Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Investigación y Educación en Enfermería
Print version ISSN 0120-5307On-line version ISSN 2216-0280
Invest. educ. enferm vol.29 no.3 Medellín Oct./Dec. 2011
ARTÍCULO ORIGINAL / ORIGINAL ARTICLE/ ARTIGO ORIGINAL
Surgical site infection incidence after a clean-contaminated surgery in Yasuj Shahid Beheshti hospital, Iran
Incidencia de infección de herida por cirugía limpia contaminada en el hospital Yasuj Shahid Beheshti, Irán
Incidência de infecção de ferida depois de cirurgia limpa-contaminada em hospital Yasuj Shahid Beheshti, Irã
Mohebbi Nobandegani Zinat1, Najafi Doulatabad Shahla2, Rambod Masoumeh3, Afraseyabi Ardeshir4
1 MSc. Department of Medical Surgical Nursing, Shiraz Medical University, Shiraz, Iran. email: mohebbi04@yahoo.com.
2 MSc. Department of Medical Surgical Nursing, Yasuj University of Medical Sciences, Yasuj, Iran. email: shahlaiss@yahoo.com.
3 MSc. Department of Medical Surgical Nursing, Shiraz Medical University, Shiraz, Iran. email: rambodma@gmail.com.
4 Yasuj University of Medical Sciences, Yasuj, Iran. email: afrasiabifara@yahoo.com.
Subvenciones y ayudas: This study had financial support of Yasuj University of Medical Science.
Conflicto de intereses: ninguno a declarar.
Cómo citar este artículo: Mohebbi Nobandegani Zinat, Najafi Doulatabad Najafi Doulatabad Shahla, Rambod Masoumeh, Afraseyabi Ardeshir. Surgical site infection incidence after a clean-contaminated surgery in the Yasuj Shahid Beheshti hospital, Iran. Invest Educ Enferm. 2011;29(3)
ABSTRACT
Objective. To determine the incidence rate of infection after a clean-contaminated surgery and its relationship with some risk factors. Methodology. Cross sectional study, in a convenience sample of 300 patients who underwent surgery classified as clean-contaminated in a hospital of Yasuj, Iran. Samples were taken directly from the wound at the first dressing change to all the patients. They were studied to determine bacteria growth. Results. The rate of infection after a clean-contaminated surgery was 53%. The most common gram positive microorganism was Staphylococcus aureus (22%), and among gram negative: Escherichia coli (26%), Klebsiella sp (26%) and Pseudomonas sp (25%). Significant correlation between the type of surgery and surgical site infection was found, it was not seen with the variables sex and surgical procedure. Conclusion. This study shows important problems regarding patients safety. Protocols should be reviewed to control infections.
Key words: bacterial infections; cross infection; surgical wound infection.
RESUMEN
Objetivo. Determinar la tasa de incidencia por infección de herida por cirugía limpia-contaminada y su relación con algunos factores de riesgo. Metodología. Estudio de corte transversal en una muestra por conveniencia de 300 pacientes sometidos a cirugía clasificada como limpia-contaminada. A todos los pacientes les tomaron una muestra para cultivo directamente de la herida en el primer cambio del apósito, la cual se estudió para determinar el crecimiento de bacterias. Resultados. La tasa de infección en heridas quirúrgicas limpias-contaminadas fue del 53%. El microorganismo gram positivo más frecuente fue Staphylococcus aureus (22%), y dentro de los gram negatives fueron: Escherichia coli (26%), Klebsiella sp (26%) y Pseudomonas sp (25%). Se encontró asociación significativa entre el tipo de cirugía y la infección de la herida quirúrgica, lo que no se observó con las variables sexo y el procedimiento quirúrgico. Conclusión. Este estudio muestra problemas importantes en el aseguramiento del paciente. Por consiguiente, es preciso revisar los protocolos para el control de las infecciones.
Palabras clave: infecciones bacterianas infección hospitalaria; infección de herida operatoria.
RESUMO
Objetivo. Determinar a taxa de incidência por infecção de ferida depois de cirurgia limpa-contaminada e sua relação com alguns fatores de risco. Metodologia. Estudo de corte transversal numa mostra por conveniência de 300 pacientes submetidos a cirurgia classificada como limpa-contaminada num hospital de Yasuj, Irã. A todos os pacientes lhes foi tomada uma mostra para cultivo diretamente da ferida na primeira mudança do apósito, a qual foi estudada para determinar o crescimento de bactérias. Resultados. A taxa de infecção em feridas depois de cirurgia limpa-contaminada foi de 53%. O microorganismo gram positivo mais frequente foi Staphylococcus aureus (22%), e dentro dos gram negativos: Escherichia coli (26%), Klebsiella sp (26%) e Pseudomonas sp (25%). Encontrou-se associação significativa entre o tipo de cirurgia e a infecção da ferida cirúrgica, o que não se observou com as variáveis sexo e o procedimento cirúrgico. Conclusão. Este estudo mostra problemas importantes na garantia do paciente. Devem revisar-se os protocolos para o controle das infecções.
Palavras chaves: infecções bacterianas; infecção hospitalar; infecção da ferida operatória.
INTRODUCTION
Surgical site infections (SSI) are the most common health care–associated infections in surgical patients1,2 and are serious surgical complications happening in approximately 2% of surgical procedures, although rates differ widely according to the type of procedure.3 In the United States, the incidence of SSI is computed to be approximately 5% to 6%, with an attributable mortality of 3.6% (40 000 to 80 000 deaths annually) in Europe.3 Studies have shown an SSI prevalence of 4.4% to 38.4%,4,5 but estimates of incidence are scarce.6
SSI can have a devastating effect on the patients course of treatment and is related to increased treatment intensity, prolonged stay in the hospital,7 and higher cost, morbidity and mortality,8 leading to a deterioration in the quality of life.9 Patients with SSI need more nursing care, additional dressing and, in some cases readmission to the hospital and further surgery.7 SSI which appears during the hospitalization period is known as nosocomial infection (NI). It is suspected to be one of the national and international problems and should be assessed urgently.10
The mortality and expenditure of nosocomial infection can be prevented by its timely diagnosis and control. Therefore, in this respect, the present study was carried out in order to determine the rate of surgical site infection in clean-contaminated wounds in the Yasuj Shahid Beheshti hospital.
METHODOLOGY
Cross- sectional descriptive study, carried out during a 12-month period from August 2006 to July 2007. 300 patients, who had undergone surgery in the general surgery ward of the Yasuj Shahid Beheshti hospital in southwest Iran, were hospitalized for more than 48 hours, and had clean-contaminated wounds participated in the study. A laboratory technician took a wet culture sample from the patients wound, using a sterile swab before changing the dressing. It was studied in order to determine bacteria growth or non- growth in blood agar and EMB (Eosine Methylene Blue) environment. All the patients information such as age, gender, surgical service, surgical site and type of surgery was obtained from medical records.
The study was approved by the ethics committee of the Yasuj University of Medical Science (YUMS). Patients were informed about the study both verbally and written. Participation was voluntary and the patients could stop their cooperation in the study without giving any reasons. The questionnaires were coded in order to guarantee anonymity.
Data were analyzed using the SPSS software (version 10) and descriptive–statistics methods (central tendency index, scatter and absolute and relative frequency), chi-square and fisher exact test.
RESULTS
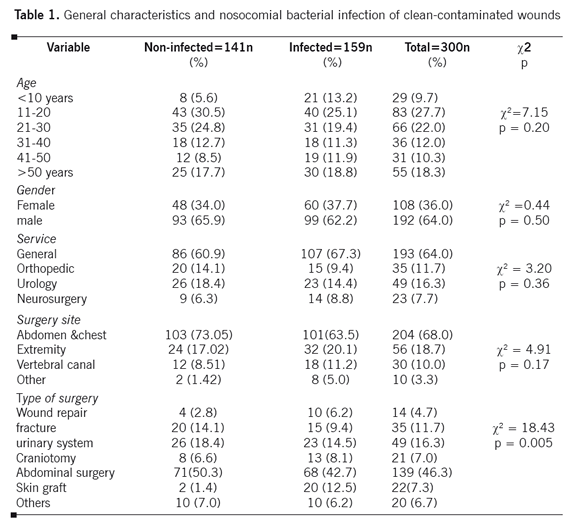
The convenience sample consisted of 300 patients, 192 (64.0%) males and 108 (36.0%) females. The mean age of the patients was 22.6±12.5 years. The surgical procedures included general surgery 193 (64.0%), orthopedic 35 (11.7%), urology 49 (16.3%) and neurosurgery 23 (7.7%). Most of the surgeries were performed on the abdomen and chest (68.0%). The six most frequent types of surgery were: abdominal surgery 139 (46.3%), urinary system 49 (16.3%), fracture 35 (11.7%), skin graft 22 (7.3%) and craniotomy 21 (7.0%). Table 1 shows the general characteristics of the patients.
In this study, the rate of bacterial infection in clean-contaminated wounds was 53.0% (n=159). In addition, the most prevalent gram-positive organism was Staphylococcus aureus bacteria (n=35, 22.0%). Escherichia coli (n= 42, 26.4%), Klebsiella sp. (n=42, 26.4%) and Pseudomonas sp. (n=40, 25.2%) were the most prevalent species among gram-negative bacteria. The most common microorganisms causing infection were Gram-negative (78.0%).
The study indicated that there was no association between age and gender with SSI (Table 1). In addition, surgical procedure and surgical site were not associated with SSI. However, the relationship between the type of surgery and SSI was statistically significant and the most common types of surgeries were gastrointestinal and skin graft surgeries.
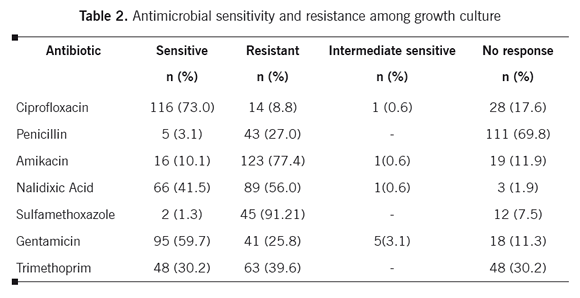
Postoperative antibiotic administration was widespread. The six most common antibiotics prescribed to the patients were: Gentamicin (55.7%), Cephalothin Sodium (53.3%), Metronidazol (15.0%), Vancomycin (6.3%), Nalidixic Acid (4.7%) and, Ciprofloxacin (3.4%). Most of the cultures were sensitive to Ciprofloxacin (73.0%), and Gentamicin (59.7%). However, the lowest sensitivity was related to sulfonamides such as sulfamethoxazole (1.3%). In addition sulfonamides such as sulfamethoxazole (91.2%), amikacin (77.4%) and Nalidixic Acid (56.0%) had the highest antibiotic resistance (Table 2).
DISCUSSION
Nosocomial infection surveillance is time consuming and requires substantial human resources. Anyway, infection control and prevention initiatives through surveillance have been found to be cost effective and improve patients safety.11,12
In this study, the rate of surgical wound infection was 53%. Hadadi et al.13 showed that it was 11% in Tehran, Iran. Szilagyi et al.14 found an overall SSI of 2.3% in their research. In Brazil and Mexico, it was reported to be 10-15%15 and in African countries it was estimated to be 16- 38.7%.16,17 High incidence of SSI in comparison to other researches could be due to the quality of infection preventive measures and differences in infection control practices, but may also be explained by variations in case randomization, distribution of surgical procedures, sample sizes, and surveillance methodology including methods used to detect SSI.
Microbiologic findings demonstrated gram-negative as the most frequently found organisms in our patients, as in previous studies.18,19 The results of a research in Ethiopia also showed that approximately 90% of micro-organisms were gram-negative and 84% were Enterobacter type.20 However, Mitt et al.21 reported that the most common pathogens in their study were Gram-positive.
In this study the most prevalent bacteria responsible for infection were Escherichia coli, Klebsiella, Pseudomonas, and Staphylococcus. Researchers have indicated that microorganisms involved in bacteremia are mainly Enterobacteriaceae (n=27), including Escherichia-coli and Proteus mirabilis. Other important microorganisms in their study in decreasing order were: Streptococcus pneumoniae, Staphylococcus aureus, and Pseudomonas aeruginosa.22 According to international studies, Staphylococcus aureus, Escherichia-coli, Pseudomonas and Staphilococus aureus are mentioned as organisms causing SSI.23
The finding that Escherichia coli was the most common pathogen for SSI seem to be consistent with other reports.24 This may be because the surgical procedures under surveillance consisted mainly of gastrointestinal tract surgery. Moreover, E. coli is normal flora of the gastrointestinal tract.
Findings suggested an association between SSI and type of surgery. The rate of infection was also higher in abdominal surgery in comparison to the other groups. This result is similar to other studies.8 It was reported that the risk of SSI in gastrointestinal surgery was 2.8% and was more common than the other surgeries.8 In this study, most of the patients who had graft surgery had SSI. This could be linked to the use of immunosuppressive drugs which are prescribed to control graft rejection.
The most common antibiotics used were gentamicin and cephalothin sodium. Researchers reported that first, or second generation cephalosporin with high anti-staphylococcal activity, such as cefazolin, is the most often used drug for antibiotic prophylaxis for clean surgery and high risk clean-contaminated surgery.7 Improving antibiotic prophylaxis is crucial and may be an important first step in reducing SSI.2,25
The current analysis suggests that most of the cultures were sensitive to fluoroquinolones (ciprofloxacin73%) and aminoglycosides such as gentamicin (59.7%). In other studies, susceptibility to cephalosporins and ciprofloxacin was more than 80, higher than the current study.26,27 Low antibiotic sensitivity rates in this study in comparison to other researches may vary substantially between regions. Therefore, local, hospital-based surveillance of the bacterial spectrum and antibiotic sensitivity is paramount for rational empiric therapy.28
Most of the cultures were resistant to aminoglycosides (amikacin), sulfonamides (sulfamethoxazole) and quinolones (nalidixic-acid). In the hospital environment, antibiotic use is extensive, resulting in selective pressure for antibiotic resistant pathogens.7 Resistance to ciprofloxacin was 8.8% in our study, and this is less than that of the study from Argentina and Brazil in which more than 80% and 25.5%, were resistant to ciprofloxacin.29,30
The present survey provides important data for patients safety. However, the main limitation of the current study was the small number of patients because it was done in one center at the southwest of Iran. Therefore it was suggested to perform more researches in other geographical areas in Iran. Another limitation is the early administration of antibiotics to some patients. This might have caused negative culture results and consequently sampling bias. Therefore, the resistance rate is likely to be slightly overestimated.
Considering these findings, it is concluded that to reduce the risk of infection among patients, better hand-washing and environmental cleaning protocols should be strengthen to diminish contamination. In addition, effective and efficient preoperative patient skin preparation is an essential intervention that may decrease the number of wound contaminations and reduce the risk of postoperative SSI. Indeed, antibiotic prophylaxis and improving host defenses are all important strategies that together will help prevent further infection. Much more studies of the causes of infection and strategies for its prevention are needed.
Acknowledgments. The authors would like to thank to all the patients who participated in the study and to Dr Nasrin Shokrpour at center for development of clinical research of Nemazee Hospital for editorial assistance.
REFERENCES
1. Horan TC, Culver DH, Gaynes R P, Jarvis WR, Edwards JR, Reid CR. Nosocomial infections in surgical patients in the United States, January 1986-June 1992. National Nosocomial Infections Surveillance (NNIS) System. Infect Control Hosp Epidemiol. 1993;14(2):73-80. [ Links ]
2. Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999. Am J Infect Control. 1999;27(2):97-132. [ Links ]
3. Raymond DP, Pelletier SJ, Crabtree TD, Schulman AM, Pruett TL, Sawyer RG. Surgical infection and the aging population. Am Surg. 2001;67(9):827-32. [ Links ]
4. Haley RW, Culver DH, White JW, Morgan WM, Emori TG. The nationwide nosocomial infection rate. A new need for vital statistics. Am J Epidemiol. 1985;121(2):159-67. [ Links ]
5. Pittet D, Allegranzi B, Storr J, Donaldson L. Clean Care is Safer Care: the Global Patient Safety Challenge 2005-2006. Int J Infect Dis. 2006;10(6):419-24. [ Links ]
6. Kanerva M, Ollgren J, Virtanen MJ, Lyytikäinen O; Prevalence Survey Study Group. Estimating the annual burden of health care-associated infections in Finnish adult acute care hospitals. Am J Infect Control. 2009;37(3):227-30. [ Links ]
7. Dohmen PM. Antibiotic resistance in common pathogens reinforces the need to minimise surgical site infections. J Hosp Infect. 2008;70 Suppl 2:15-20. [ Links ]
8. Astagneau P, LHériteau F, Daniel F, Parneix P, Venier AG, Malavaud S, et al. Reducing surgical site infection incidence through a network: results from the French ISO-RAISIN surveillance system. J Hosp Infect. 2009;72(2):127-34. [ Links ]
9. Kanellakopoulou K, Papadopoulos A, Varvaroussis D, Varvaroussis A, Giamarellos-Bourboulis EJ, Pagonas A, et al. Efficacy of teicoplanin for the prevention of surgical site infections after total hip or knee arthroplasty: a prospective, open-label study. Int J Antimicrob Agents. 2009;33(5):437-40. [ Links ]
10. Smeltzer SC, Bare BG, Hinkle JL, Cheever KH. Brunner & Suddarths Textbook of medical surgical nursing. 11th (Ed). China: Lippincott Williams & Wilkins a Wolters Kluwer Company; 2008. p. 1521-52. [ Links ]
11. Haley RW, Culver DH, White JW, Morgan WM, Emori TG, Munn VP, Hooton TM. The efficacy of infection surveillance and control programs in preventing nosocomial infections in US hospitals. Am J Epidemiol. 1985;121(2):182-205. [ Links ]
12. Brandt C, Sohr D, Behnke M, Daschner F, Rüden H, Gastmeier P. Reduction of surgical site infection rates associated with active surveillance. Infect Control Hosp Epidemiol. 2006;27(12):1347-51. [ Links ]
13. Hadadi A, Rasoulinejad M, Maleki Z, Yonesian M, Shirani A, Kourorian Z. Antimicrobial resistance pattern of Gram-negative bacilli of nosocomial origin at 2 university hospitals in Iran. Diagn Microbiol Infect Dis. 2008;60(3):301-5. [ Links ]
14. Szilágyi E, Böröcz K, Gastmeier P, Kurcz A, Horváth-Puhó E.The national nosocomial surveillance network in Hungary: results of two years of surgical site infection surveillance. J Hosp Infect. 2009;71(1):74-80. [ Links ]
15. Santos KR, Bravo Neto GP, Fonseca LS, Gontijo Filho PP. Incidence surveillance of wound infection in hernia surgery during hospitalization and after discharge in a university hospital. J Hosp Infect. 1997;36(3):229-33. [ Links ]
16. Moreea S, Rajah F, Rycken J. Pilot study of post operative infections in incisional wounds and split skin grafts in a provincial hospital in Zimbabwe. Trop Doct. 1993;23(4):160-4. [ Links ]
17. Kotisso B, Aseffa A. Surgical wound infection in a teaching hospital in Ethiopia. East Afr Med J. 1998;75(7):402-5. [ Links ]
18. Olsen MA, Sundt TM, Lawton JS, Damiano RJ Jr, Hopkins-Broyles D, Lock-Buckley P, et al. Risk factors for leg harvest surgical site infections after coronary artery bypass graft surgery. J Thorac Cardiovasc Surg. 2003;126(4):992-9. [ Links ]
19. LEcuyer PB, Murphy D, Little JR, Fraser VJ. The epidemiology of chest and leg wound infections following cardiothoracic surgery. . Clin Infect Dis. 1996;22(3):424-9. [ Links ]
20. Habte-Gabr E, Gedebou M, Kronvall G. Hospital-acquired infections among surgical patients in Tikur Anbessa Hospital, Addis Ababa, Ethiopia Am J Infect Control. 1988;16(1):7-13. [ Links ]
21. Mitt P, Adamson V, Lõivukene K, Lang K, Telling K, Päro K, et al. Epidemiology of nosocomial bloodstream infections in Estonia. J Hosp Infect. 2009;71(4):365-70. [ Links ]
22. Akpabie A, Duché C, Le Gaudion M. Nosocomial bacteremia: impact of empirical antimicrobial treatment on the patients outcome. Pathol Biol (Paris). 2009;57(1):51-5. [ Links ]
23. Mayon-White RT, Ducel G, Kereselidze T, Tikomirov E. An international survey of the prevalence of hospital-acquired infection. J Hosp Infect. 1988;11 Suppl A:43-8. [ Links ]
24. Kasatpibal N, Jamulitrat S, Chongsuvivatwong V. Standardized incidence rates of surgical site infection: a multicenter study in Thailand. J Infect Control. 2005;33(10):587-94. [ Links ]
25. Brown S, Kurtsikashvili G, Alonso-Echanove J, Ghadua M, Ahmeteli L, Bochoidze T, et al. Prevalence and predictors of surgical site infection in Tbilisi, Republic of Georgia. J Hosp Infect. 2007;66(2):160-6. [ Links ]
26. Orrett FA. Resistance patterns among selective Gram-negative bacilli from an intensive care unit in Trinidad, West Indies. Saudi Med J. 2004;25(4):478-83. [ Links ]
27. Rhomberg PR, Fritsche TR, Sader HS, Jones RN. Comparative antimicrobial potency of meropenem tested against Gram-negative bacilli: report from the MYSTIC surveillance program in the United States (2004). J Chemother. 2005;17(5):459-69. [ Links ]
28. Wagenlehner FM, Weidner W, Naber KG. Emergence of antibiotic resistance amongst hospital-acquired urinary tract infections and pharmacokinetic/pharmacodynamic considerations. J Hosp Infect. 2005;60(3):191-200. [ Links ]
29. Rodríguez CH, Juárez J, de Mier C, Pugliese L, Blanco G, Vay C, et al. Bacterial resistance to antibiotics in gram-negative rods isolated from intensive care units. Comparative analysis between two periods (1998 and 2001). Medicina (B Aires). 2003;63(1):21-7. [ Links ]
30. Mendes C, Oplustil C, Sakagami E, Turner P, Kiffer C; MYSTIC Brazil Group. Antimicrobial susceptibility in intensive care units: MYSTIC Program Brazil 2002. Braz J Infect Dis. 2005 Feb;9(1):44-51 [ Links ]
Fecha de Recibido: 17 de enero de 2011. Fecha de Aprobado: 16 de agosto de 2011.