Paracoccidioidomycosis is the most important systemic mycosis in South America. It is caused by thermally dimorphic fungi belonging to the genera Paracoccidioides1-3. Approximately 80% of these cases were described in Brazil, followed by Colombia, Venezuela, and Argentina 4.
This mycosis presents a broad spectrum of clinical manifestations and occurs more frequently in males than females. The ratio is 1.7:1.0 among patients with the acute/subacute form and 22:1among those with the chronic form of the disease, affecting people aged 30 to 60 years, mostly in rural areas 5.
The definitive diagnosis of Paracoccidioides spp. infection is based on direct microscopic examination of biological specimens and their culturing, followed by macro-and microscopic observation for fungus identification 2,6. However, these procedures are insensitive and time-consuming, and it is difficult to obtain clinical specimens 7. In contrast, serological assays have played an important role in the early diagnosis of paracoccidioidomycosis and in monitoring its evolution and response to treatment 6-9. The standard serological assay involves double immunodiffusion; however, counterimmunoelectrophoresis, complement fixation, enzyme-linked immunosorbent assay (ELISA), immunoblotting, and dot ELISA may also be used 2,6-9.
The existing methods for producing Paracoccidioides spp. antigens are problematic in terms of standardization, specificity, stability, repeatability, and reproducibility 10-12. Thus, in this study, we sought to optimize the methodology for antigen production described by Freitas et al., evaluating its applicability in paracoccidioidomycosis immunodiagnosis using a double immunodiffusion assay 13.
Material and methods
Isolates of Paracoccidioides spp.
We seleted one P. brasiliensis senso strictu (S1-Pb 113), one P. restrepiensis (PS3-B-339), and three P. lutzii (01, 66, and 8334) isolates for this study. The isolates were cultured on Fava-Netto modified agar medium at 36 °C ± 1 °Cand were subcultured every 15 days.
Preparation of Paracoccidioides spp. antigens
We employed the method described by Freitas et al. to prepare antigens from Paracoccidioides spp. isolates 13. Briefly, P. lutzii yeast cells were grown on Fava-Netto modified agar medium at 36 °C ± 1 °C for 5, 10, and 20 days. Paracoccidioides brasiliensis senso strictu and P. restrepiensis were grown in the same medium and temperature for seven days. Following the incubation period, 20 ml of a thimerosal-borate aqueous solution (1:5,000; Sigma Chemical Co. St. Louis, MO, USA) was added to each culture tube.
Culture tubes containing the fungal cells were incubated and submerged in a thimerosal-borate aqueous solution at room temperature for 24 hours. The supernatants were then aspirated using a sterile serological pipette and filtered through a sterile filter unit of 0.22 μm (Millipore Corporation, Billerica, MA, USA). The antigen solutions were concentrated 10-20-fold by lyophilization. After the protein dosage was determined using the Bradford method 14, the antigenic preparations were stored at -20 °C until use.
Serum samples
We evaluated 24 serum samples from patients with proven paracoccidioidomycosis and previously reactive to P. brasiliensis antigens; ten serum samples from patients with serological reactivity, by double immunodiffusion, for Histoplasma capsulatum; ten serum samples from patients with serological reactivity, by double immunodiffusion for Aspergillus fumigatus, and nine serum samples from random blood donors. Paracoccidioidomycosis cases were classified according to the clinical practice guidelines for this disease 3. Anti-P. brasiliensis and anti-P. lutzii polyclonal antibodies obtained from rabbits were used as positive controls, and anti-Histoplasma capsulatum and anti-Aspergillus fumigatus polyclonal antibodies were used as negative controls.
The paracoccidioidomycosis serum samples evaluated in this study were obtained from patients residing in the state of São Paulo, a region endemic for P. brasiliensis sensu stricto. The serum samples evaluated in this study were stored at -20 °C in the biorepository of Laboratório de Imunodiagnóstico das Micoses, Instituto Adolfo Lutz, São Paulo, SP, Brasil.
Ethics
The study was approved by the Internal Scientific Commissions and Bioethics in Human Research Committee of Instituto Adolfo Lutz (CEPIAL# 026/11).
Double immunodiffusion assay
Double immunodiffusion assays were performed using the modified Ouchterlony method 15. The glass slides were covered with 3 ml of 1% agarose gel type II medium (Sigma Chemical Co., St. Louis, MO, USA) diluted in a buffered saline solution (pH 6.9) containing 0.4% sodium citrate and 7.5% glycine. Antigens (10 μl) were placed in the central well, while control and patient sera (10 μl) were added to the surrounding wells. The slides were incubated in a humid chamber at room temperature for 48 hours. Subsequently, they were washed with saline solution several times over a 24 hour-period. The gels were dried and stained in an ethanol-acetic acid-water mixture with 0.4% Coomassie brilliant blue R-250® (Sigma Chemical Co., St. Louis, MO, USA).
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS- PAGE) was performed as previously described by Laemmli (1970) 16. Paracoccidioides spp. antigens were diluted in a buffer containing 62 mM Tris- HCl (pH 6.8), 2% (w/v) SDS, 50 mM 2-mercaptoethanol, 10% glycerol, and 0.01% bromophenol blue; boiled for three minutes, and centrifuged before being seeded into the gels. The antigens were subjected to electrophoresis (20 mA at room temperature) in a 10% discontinuous SDS buffer system in a Mini-Protean II Electrophoresis Cell (Bio-Rad Laboratories, Hercules, CA, USA). The molecular mass was determined using a 10-250 kDa standard prestained protein marker (Bio-Rad Precision Plus Protein All Blue Standards, cat. # 161-037, Bio-Rad Laboratory, Hercules, CA, USA). The electrophoretic profile was visualized using silver nitrate (Sigma-Aldrich Co., St. Louis, MO, USA) stain, according to the protocol described by Ansorge (1985) 17.
Polyclonal antibodies against Paracoccidioides spp. antigens
Two New Zealand white rabbits were immunized subcutaneously on the back with 100 μg of P. brasiliensis (Pb 113) and P. lutzii (Pb 01) antigens, emulsified (3:1 v/v) with complete Freund’s adjuvant (Sigma-Aldrich Co., St. Louis, MO, USA) in the first immunization, and with incomplete Freund’s adjuvant (Sigma-Aldrich Co., St. Louis, MO, USA) in the subsequent ones, repeated every week for four weeks. Rabbit sera were collected one week after the fourth immunization to assess the production of circulating antispecies-specific polyclonal antibodies using an immunodiffusion assay. One week after the booster dose, the rabbits were anesthetized intramuscularly with 10 mg/kg ketamine. The same protocol was used to obtain polyclonal antibodies against H. capsulatum and A. fumigatus antigens.
Stability of Paracoccidioides spp. antigens
The stability of the antigens lyophilized and stored at -20oC was analyzed using a double immunodiffusion assay against species-specific polyclonal antibodies after three, six, and nine months.
Screening of Paracoccidioides spp. antigens
Screening of P. brasiliensis sensu strictu (Pb 113), P. restrepiensis (B- 339), and P. lutzii (01, 66, and 8334) antigenic preparations was performed by determining the protein content, electrophoretic profile, and immunoreactivity against anti-P. brasiliensis, anti-P. lutzii, anti-H. capsulatum, and anti-A. fumigatus polyclonal antibodies and serum samples from random blood donors.
Results
Using different cultivation times (5, 10, and 15 days) and antigen concentrations (in natura, 10- and 20-fold), we obtained 18 antigens from P. lutzii. After the evaluation of the protein content, electrophoretic profile, and immunoreactivity against polyclonal species-specific antibodies, three antigenic preparations were selected: antigen obtained from 01 isolate, cultured for 20 days and 10X (Th 01 20d 10X), antigen obtained from 66 isolate, cultured for five days, 20X (Th 66, 5d 20X), and antigen obtained from 8334 isolates cultured for 10 days, 20X (Th 8334 10 d 20X ) (table 1).
Table 1 Profile of the selected antigens
| Antigens | Protein dosage (mg/mL) | Reactivity with C+ Pb | Reactivity with C+ Pl | Reactivity with C+ Hc | Reactivity with C+ Af |
|---|---|---|---|---|---|
| Th 01 20d 10X (FN) | 71.09 | R | R | NR | NR |
| Th 66 5d 20X (FN) | 66.34 | R | R | NR | NR |
| Th 8334 10d 20X (FN) | 230.19 | R | R | NR | NR |
| Th 113 7d 10X (FN) | 99.58 | R | R | NR | NR |
| Th 339 7d 10X (FN) | 102.50 | R | R | NR | NR |
Th: Thimerosal; FN: Modified Fava-Netto agar; 10X: 10X concentrate; 20X: 20X concentrate; R: Reagent; NR: Non-reactive
C+Pb: policlonal antibody anti-antigen of P. brasiliensis sensu strictu
C+Pl: policlonal antibody anti-antigen of P. lutzii
C+Hc: policlonal antibody anti-antigen of H. capsulatum
C+Af: policlonal antibody anti-antigen of A. fumigatus
Four antigens from P. brasiliensis sensu strictu and P. restrepiensis were obtained, all produced after seven days of culture, following the laboratory’s previous experience with the isolates employed (113 and B-339). After evaluating the same parameters mentioned for P. lutzii, 10-fold concentrated antigens were selected (Th 113 7d 10X and Th 339 7d 10X ) (table 1).
Evaluation of antigenic preparations using double immunodiffusion assay demonstrated strong cross-reactivity when P. brasiliensis sensu strictu and P. restrepiensis antigens were evaluated against anti-P. lutzii polyclonal antibody; and when P. lutzii antigens were evaluated against the anti-P. brasiliensis polyclonal antibody (figure 1). Human serum samples with proven paracoccidioidomycosis also showed reactivity using double immunodiffusion assay against antigenic preparations of P. lutzii, reinforcing the desirable cross-reactivity between Paracoccidioides spp. and polyclonal antibodies. Out of the evaluated samples, 21 (87.5%) showed reactivity to Th 01 20d 10X antigen, 16 (66.66%) to Th 66 5d 20X antigen, and 20 (83.33%) to Th 8334 10d 20X antigen (figure 1 and table 2).
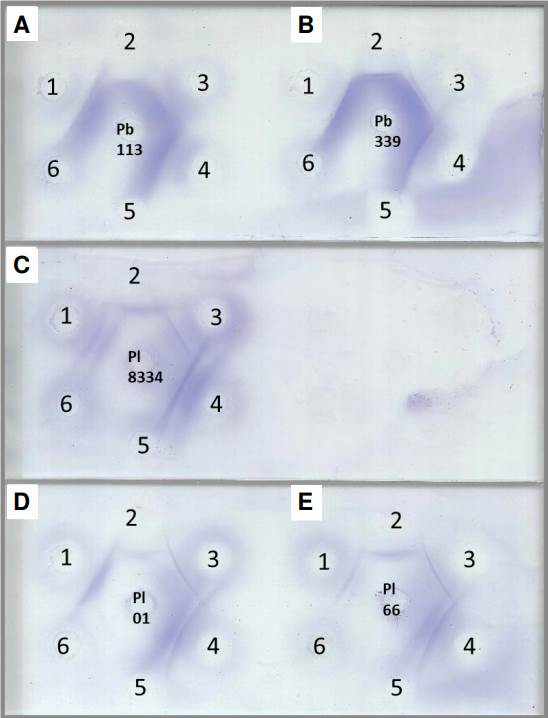
Figure 1 Double immunodiffusion assay with different Paracoccidioides spp. antigens. A) Th 113 7d 10X, B) Th 339 7d 10X, C) Th 8334 10d 20X, D) Th 01 20d 10X, and E) Th 66 5d 20X. 1) Anti-P. lutzii polyclonal antibody, 2) anti-P. brasiliensis polyclonal antibody, 3 and 4) paracoccidioidomycosis serum samples, 5) anti-H. capsulatum polyclonal antibody, and 6) anti-A. fumigatus polyclonal antibody.
Table 2 Sensitivity and specificity of Paracoccidioides spp. antigens
| Antigens | Reactivity with proven PCM | Sensitivity % | Specificity % |
|---|---|---|---|
| Th 01 20d 10X (FN) | 21/24 | 87.5 | 85.29 |
| Th 66 5d 20X (FN) | 16/24 | 66.66 | 85.29 |
| Th 8334 10d 20X (FN) | 20/24 | 83.33 | 85.29 |
| Th 113 7d 10X (FN) | 24/24 | 100.00 | 100.00 |
| Th 339 7d 10X (FN) | 24/24 | 100.00 | 100.00 |
Th: Thimerosal; FN: Modified Fava-Netto agar; 10X: 10X concentrate; 20X: 20X concentrate;
PCM: Paracoccidioidomycosis
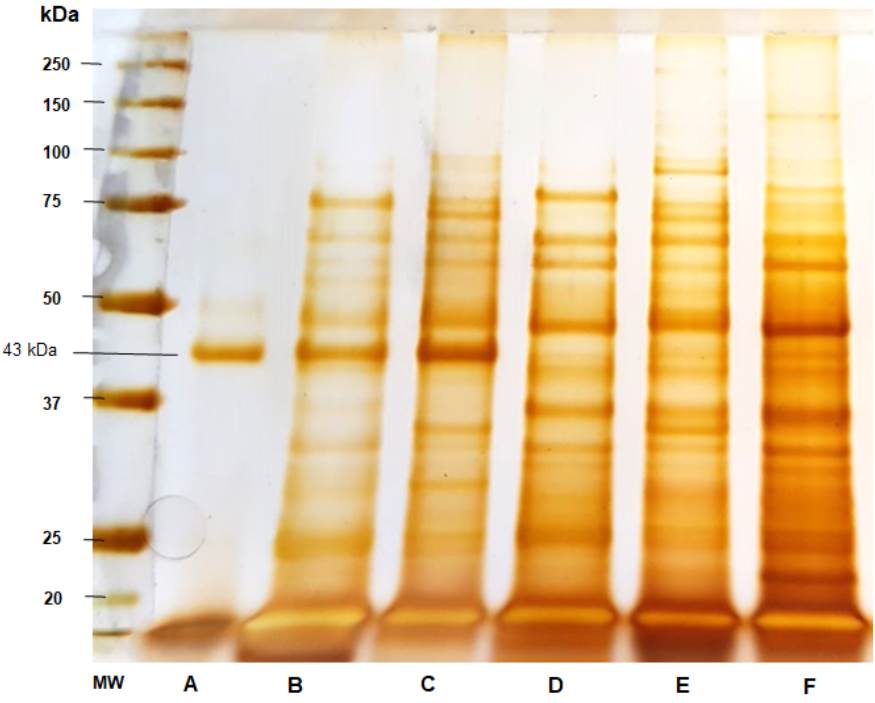
Figure 2 Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) of the Paracoccidioides spp. antigens. A) Paracoccidioides brasiliensis 113 culture-filtrate, B) Thimerosal 113 7d 10X, C) thimerosal B-339 7d 10X, D) Thimerosal 01 20d 10X, E) Thimerosal 66 5d 20X, and F) Thimerosal 8334 10d 20X.
There was no cross-reactivity between the Paracoccidioides antigens and the polyclonal antibodies anti-H. capsulatum and anti-A. fumigatus, neither with the random blood donors. However, we observed that three (3/10) serum samples from patients with histoplasmosis and two (2/10) from patients with aspergillosis showed cross-reactivity using double immunodiffusion assay when evaluated against P. lutzii antigens (Th01 20d 10X, Th 66 5d 20X, and Th 8334 10d 20X).
The stability results of the Paracoccidioides spp. antigens lyophilized and stored at -20 °C were analyzed after three, six, and nine months using double immunodiffusion assay against species-specific polyclonal antibodies. The results revealed that all the antigens retained their activity.
Analysis of the electrophoretic profile of the Paracoccidioides spp. antigens revealed several protein fractions with apparent molecular masses between 14 and 150 kDa. Some protein fractions were shared among P. brasiliensis sensu strictu, P. restrepiensis, and P. lutzii when obtained using the method described by Freitas et al. 13. Figure 2 shows gp43 production and secretion preferentially by B-339 and 113 Paracoccidioides spp. isolates (lanes A, B, and C). The culture-filtrate antigen of strain 113 predominantly comprised the fraction of gp43 (lane A).
Discussion
Different antigens have been used for the immunodiagnosis of paracoccidioidomycosis using a double immunodiffusion assay. However, these antigen preparations were not standardized from one laboratory to another and included cytoplasmic extracts of yeast forms, concentrated filtrates, lyophilized filtrates, cell wall components, purified molecules, and recombinant antigens 7,18. These antigens were prepared from different Paracoccidioides spp. isolates, grown in different culture media and under different growth conditions 12,18. Besides, distinct Paracoccidioides spp. isolates can produce remarkably diverse qualities and quantities of antigens, a response that is also influenced by the culture medium 11,18.
Considering all the above in Paracoccidioides spp. antigen production, considerable disagreement regarding the sensitivity and specificity of the double immunodiffusion assay is not surprising. Vidal et al. compared the performance of laboratories from six medical mycology reference centers in Brazil that conducted routine serology with P. brasiliensis antigen preparations for the paracoccidioidomycosis diagnosis 11. The results showed inconsistencies among laboratories strong enough to result in conflicting information regarding patient management.
In addition to these methodological variations, in the last few years, false negative results have been reported in patients with proven paracoccidioidomycosis, especially in endemic regions of P. lutzii, suggesting antigen differences between species. Therefore, a comparative assessment of the antigen profiles of P. brasiliensis sensu strictu, P. restrepiensis, and P. lutzii is urgently needed. However, it must be emphasized that the protocol for obtaining these antigen preparations needs to be carefully considered because different parameters can alter the expression or secretion of protein fractions, both important for immunodiagnosis. According to the latter, this study developed a useful methodology for obtaining antigens from P. brasiliensis and P. lutzii, in contrast to the culture-filtered exoantigen, which, despite being indicated for the complex P. brasiliensis, does not show good results for P. lutzii19,20.
For several years, culture-filtrate preparations obtained from P. restrepiensis B-339 strain, rich in gp43, an immunodominant molecule of the species, was considered by several groups 12,21,22 as the “gold standard” antigen preparation, being distributed gracefully to different countries in South America like Colombia, Venezuela, and Argentina. However, Leitão et al. (2014) demonstrated that the gp43 of P. lutzii is produced in different amounts and has different epitopes according to the species 23. It strongly suggests that this molecule does not have the same serological importance between species.
These data confirm the need to reevaluate the parameters used to select antigen preparations to diagnose suspected cases. For example, it must be considered the migrating populations in Brazil. In addition, recent eco- epidemiological studies suggested that species can cohabit inthe same ecological niche 24,25. In this study, we observed cross-reactivity when P. brasiliensis sensu strictu and P. restrepiensis antigens were evaluated against anti-P. lutzii polyclonal antibody, and when P. lutzii antigens were evaluated against the anti-P. brasiliensis polyclonal antibody, suggesting that both species share protein and/or glycoprotein fractions.
Our results agree with those of Buccheri et al. (2018) 26 and Lenhard-Vidal et al. (2018) 27. Buccheri et al. (2018) described a patient with the acute form of paracoccidioidomycosis caused by P. brasiliensis with negative results on two reference centers’ routine screening for P. brasiliensis antibodies but positive for P. lutzii antigens implying that antibodies elicited during P. brasiliensis infection recognized the antigen fractions shared by both species 25. Lenhard-Vidal et al. (2018) showed that rabbit and human immune sera recognized several antigens from P. lutzii and P. brasiliensis complex isolates 27.
Regarding the three serum samples that did not show reactivity to P. lutzii, we must emphasize that other factors may contribute to the occurrence of false negative results, such as the clinical form of the disease, patient’s immunological status, and duration of antifungal therapy, all of which decrease the number of circulating antibodies and prevent their detection using serological methods, especially those with lower sensitivity 28. In such cases, immunoenzymatic assays, such as immunoblotting, can be used to improve the accuracy of immunodiagnostics. SDS-PAGE analysis of the antigen preparations revealed the presence of a wide variety of proteins in Paracoccidiodes spp. This method showed numerous protein and glycoprotein fractions in common for the three fungal species, corroborating the cross-reactivity observed in serological evaluations. It was also observed high gp43 secretion by P. brasiliensis sensu stricto and P. restrepiensis, with the majority being in the culture-filtered exoantigen. False negative results with this antigen preparation may be related to differences between species and the methods used to obtain the antigen preparations.
In this study, we evaluated the antigens obtained from three species of Paracoccidioides: P. brasiliensis sensu stricto, P. restrepiensis, and P. lutzii. Evaluation of the electrophoretic profile using SDS-PAGE revealed the presence of common protein fractions among different species, explaining the occurrence of cross-reactivity. Our results indicate the existence of genusspecific recognition, and we believe this characteristic is highly beneficial for the immunodiagnosis of paracoccidioidomycosis. In addition, we verified that using an antigen preparation composed of all the antigens obtained significantly increased the sensitivity of the agarose gel immunodiffusion assay (data not shown), thus contributing to not losing cases in serological evaluation.
Cross-reactivity between sera from patients with histoplasmosis or aspergillosis and Paracoccidioides spp. antigens has already been described in numerous studies and is related to antigen similarity between genera, translating it into a diagnostic problem, even when recombinant antigens are used in assays 29,30. Furthermore, overlapping ecological niches of different fungal species, cell wall composition, ubiquity of antigen determinants, and heterophile antibodies may contribute to cross-reactivity 31. Also, identified cross-reactivity may be influenced by memory antibodies or even rare co-infection by the two pathogens.
Dos Santos et al. (2015) evaluated latex assay performance using purified gp43 to identify circulating antibodies in serum samples from 65 patients with paracoccidioidomycosis. The specificity assay was determined using serum samples from 18 patients with histoplasmosis, 18 with aspergillosis, 13 with candidiasis, and 38 “healthy” individuals. The authors reported a sensitivity of 98.4% and a cross-reactivity of 16.6% for histoplasmosis, 11.11% for aspergillosis, and 7.7% for candidiasis.
In other study, the original 27 kDa recombinant protein was cloned and expressed in Escherichia coli to produce the 27 kDa molecular weight recombinant Paracoccidioides antigen. Indirect enzyme immunoassays were performed with the antigen against 160 human serum samples, of which 64 were from patients with paracoccidioidomycosis. The sensitivity mean was 73.4% compared with healthy individuals; specificity for patients with paracoccidioidomycosis was 87.5% but for patients with other fungal infections was 58.7%. These results showed high cross-reaction with the sera of patients infected with other mycoses 32,33.
Studies with a larger number of sera should be conducted. However, so far the preliminary evaluation of the antigen capacity by double immunodiffusion assay and the observed antigen profile by SDS-PAGE suggested a Paracoccidioides spp. antigen production with great potential for its use in the immunodiagnosis of patients with clinical suspicion of paracoccidioidomycosis.
This study proposes an alternative protocol for Paracoccidioides spp. antigen production. Among the advantages of the suggested methodology is the antigen obtention from Paracoccidioides spp. yeast stationary phase culture (without agitation). To dispense with a shaking incubator to obtain antigens results in savings for laboratories because this equipment costs an average of four to five times more than a conventional microbiological oven and requires more frequent corrective maintenance.
This protocol does not need an additional inactivation step of the fungal culture with thimerosal-borate solution (1:5,000), saving four days compared to obtaining culture filtrate. In this protocol, a thimerosal-borate aqueous solution (1:5,000) was used to extract and/or dissolve the soluble proteins (antigens) of Paracoccidioides spp. wall, for 24 hours. During that time, the yeasts were inactivated since this solution also has fungicidal and fungistatic functions, guaranteeing the safety of the obtained product.
In addition, most protocols aimed at obtaining culture filtrate recommend fungal culture for 20 days, culture inactivation, filtration on filter paper, and supernatant centrifugation in a refrigerated centrifuge (expensive equipment compared to a non-refrigerated centrifuge, which also requires a preventive maintenance contract). All of that to remove tiny fungal Paracoccidioides spp. cells, that escaped the filter paper, and thimerosal crystals.
In the proposed protocol, we obtained good results with antigens prepared after five days of culture (Th 66 5d 20X, P. lutzii) and seven days for P. brasiliensis and P. restrepiensis without the supernatant centrifugation and long filtering processes.
Finally, antigen concentration is often used to obtain culture-filtered antigens and other types of Paracoccidioides spp. antigens. Therefore, concentration does not make the process more expensive, as it is a common and necessary practice to obtain good antigen preparations. Although we used a lyophilizer, there are other economical and feasible methods for laboratories without this equipment 22.
We believe the advantages presented here support this simple, rapid, and accessible methodology for most mycoserology laboratories, particularly those with limited technological resources. The antigens obtained in this study have common antigen fractions among different species, allowing genus-specific recognition. Therefore, we think this protocol can be developed and reproduced in laboratories in Brazil and South America, where paracoccidioidomycosis is a neglected disease, contributing to an early diagnosis and favoring initiation of specific treatments, especially in endemic regions, regardless of the fungal species causing paracoccidioidomycosis.














