were non-significant diferences between groups according to Tukey's test (eight rats per group).
During the macroscopic evaluation, the rats of the basal control and M. caesalpiniifolia control showed total integrity of the colon, without signs of edema, inflammation, or ulcers. In contrast, the untreated DSS-induced colitis group showed thickening, edema, and ulcers. Rats with DSS-induced colitis treated with M. caesalpiniifolia pre-formulation showed mild edema and hyperemia. The macroscopic representation of the colon from each group can be viewed in figure 2.

Figure 2 Images of Wistar rat’s intestine in each of the four groups. A) Basal control - without macro-scopic changes; B) 5% untreated DSS-induced colitis presented intestinal thickening, edema, and ulcers; C) Mimosa caesalpiniifolia pre-formulation control (125 mg/kg/ day) did not present macroscopic chang-es; and D) 5% DSS-induced colitis treated with Mimosa caesalpiniifolia pre-formulation (125 mg/kg/day) presented mild edema and hyperemia
Effects of Mimosa caesalpiniifolia pre-formulation on the microscopic score
There were no changes in the colonic cellular architecture of the basal and M. caesalpiniifolia groups. In the DSS-induced colitis group, we observed intense inflammatory exudate and the presence of ulcers, permeated by healthy areas. On the other hand, rats treated with M. caesalpiniifolia preformulation showed a decrease in histological score. There was a reduction of the inflammatory infiltrate cells and the absence of ulcers, but no significant differences. The representative photomicrographs of each group and the histological score are in figure 3.
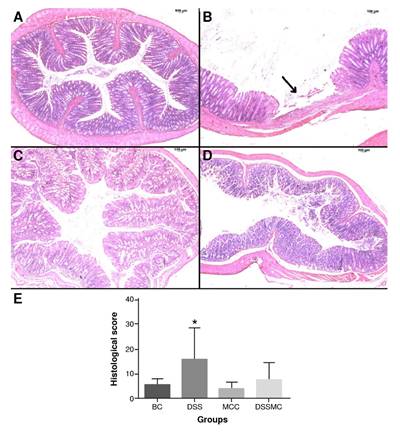
BC: basal control; DSS: dextran sulphate sodium; MCC: Mimosa caesalpiniifolia pre formulation control; DSSMC: DSS-induced colitis treated with Mimosa caesalpiniifolia pre-formulation
There were significant differences (p=0.0187) between * 5% untreated DSS-induced colitis and Mimosa caesalpiniifolia pre-formulation control according to Tukey's test (eight rats per group).
Figure 3 Effects of Mimosa caesalpiniifolia pre-formulation on intestinal tissue stained with hematoxilin-eosin, 250X. A) Basal control with typical cellular architecture; B) 5% untreated DSS-induced colitis showed intense inflammation and presence of ulcer (arrow) alternated by healthy areas; C) Mimosa caesalpiniifolia pre-formulation control with typical cellular architecture; D) 5% DSS-induced colitis treated with Mimosa caesalpiniifolia pre-formulation with typical cellu-lar architecture; and E) Histological score.
Effects of Mimosa caesalpiniifolia pre-formulation on goblet cell's index
There were no significant differences in the goblet cells index between the group treated with M. caesalpiniifolia pre-formulation when compared with the DSS-induced colitis group. The results are summarized in figure 4.
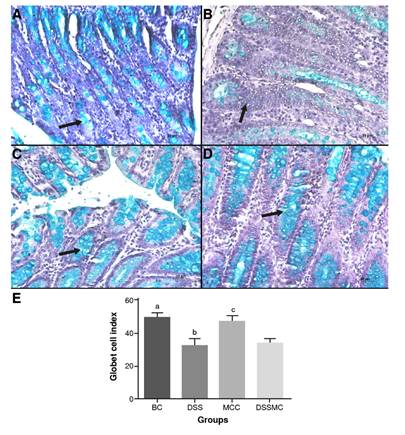
BC: basal control; DSS: dextran sulphate sodium; MCC: Mimosa caesalpiniifolia pre formulation control; DSSMC: DSS-induced colitis treated with Mimosa caesalpiniifolia pre-formulation
Statistically significant differences (p<0.0001) were found comparing BC versus DSS and DSSMC; DSS versus MCC; MCC versus DSSMC. Analyses were performed with five rats per group applying ANOVA and Tukey's post-hoc test.
Figure 4 Effects of Mimosa caesaipiniifolia pre-formulation on goblet cell's index. Samples stained with Alcian blue staining, 500X. A) Basal control with preserved goblet cells (arrow); B) 5% untreated DSS induced colitis showed depletion of goblet cells (arrow); C) Mimosa caesaipiniifolia pre-formulation control with preserved goblet cells (arrow); D) 5% DSS-induced colitis treated with Mimosa caesaipiniifoiia preformulation with preserved goblet cells; and E) Goblet cells index.
Effects of Mimosa caesalpiniifolia pre-formulation on ultrastructural changes
The basal control group showed preserved organelles, absorptive epithelium, and tight junctions. The DSS-induced colitis group displayed disorganization of the mitochondrial crest, endoplasmic reticulum edema, nucleus fragmentation, collagen fiber deposit (healing process indicative), junctions, and absorptive epithelium loss. We observed that the treatment with M. caesalpiniifolia pre-formulation stalled the inflammatory process, but was not able to restore the absorptive epithelium and the tight junctions. The ultrastructural changes can be viewed in figure 5.
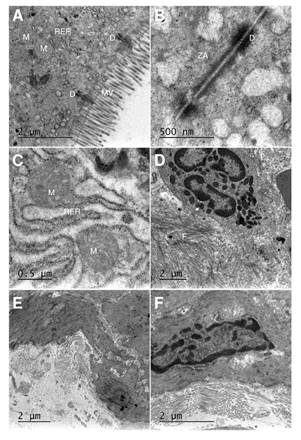
MV: mucosal villus; ZA: zonula adherens; RER: rugous endoplasmic reticulum; D: desmosome; M: mito-chondria; F: collagen fiber
Figure 5 Representative transmission electron micrographs: Basal control displaying typical morphology of mucosal villus: zonula adherens preserved, normal rugous endoplasmic reticulum, preserved desmosome, and mitochondria. A) 2 µm scale, and B) 0.5 µm scale; 5% untreated DSS-induced colitis displaying edema of rugous endoplasmic reticulum, swollen mitochondria, a deposit of collagen fiber, loss of mucosal villus and zonula adherens. C) 0.5 µm scale and D) 2 µm scale; E) and F) 5% DSS induced colitis treated with Mimosa caesaipiniifolia pre-formulation displaying a deposit of collagen fiber, loss of mucosal villus and zonula adherens, at a 2 µm scale.
Effects of Mimosa caesalpiniifolia pre-formulation on SCFAs measurement
The retention time for the short-chain fatty acids was 7.53 minutes for acetic acid, 8.28 for propionic acid, and 9.18 for butyric acid. The total run time was 13 minutes. Overall, the results show that the levels of short-chain fatty acids are decreased in rats of the DSS-induced colitis group, mainly for butyrate levels (p=0.0040). Although no significant differences were evident, we observed that treatment with M. caesaipiniifoiia pre-formulation demonstrated a trendy increase of short-chain fatty acids (figure 6).
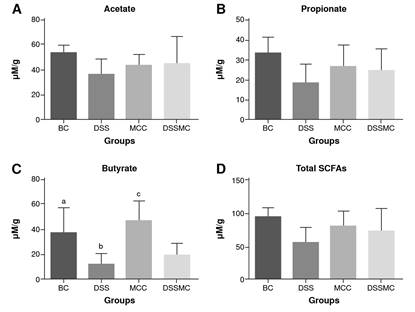
BC: basal control; DSS: dextran sulphate sodium; MCC: Mimosa caesalpiniifolia pre formulation control; DSSMC: DSS-induced colitis treated with Mimosa caesalpiniifolia pre-formulation
Statistically significant differences (p=0.0040) were found for butyrate levels (C) comparing: BC versus 5% untreated DSS-induced colitis; DSS versus Mimosa caesalpiniifolia pre-formulation control, and MCC versus 5% DSS-induced colitis treated with Mimosa caesalpiniifolia preformulation (DSSMC). Analyses were per-formed with six rats per group applying ANOVA and Tukey's post-hoc test.
Figure 6 Effects of Mimosa caesalpiniifolia pre-formulation on short-chain fatty acids (SCFAs) measurements. Non-significant differences were found in SCFAs for acetate (A), propionate (B) and total SCFAs levels (D) in each of the four assessed groups
Discussion
Researchers are highly attracted to using plants in folk medicine, as this is ancient knowledge. Bioactive compounds such as flavonoids and catechin derivatives that have antioxidant, anti-inflammatory, and healing actions, among others, are revealed through phytochemical screening of different species 20.
Other species of Mimosa have also been applied to treat other diseases 21. Mimosa pudica, for example, is used in the treatment of various activities and has shown anthelmintic action 22 and anti-inflammatory properties 23. The M. pudica root extract contains high levels of antioxidants 24.
Mimosa tenuiflora is a popular remedy used in Mexico and Brazil. The bark of this plant is an effective remedy for treating burns and wounds, and is also used to prevent inflammation 23-26.
Another species, the Mimosa hostilis, also known as jurema, is culturally used in Candomblé rituals of indigenous heritage 27. The ingestion of jurema wine, or the consumption of the stem and root in the form of smoke, triggers psychotropic effects such as euphoria, visual and oneiric hallucinations, and perception distortion of time, space, shape and colors, among other symptoms. In addition, M. hostilis is also used against coughs and it aids in wound healing 27.
In a previous study, we found that administration of hydroalcoholic extract of M. caesalpiniifolia at a dose of 125 mg/kg after induction of colitis by TNBS was able to mitigate the harmful effects by decreasing colon inflammatory process through negative regulation of TNF-α expression 16,28. With these findings and to evolve in the development of a plantbased medication, the extract of M. caesalpiniifolia was incorporated into the adjuvant Aerosil®, obtaining the pre-formulation at a promising dose of 125 mg/kg. We tested the preformulation as a possible restorer of the intestinal barrier in rats with DSS-induced colitis.
It is well-established that during the clinical activity of inflammatory bowel diseases in humans, there is disruption of the intestinal barrier caused by the intense inflammatory response triggered, powering the process of intestinal injury 4,29. In this context, the DSSinduced colitis is the most assertive way to evaluate the mechanisms of intestinal barrier injury 30-32. Initially, the disruption of the colon epithelial monolayer allows the dissemination of pro- inflammatory stimuli (microorganisms and their secreting proteins) into the underlying tissue 30-32. Indeed, in our study we successfully induced colitis with bloody diarrhea and weight loss as main symptoms. We emphasized the therapeutic safety of the M. caesalpiniifolia pre-formulation, since the control group did not show intestinal alterations. The rats with DSSinduced colitis and treated with the pre-formulation demonstrated a significant decrease in the clinical signs of colitis, eliminating episodes of diarrhea and promoting gradual weight gain.
In the clinical management of inflammatory bowel diseases, the goal is remission of the clinical symptoms and complete healing of the intestinal mucosa. In this point of view, the M. caesalpiniifolia pre-formulation might be a good option due to its healing and antiinflammatory properties. Although there were no significant differences, we observed decreasing histological damage, stalling of the inflammatory process, and a massive deposit of collagen fiber in animals treated with M. caesalpiniifolia pre-formulation. However, there was no evidence of barrier restoration. Like the untreated DSS-induced colitis group, there was a loss of absorptive epithelium and tight junctions, endoplasmic reticulum stress, and disorganization of the mitochondrial crest.
These findings corroborate the mechanisms of injury in classical human inflammatory bowel diseases, where disturbances in the intestinal barrier lead to mitochondrial damage, alteration in the ATP production causing reactive oxygen species (ROS) accumulation, hindering cellular metabolism, and macromolecules anabolism for effective repair 33-38. Also, the endoplasmic reticulum stress promotes ROS increasing, which leads to the goblet cells’ death, affecting the processes of synthesis, modification and folding of proteins, particularly those of the mucin 2 protein (MUC-2), secreted continuously to replenish the mucus layer 39. Based on this fact, we also evaluated the effects of M. caesalpiniifolia pre-formulation in the goblet cells index, but there was no significant differences. Therefore, our findings suggested that the treatment could prevent the death of goblet cells by minimizing the damage caused by DSS.
Butyrate, acetate, and propionate are considered energy sources for colonocytes, stimulating smooth muscle contractions, intestinal blood flow, transepithelial chloride secretion chloride, and exerting stimuli of colonic epithelial cells 40,41. In addition, some studies reported that low concentrations of butyrate stimulate MUC2 expression suggesting a protective effect on intestinal barrier function 40,41. Although no significant differences were observed, our findings of the short-chain fatty acid measurement suggest that M. caesalpiniifolia preformulation treatment positively affected the levels of acetate, propionate, and butyrate.
The M. caesalpiniifolia pre-formulation minimized the clinical symptoms of colitis and intestinal inflammation but did not minimize damage to the intestinal barrier. We hypothesized that a new experimental protocol with a prolonged treatment period could demonstrate more effective results regarding the role of M. caesalpiniifolia pre-formulation on epithelial barrier restoration.
Therefore, we can conclude that the treatment with the pre-formulation of the M. caesalpiniifolia plant extract in the experimental colitis induced by the DSS reduced the clinical symptoms when administered alone, and helped reduce microscopic lesions. On the other hand, it did not restore the damage to the intestinal barrier and did not modify the index of goblet cells, nor interfere significantly with the levels of short-chain fatty acids. In addition, we suggest that the DSS model may demonstrate a better reproducibility of UC in the animals compared to the TNBS model, which would be better for a colitis disease model.
Phytotherapy requires more in-depth studies and care by qualified professionals, remembering that it is not because it is natural that it is not harmful. Popular medicine in Brazil has not finished its course, it is in constant formation and transformation, incorporating new elements at every moment.














