Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Acta Biológica Colombiana
Print version ISSN 0120-548X
Acta biol.Colomb. vol.11 no.2 Bogotá June 2006
Patrones electroforéticos de proteínas y actividad anticongelante en el apoplasto de la hoja de la especie andina tropical Senecio niveoaureus
ÁLVAREZFLÓREZ F,1 MELGAREJO L.M,1 ROMERO H.M,1 DOUCET L2
- The Plant Physiology Laboratory, Department of Biology, Universidad Nacional de Colombia. Bogotá, Colombia.
- The Plant Laboratory, Department of Plant Biology, University of York, England.
Presentado septiembre 9 de 2005 aceptado mayo 9 de 2006, correcciones agosto 8 de 2006.
ABSTRACTTropical high mountain plants have different adaptations to survive extreme daily temperature fluctuations and specially freezing night conditions. In winter plant species, survival to low temperatures is related to the ability of the cell to produce specific low molecular weight proteins (antifreezing proteins) and to export them to the apoplast. In order to see if high mountain tropical plants survive to low temperatures through the same mechanism we collected, during a 24 hourperiod, leaves from Senecio niveoaureus growing at 3,300 and 3,600 m.o.s.l, in the Páramo de Palacio, Chingaza, Colombia. Leaf apoplast proteins had MW between 3512 kDa. Electrophoretic patterns were different depending on the altitude and the time of sampling. However the observed variations could not be linked to changes in temperature or to the altitudinal gradient. Antifreeze activity was detected in leaf apoplast of plants at different altitudes. This is the first report of antifreeze activity in a high mountain tropical species.
Key words: antifreezing proteins, Senecio niveoaureus, high tropical Andes (páramo).
RESUMENLas plantas de alta montaña tienen diferentes adaptaciones para sobrevivir a cambios drásticos de temperatura, especialmente a condiciones de congelamiento. En plantas de invierno, la supervivencia a temperaturas bajas está relacionada con la capacidad de las células para producir proteínas específicas de bajo peso molecular (proteínas anticongelantes) y exportarlas al apoplasto. Para establecer si plantas tropicales de alta montaña sobreviven las temperaturas bajas a través del mismo mecanismo, se colectaron hojas de plantas de Senecio niveoaureus durante 24 horas y a dos alturas 3.300 y 3.600 msnm en el Páramo de Palacio, Chingaza, Colombia. Se observaron proteínas apoplásticas de pesos moleculares entre 3512 kDa. Los patrones electroforéticos fueron diferentes dependiendo de la altura y la hora de muestreo, sin embargo, se observaron variaciones en el patrón de bandeo que no pueden ser atribuidas ni a la temperatura ni al gradiente altitudinal únicamente. Se detectó actividad anticongelante en el apoplasto de hojas de S. niveoaureus, siendo este el primer reporte en especies tropicales de alta montaña.
Palabras clave: proteinas anticongelantes, Senecio niveoaureus, páramo.
Abbreviations: antifreeze proteins (AFP)
INTRODUCTIONTropical high mountain regions present drastic changes in daily temperature, exposing plants to extreme conditions in which they must develop morphological, physiological and biochemical characteristics that allow them to survive. Tropical high mountain ecosystems, located at the upper limit of forests (2,800 m.o.s.l) and below the level of perpetual snow (4,200 m.o.s.l) are known as páramos, a term restricted to the Andes and mountains in the south of CentralAmerica (Luteyn, 1999). Due to their location, páramos are characterized by wide daily thermal alternation where temperatures oscillate between 3 ºC and 12 ºC. The annual rainfall in páramos varies between 500 mm and 3,000 mm and there is intense ultraviolet radiation and strong winds (MoraOsejo and Sturm, 1994; Lüttge, 1997; Luteyn, 1999). These ecological conditions of the páramos allow limited growth of specific plants highly adapted to the extreme conditions. Several protection mechanisms against low temperatures have been identified in páramo plants including morphological aspects such as rossetted habitat, the presence of necromass (Beck et al., 1982; Rada et al., 1985; Körner, 1999), coriacity, leaf orientation (Goldstein et al., 1989), increased sugar content, mucilage secretion, reduced leaf bud freezing point (Beck et al., 1982; Körner, 1999; Lüttge, 1997), increased lineal supercooling ability as altitude increases and avoiding or resistance to low temperatures (Rada et al., 1985; Rada et al., 1987; Rada et al., 2001). However, few biochemical studies have been done on páramo species and up to date there are no reports of antifreeze protein activity.
The presence of antifreeze proteins (AFP) is one of the mechanisms of plants to tolerate low temperatures. AFPs have been found in wheat, barley, rye, alfalfa (Hon et al., 1994; Antikainen et al., 1996; Antikainen and Griffith, 1997) and carrots (Worrall et al., 1998; Smallwood et al., 1999). Plant AFPs present partial homology with AFPs described in Atlantic flounder. AFPs have two related effects on aqueous solution: thermal hysteresis (TH) and inhibition of ice recrystallization. TH is the noncolligative depression of the freezing temperature of solutions below their melting temperature. Ice recrystallization is the growth of large ice crystals at the expense of the smaller ones in partially frozen solutions (Smallwood et al., 1999). AFPs inhibit the growth and recrystallization of intercellular ice by adsorbing it onto the surface of ice crystals via van der Waals interactions and/or hydrogen bonds (De Vries, 1986). In plants, AFPs are present in epidermal cell walls, in cell walls surrounding intercellular spaces and in secondary cell walls of leaf xylem (Antikainen et al., 1996; PihakaskiMaunsbach et al., 1996). Different studies have shown that some of the apoplast AFPs in rye have also glucanase and chitinase activities, which indicates a bifunctionality of these proteins acting as defense proteins against pathogens and against ice formation (Hon et al., 1995; Griffith et al., 1997). However, since glucanases and chitinases normally do not have antifreeze activity, it has been hypothesized that AFPs evolved from the pathogenesis related proteins (Griffith et al., 1997).
Senecio niveoaureus is a rossete plant from the Colombian páramos, found between 3,200
m.o.s.l and 4,300 m.o.s.l. In this species the mechanisms of adaptation to low temperatures are not well known, however, research with giant Senecio species from African alpine ecosystems has shown that leaves close to the apical bud move towards it keeping the temperature in the bud some degrees higher than the air. Also, the freezing point in apical bud cells is lower than in mature leaves. Furthermore, the presence of cryoprotectans such as sugars has been reported (Beck et al., 1982; Körner, 1999). This study analyzed the electrophoretic patterns and the antifreeze activity of proteins from the leaf apoplast of S. niveoaureus and their relationship with the altitudinal gradient and daily temperature changes in a daynight cycle in the páramo, in order to check if the presence of antifreezing activity in the apoplast constitutes a mechanism of tolerance to low temperatures of the tropical high mountain plants.
METHODOLOGYPlant material
The sampling was done in the Páramo de Palacio located in the Chingaza Natural National Park (Colombia) at two different elevations, 3,300 m.o.s.l. (Valle de los Tunjos) and 3,600 m.o.s.l. (La Mina), between July and September. Mature leaves from three different plants at each site were taken every three hours throughout 24 hour periods. The plants were adult and in good overall conditions. The leaf surface temperature was measured with a KEDLEZ thermocouple probe. Sampling was done twice in each site.
ExtractionBits of leaf (12 cm2) were kept in plastic bags at 70 ºC for 4 days. They were then moved at room temperature and 50 mM TrisHCl pH 7.4, 5 mM EDTA and 20 mM ascorbic acid buffer were added. After thirty minutes, the bag contents were gently squeezed to release only the contents of the apoplast. The extracts were centrifuged at 4,000 rpm for 15 minutes at 4 ºC and concentrated by lyophilisation. Total protein content was quantified by Bradford’s method (1976) as modified by Stoscheck (1990).
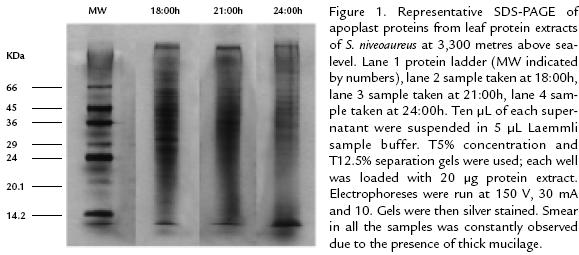
Unidimensional SDS-PAGE electrophoresisLyophilized samples were suspended in 200 µL 5 mM TrisHCl pH 7.4, 0.5 mM EDTA, 2 mM ascorbic acid buffer; they were centrifuged at 13,000 rpm for 10 minutes. Ten µL of each supernatant were suspended in 5 µL Laemmli sample buffer (Laemmli, 1970). T5% concentration and T12.5% separation gels were used; each well was loaded with 20 µg protein extract. Electrophoreses were run at 150 V, 30 mA and 10 watts in a Protean cell chamber (BioRad). Gels were then silver stained (Blum et al., 1987). One DScan software (BioRad, version 1.3) was used for determining protein molecular weight. ∴lactoalbumin
(14.2 kDa), trypsin inhibitor (20.1 kDa), trypsinogen (24 kDa), carbon anhydrase (29 kDa), glyceraldehyde 3phosphate dehydrogenase (36 kDa), eggwhite (45 kDa) and bovine albumin (66 kDa; SIGMA) were used as molecular weight patterns.
| 12:00 h | 15:00 h | 18:00 h | 21:00 h | 24:00 h | 3:00 h | 9:00 h |
|---|---|---|---|---|---|---|
| 80 | 83 | 65 | 74 | 70 | 81 | 74 |
| 70 | 62 | 59 | 51 | 64 | 64 | 61 |
| 63 | 50 | 41 | 47 | 57 | 59 | 55 |
| 57 | 35 | 36 | 42 | 54 | 42 | 46 |
| 50 | 31 | 34 | 40 | 41 | 37 | 43 |
| 49 | 30 | 26 | 39 | 36 | 33 | 39 |
| 45 | 28 | 22 | 34 | 32 | 30 | 34 |
| 37 | 27 | 22 | 31 | 25 | 28 | 31 |
| 36 | 24 | 21 | 28 | 21 | 22 | 26 |
| 35 | 23 | 19 | 25 | 19 | 20 | 22 |
| 29 | 22 | 16 | 24 | 15 | 16 | 21 |
| 26 | 19 | 15 | 20 | 12 | 15 | 20 |
| 23 | 17 | 14 | 18 | 14 | 19 | |
| 22 | 14 | 13 | 16 | |||
| 21 | 13 | 12 | 14 | |||
| 20 | 12 | 13 | ||||
| 17 | 12 | |||||
| 16 | ||||||
| 15 | ||||||
| 13 |
Table 1. Molecular weights of apoplast proteins from Senecio niveoaureus leaves collected at 3,300 meters above sealevel. Molecular weights obtained on SDSPAGE gels and determined by One Dscan software (version 1.3).
ANTIFREEZE ACTIVITY ASSAY The sucrosesandwichsplat assay based on Worrall et al. (1998) and Smallwood (personal communication) was used. 0.5 µg/µL of apoplast extracts of S. niveoaureus in 30% (w/w) sucrose was squashed between circular coverslips. The sandwich was dropped into a bath of heptane kept at 80 ºC and transferred into a chamber containing heptane at 6 ºC. Ice crystals were viewed using a 20X object on an Optiphot microscope (Nikon); images were captured after 3060 min incubation at 6 ºC using a video camera. Recombinant fish AFPIII was used as positive control.;
| 12:00 h | 15:00 h | 18:00 h | 21:00 h | 24:00 h | 3:00 h | 9:00 h |
|---|---|---|---|---|---|---|
| 85 | 80 | 79 | 86 | 127 | 77 | 115 |
| 76 | 74 | 73 | 83 | 88 | 65 | 93 |
| 70 | 66 | 64 | 78 | 76 | 54 | 81 |
| 65 | 60 | 58 | 73 | 62 | 48 | 78 |
| 58 | 55 | 53 | 68 | 57 | 40 | 73 |
| 44 | 47 | 46 | 65 | 53 | 37 | 65 |
| 43 | 42 | 42 | 61 | 48 | 33 | 60 |
| 42 | 37 | 36 | 56 | 45 | 30 | 54 |
| 41 | 35 | 33 | 52 | 42 | 29 | 51 |
| 39 | 32 | 31 | 47 | 38 | 27 | 46 |
| 36 | 30 | 29 | 42 | 33 | 25 | 42 |
| 34 | 28 | 26 | 38 | 32 | 23 | 40 |
| 27 | 26 | 23 | 34 | 29 | 22 | 36 |
| 25 | 24 | 23 | 33 | 24 | 21 | 35 |
| 23 | 23 | 21 | 31 | 22 | 19 | 33 |
| 19 | 22 | 19 | 29 | 19 | 16 | 32 |
| 18 | 19 | 18 | 26 | 17 | 15 | 31 |
| 15 | 18 | 16 | 25 | 13 | 27 | |
| 16 | 14 | 23 | 12 | 26 | ||
| 15 | 12 | 22 | 24 | |||
| 13 | 21 | 24 | ||||
| 18 | 22 | |||||
| 16 | 20 | |||||
| 18 | ||||||
| 17 | ||||||
| 15 |
Table 2. Molecular weights of apoplast proteins from Senecio niveoaureus leaves collected at 3,600 meters above sealevel. Molecular weights obtained on SDSPAGE gels and determined by One Dscan software (version 1.3).
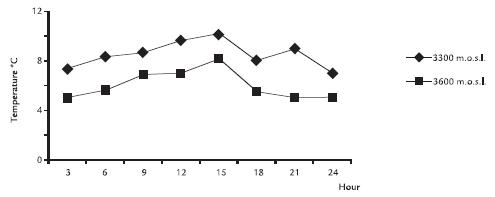
RESULTS AND DISCUSSIONElectrophoretic patterns of leaf apoplast proteins from S. niveoaureus showed the presence of 85 kDa to 12 kDa proteins (Fig. 1). Most of the proteins were in the 3512 kDa range (Tables 1 y 2; Figs. 2A y 2B). The proteins observed in the 3512 kDa molecular range were found mainly when temperatures dropped during the night between 18:00 and 3:00 hrs. and when the temperatures increased during the day between 9:00 and 15:00 hrs. (Fig. 3). More proteins were observed in the material collected at 3,600 m than at 3,300 m (Tables 1 y 2; Figs. 2A y 2B). AFPs described in apoplast from different plant species present molecular weights between 3511 kDa (Hon et al., 1994; Hon et al., 1995; Griffith and Ewart, 1995; Antikainen et al., 1996; Antikainen and Griffith, 1997; Smallwood et al., 1999), this being in a similar range to that found in S. niveoaureus extracts.
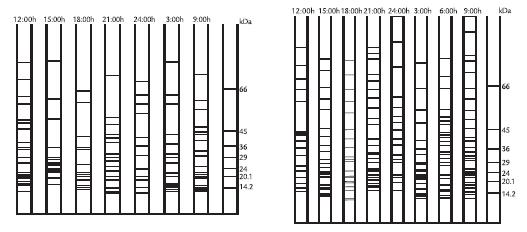
Figure 2A. Molecular weight scheme for Senecio niveoaureus leaf protein extracts obtained at 3,300 metres above sealevel. Molecular weights obtained on SDSPAGE gels and determined by One Dscan software (version 1.3). B. Molecular weight scheme for Senecio niveoaureus leaf protein extracts obtained at 3,600 metres above sealevel. Molecular weights obtained on SDSPAGE gels and determined by One Dscan software (version 1.3).
The analysis of the molecular weights of the apoplast proteins of S. niveoaureus leaves, during the daynight cycle and at different elevations showed significant variations among the samples. However, it is not clear if these variations in the electrophoretic patterns are due to the daily variation in temperature during the daynight cycle or to the altitudinal gradient. In fact, some reports have indicated that one limitation of these studies is that they do not demonstrate a causeandeffect relationship between AFPs and freezing tolerance or winter survival (Griffith et al., 2005).
Figure 3. Temperature variation of S. niveoaureus leaves at 3,300 and 3,600 metres above sealevel.
To determine the cryoprotective activity of the apoplast extracts, the sucrosesandwichsplat technique was used. This technique is used for creating small icecrystals by flashfreezing the solution whose growth and appearance are monitored by microscope (Smallwood et al., 1999).
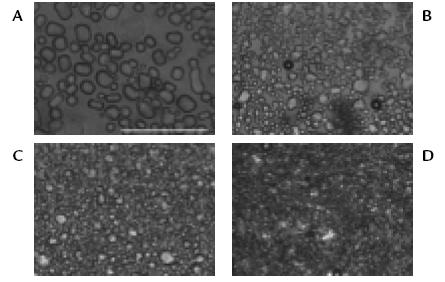
Figure 4. Sucrosesandwichsplat assay for inhibition of ice recrystallisation. Object 20X. A. negative control; B. S. niveoaureus extract at 3,300 metres above sealevel; C. S. niveoaureus extract at 3,600 meters above sealevel; D. recombinant fish AFPIII positive control. Bar represents 125µm.
figure 4 presents antifreezing activity results. The appearance of icecrystals can be observed in a negative control solution which has no antifreezing activity (Fig. 4A), a positive control solution containing recombinant fish AFPIII having antifreezing activity (Fig. 4D) and antifreezing activity induced by low temperature in S. niveoaureus leaf extracts (Figs. 4B y 4C). It can be observed that the crystals are smaller in S. niveoaureus extracts than in the solution without antifreezing activity. Moreover, the size of the crystals of S. niveoaureus extracts is comparable to that observed in the positive control. The results indicate the presence of AFPlike proteins in S. niveoaureus at low temperatures, which could be a mechanism of adaptation of these plants to the extreme environmental conditions of the high Andes. The presence of AFP proteins and activity regardless the temperature or the altitude indicates that S. niveoaureus constitutively expresses this type of proteins in order to cope with the wide and unpredictable daily thermal alternation presented in the páramo.
ACKNOWLEDGEMENTSWe would like to thank Marylin Griffith and Maggie Smallwood for their suggestions regarding some experiments and Doctor Körner for his comments. The Universidad Nacional de Colombia partly financed this work.
REFERENCESANTIKAINEN M, GRIFFITH M, ZHANG J, HON W, YANG D, PIHAKASKIMAUNSBACH K. Immunolocalisation Of Antifreeze Proteins In Winter Rye Leaves, Crowns, And Roots By Tissue Printing. Plant Physiol. 1996;110:845857.
[ Links ]ANTIKAINEN M, GRIFFITH M. Antifreeze Protein Accumulation in FreezingTolerant Cereals. Physiol Plant. 1997;99:423432.
[ Links ]BECK E, SENSER M, SCHEIBE R, STEIGER H, PONGRATZ P. Frost Avoidance And Freezing Tolerance In Afroalpine “Giant Rosette” Plants. Plant Cell Environ. 1982;5:215222.
BLUM H, BEIER H, GROSS HJ. Improved Silver Staining of Plant Proteins Rna and Dna in Polyacrylamide Gel Electrophoresis. Electrophoresis. 1987;8:9399.
[ Links ]BRADFORD M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of ProteinDye Binding. Anal Biochem. 1976;72:248254.
[ Links ]DE VRIES AL. Antifreeze Glycopeptides And Peptides: Interactions With Ice And Water. Meth Enzymol. 1986;127:293303.
[ Links ]GOLDSTEIN G, RADA F, CANALES M, ZABALA O. Leaf Gas Exchange of Two Giant Caulescent Rosette Species. Acta Oecologica. 1989;10:359370.
[ Links ]GRIFFITH M, EWART V. Antifreeze Proteins and their Potential Use in Frozen Foods. Biotechnol Adv. 1995;13:375402.
[ Links ]GRIFFITH M, ANTIKAINEN M, HON W, PIHAKASKIMAUNSBACH K, YU X, CHUN J, et al. Antifreeze Proteins in Winter Rye. Physiol Plant. 1997;100: 327332.
[ Links ]GRIFFITH M, LUMB C, WISEMAN SB, WISNIEWSKY M, JOHNSON R, MARANGONI A. Antifreeze Proteins Modify The Freezing Process in Planta. Plant Physiol. 2005;138:330340.
[ Links ]HON W, GRIFFITH M, CHONG P, YANG D. Extraction and Isolation of Antifreeze Proteins from Winter Rye (secale cereale l.) Leaves. Plant Physiol. 1994;104:971980.
[ Links ]HON W, GRIFFITH M, MLYNARZ A, KWOK Y, YANG D. Antifreeze Proteins in Winter Rye are Similar to PathogenesisRelated Proteins. Plant Physiol. 1995;109:879889.
[ Links ]KÖRNER C. Alpine Plant Life. Germany: Springer; 1999.
[ Links ]LAEMMLI U K. Cleavage of Structural Proteins During the Assembly of the Head of Bacteriophage T4. Nature. 1970;227:680685.
[ Links ]LUTEYN J. Paramos A Checklist Of Plant Diversity, Geographical Distribution, and Botanical Literature. The New York Botanical Garden, USA; 1999.
[ Links ]LÜTTGE U. Physiological Ecology of Tropical Plants. Germany: Springer; 1997. p. 349371.
[ Links ]MORAOSEJO L, H. STURM. Estudios ecológicos del páramo y del bosque altoandino cordillera Oriental Colombiana. Academia Colombiana de Ciencias Exactas, Físicas y Naturales. Bogotá, Colombia. 1994. p. 525.
[ Links ]PIHAKASKIMAUNSBACH K, GRIFFITH M, ANTIKAINEN M, MAUNSBACK AB. Immunogold Localization of GlucanaseLike Antifreeze Protein in Cold Acclimated Winter Rye. Protoplasma. 1996;191:115125.
[ Links ]RADA F, GOLDSTEIN G, AZOCAR A, MEINZER F. Freezing Avoidance in Andean Giant Rosette Plants. Plant Cell Environ. 1985;8:501507.
[ Links ]RADA F, GOLDSTEIN G, AZOCAR A, TORRES F. Supercooling Along an Altitudinal Gradient In Espeletia schultzii, A Caulescent Giant Rosette Species. J Exp Bot. 1987;38:491497.
[ Links ]RADA F, GARCÍA NC, BOERO C, GALLARDO M, HILAL M, GONZÁLEZ J, et al. Low Temperature Resistance In Polylepis tarapacana, A Tree Growing at the Highest Altitudes in the World. Plant Cell Environ. 2001;24:377381.
[ Links ]SMALLWOOD M, WORRALL D, BYASS L, ELIAS L, ASHFORD D, DOUCET J, et al. Isolation and Characterization of a Novel Antifreeze Protein from Carrot (Daucus carota). Biochem J. 1999;340:385391.
[ Links ]STOSCHECK CM. Increased Uniformity in the Response of the Coomasie Blue G Protein Assay to Different Proteins. Anal Biochem. 1990; 184:111116.
[ Links ]WORRALL D, ELIAS L, ASHFORD D, SMALLWOOD M, SIDEBOTTOM C, LILLFORD P, et al. A Carrot LeucineRichRepeat Protein that Inhibits Ice ReCrystallization. Science. 1998;282:115117.
[ Links ]