Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Acta Biológica Colombiana
Print version ISSN 0120-548X
Acta biol.Colomb. vol.12 no.1 Bogotá Jan./June 2007
BIOCONTROL OF Rhizoctonia solani IN NATIVE POTATO (Solanum phureja) PLANTS USING NATIVE Pseudomonas fluorescens
Control biológico de Rhizoctonia solani en plantas de papa criollaSolanum phureja usando cepas nativas de Pseudomonas fluorescens
GLORIA BAUTISTA1, M. Sc.; HENRY MENDOZA2, M. Sc.; DANIEL URIBE1, Ph. D. 1Laboratorio de Microbiología Agrícola, Instituto de Biotecnología, Universidad Nacional de Colombia, Sede Bogotá, ciudad universitaria, Carrera 30 No. 4503. Bogotá, Colombia. duribev@unal.edu.co 2Departamento de Matemáticas y Estadística, Facultad de Ciencias, Universidad Nacional de Colombia, Sede Bogotá, ciudad universitaria, Carrera 30 No. 4503. Bogotá, Colombia. hmendozar@unal.edu.co
Presentado 14 de junio de 2006, aceptado 25 de octubre de 2006, correcciones 31 de enero de 2007.
ABSTRACT
Rhizoctonia solani is a soil borne phytopathogen associated with reduced plant vigor and tuber production in potato crops. There is a huge interest to search alternatives of biological control management of this disease, because the potato crops in Colombia are the highest consumers of chemical pesticides in Colombia. In order to obtain a fluorescent Pseudomonas strain with the capacity to reduce the disease symptoms produced by R. solani, determination and isolation of the predominant fluorescent Pseudomonas in several potato crops of the main Colombian producing region was done in a previous study. Six different P. fluorescens strains with none, moderate and high fungal growth inhibition capacity in vitro, were used in this study. Despite of the differences found in the dynamics of colonization and colonization capacity, all evaluated strains induced S. phureja growth and reduced disease symptoms produced by R. solani. Our results support the conclusion that association of P. fluorescens strains with S. phureja rhizosphere is a feasible alternative for the management of R. solani symptoms.
Key words: Rhizobacteria, Rhizoctonia solani, fluorescent Pseudomonas, Solanum phureja, antagonism.
RESUMEN
Rhizoctonia solani es un hongo fitopatógeno del suelo, el cual produce una reducción significativa del vigor de las plantas y de la producción de tubérculos en cultivos de papa. Es de gran interés la búsqueda de alternativas de manejo de esta enfermedad, especialmente desde la perspectiva de control biológico ya que los cultivos de papa son los mayores consumidores de plaguicidas de origen químicos en Colombia. Con el objeto de obtener una cepa del grupo de las Pseudomonas fluorescentes con la capacidad para reducir los síntomas de la enfermedad producidos por R. solani, se realizó en un estudio previo el aislamiento y caracterización de una colección de aislamientos de Pseudomonas fluorescentes provenientes de diferentes cultivos de la región papera más productiva del país. Seis cepas nativas de P. fluorescens con buena, moderada o ninguna capacidad para inhibir el crecimiento fúngico in vitro fueron seleccionadas. A pesar de las diferencias encontradas en términos de la dinámica y capacidad de colonización, todas las cepas evaluadas indujeron el crecimiento en las plantas de S. phureja y redujeron los síntomas de la enfermedad producidos por R. solani a nivel de invernadero. Nuestros resultados sustentan la conclusión que la asociación de cepas de P. fluorescens con la rizosfera de S. phureja es una alternativa para el manejo de R. solani en papa.
Palabras clave: Rizobacterias, Rhizoctonia solani, fluorescent Pseudomonas, Solanum phureja, antagonismo.
INTRODUCTION
The inoculation of seeds or roots with fluorescent Pseudomonas to increase plant vigour and productivity has been a worldwide studied practice (Burr et al., 1978; Kloepper et al., 1980; Wei et al.,1991; Bagnasco et al., 1998). Investigation into the cause of the beneficial effect of this kind of bacteria has implicated them in the control of a wide range of root phytopathogens, amongst which Gaeumannomyces graminis var tritici (Poplawsky and Ellingboe, 1989; Slinninger et al., 1996), Rhizoctonia solani (Howell and Stipanovic, 1979; Krechel et al., 2002; Nielsen et al.,1999), Erwinia carotovora var carotovora (Kloepper et al., 1980), Pythium ultimum and Fusarium oxysporum (Mao et al., 1991; Benhamou et al., 1996) can be singled out. The mechanisms suggested for achieving such inhibition include: production of antibiotics, iron chelating compounds, hydrolytic enzymes and biosurfactants (Hammer et al., 1997; Raaijmakers et al., 1997; Mazzola, 1998; Nielsen et al., 2002); competition for favourable nutritional sites (Suslow and Schroth 1982); induction of systemic resistance (Benhamou et al., 1996; Parmar and Dadarwal, 1999; Nandakumar et al., 2001) and even due to their action as mycorrhizationhelper bacteria (Gamalero et al., 2003). However, all disease suppressive mechanisms exhibited by rhizobacteria are essentially of no real value unless these bacteria can successfully establish themselves in the root environment (Nautiyal, 1997; Bagnasco et al., 1998). The fluorescent Pseudomonas can be found in the rhizosphere where microflora colonization depends on characteristics such as soil texture, rhizosphere pH, temperature, soil matric potential, soil water flow, plant species and even plant genotype (Bahme and Schroth 1987; Lemanceau et al., 1995; Latour et al., 1996; Berg et al., 2002).
Rhizoctonia solani is a widely distributed pathogen affecting different economically important crops, including the native Colombian potato, Solanum phureja where the tuber quality and production are highly decreased by the action of this fungus (Krechel et al., 2002). In this study we attempted to identify fluorescent Pseudomonas strains, useful for the suppression of R. solani in the S. phureja rhizosphere. Several activities were followed to fulfill our goal, firstly, from a previous study where a Pseudomonas fluorescent strain collection was obtained (Uribe et al., 1999), twelve isolates of P. fluorescens were selected and evaluated in terms of their antagonistic activity against R. solani in vitro conditions. Then, six of those isolates with none, moderate and high fungal growth inhibition capacity in vitro were selected in order to analyze: a) the root colonization dynamics, b) growth promotion capacity and c) their antagonistic activity against R. solani in greenhouse conditions.
MATERIALS AND METHODS
BACTERIAL AND FUNGAL STRAINS
Pseudomonas fluorescens strains obtained from S. tuberosum roots (IBPF.23, IBPF.26, IBPF.29, IBPF.30, IBPF.47, IBPF.63, IBPF.66, IBPF.78, IBPF.96, IBPF.102) and S. phureja roots (IBPF.25, IBPF.33, IBPF.34, IBPF.60, IBPF.62) were selected. Strains were cryopreserved at 70ºC on Gherna medium (Gherna, 1986). Potato active Rhizoctonia solani isolate was obtained from a tuber affected by sclerotia using PotatoDextrose Agar (PDA, Oxoid, UK). Its identification was made according to Sneh et al. (1991). Trichoderma hamatum T21 strain was provided by Professor Emira Garces from the Phytopathology strainbank, Department of Biology, Universidad Nacional de Colombia. This strain was selected for its capacity to reduce the incidence of disease caused by R. solani in beans (Phaseolus vulgaris L) by 80%, according to Leal and Plata (1996).
In vitro Rhizoctonia solani GROWTH INHIBITION ASSAYS
The methodology of Carruthers et al., (1994), with modifications, was used to determine fungal growth inhibition capacity of P. fluorescens isolates. Bacterial colonies grown for 48 h were streaked on the edges of Potato PDA plates and incubated at 25ºC for 72 h. A 0.5 cm2 plug of a six days old R. solani culture was inoculated in the middle of the plate. Finally the plates were incubated at 25º C for six days and scored for inhibition of fungal growth by measuring the halo of growth daily in millimetres. The fungi inoculated with a nonantagonistic strain of Escherichia coli were used as negative control. This assay was done as a Completely Random Design (CRD), having five replicas and two repetitions. The isolates used for this test were: IBPF.25, IBPF.29, IBPF.30, IBPF.33, IBPF.60, IBPF.62, IBPF.63, IBPF.66, IBPF.78, IBPF.96 and IBPF.102. The results were analyzed using SAS’s ANOVA with SAS PROC GLM (SAS Institute 1994).
Pseudomonas fluorescens ANTAGONISM AGAINST R. solani UNDER GREENHOUSE CONDITIONS
Six P. fluorescens strains characterized by presenting none (IBPF.63, IBPF.96), moderate (IBPF.25, IBPF.62) and high (IBPF.33, IBPF.60) fungal growth inhibition in vitro, were selected for the evaluation in greenhouse conditions. All strains showed negative potential for the potato tissue maceration test (Sands and Hankin, 1975). This test was done to eliminate the risk of working with any isolate capable of causing potato rot under storage conditions. Colonies with natural resistance to rifampicin were selected in order to be able to identify the Pseudomonas strains of our interest and distinguish them from the natural population present in the soil. Selected strains presented total sensitivity at concentrations of 25 µg ml1 or higher. No changes, in growth curves, colony morphology, duplication time, fluorescence pattern or in vitro antagonism, were found after the antibiotic selection using a concentration of 10 µg ml1.
The methodology reported by van Peer et al., (1990) and Kloepper et al., (1980), was used for bacterial suspension preparation. A combined treatment maintaining the same amount of cfu ml1 (108 cfu ml1), was prepared by mixing equal volumes of the bacterial suspension of each isolate. The production of R. solani and T. hamatum was carried out by inoculation in sterile wheat grains (previously soaked for 24 h in tap water), with 1 cm2 piece of mycelia grown in PDA. The wheat grains were then incubated at 25ºC for three weeks. Rhizoctonia solani and T. hamatum inoculums were added to soil as previously described by De Freitas and Germida (1991). Trychoderma hamatum strain was added to the soil eight days before planting the potato seeds, whilst the pathogen was inoculated one day before. Plastic plantpots, containing approximately 2 kg of sterilized soil (soil texture sandy clay loam (sand 56%silt 9.12%clay 34.9%), pH: 5.0), were used to carry out root colonization and antagonism tests. The soil was sterilized in extrathick cardboard boxes, in two cycles of one hour at 121ºC and 15 psi. Tuberseeds of S. phureja variety “clone one” were inoculated by submersion in the bacterial suspension. The untreated control and the treatment with R. solani alone were submerged in sterile 0.1 M MgSO4.
A CRD having seven replicas, was used for the evaluation of the seven treatments (six P. fluorescens isolates and the mix of isolates), against R. solani. A negative control (with neither pathogen nor antagonist), a disease marker (pathogen without antagonist) and a positive biological control (T. hamatum T21) were used. Disease presence was determined by the evaluation of tuber deformation, sclerotia and scarification formation in the tuber. Each symptom was divided in three categories as proposed by Delgado and Vargas (1989): deformed tubers: was divided in tuber without damage, moderate deformation (having depressions) and advanced deformation (abnormal lumps and growth). Sclerotia and scarification were divided in healt, moderate (less than 40% of tuber surface affection) and severe (more than 40% of tuber surface affection).
ROOT COLONIZATION
The rhizospheric fluorescent Pseudomonas population resistant to rifampicin (10 µg ml1) was determined as cfu g1 of root in each one of these samples, following the methodology proposed by Kloepper et al. (1980) and Lemanceau et al. (1995). The dynamics of root colonization of the isolates was proposed as a CRD with seven treatments using a control (inoculated with 0.1 M MgSO4). Three replicas and five sampling points each 2225 d, were analyzed per treatment. A CRD having 2x8 factorial structure, with 16 treatments (six isolates, the bacterial mix and the control with and without R. solani,) and three replicas, was carried out to determine the possible influence of R. solani on P. fluorescens isolates colonization. The values were collected at the end of the culture cycle (day 122) and transformed logarithmically (log 10) for the root colonization analysis.
PLANT PRODUCTION AND GROWTH PROMOTION
Plant productivity was evaluated after harvesting by taking the number and average weight of tubers produced per plant. At this point, foliage height (in cm) and dry weight (in g) were also determined for plant growth promotion in the presence and absence of pathogen. The dry weight was determined by leaving the fresh plants in an oven at a temperate room at 40ºC during eight days. These assays were carried out as a CRD having a 2x8 factorial structure, with 16 treatments and seven replicas. The T. hamatum isolate (T21) was used as a positive biological control.
STATISTICAL ANALYSIS
Root colonization and plant growth during culture cycle were carried out using growth measurements that were analyzed with SAS’s PROC GLM. Other assays were analyzed using one and two ways ANOVA analysis, with SAS’s PROC GLM (SAS Institute 1994).
RESULTS
Pseudomonas fluorescens ANTAGONIST EFFECTIVITY AGAINST R. solani in vitro
Eight of the eleven P. fluorescens strains evaluated, inhibited in vitro growth of R. solani (p=0.005*); the most significant inhibition occurred with the strains IBPf.29, IBPf.33 and IBPf.60 (77,8 and 80 % respectively). Then, there were five more strains with a moderate activity showing from 10% to 69% of growth inhibition and finally three more strains with none activity against R. solani showing a similar pattern that the negative control (E. coli; Fig. 1). From the eleven strains, six were chosen to be evaluated in terms of colonization capacity, plant growth promotion and antagonism against R. solani in greenhouse conditions. Two of those strains presented none (IBPf.63, IBPf.96), two moderate (IBPf.25, IBPf. 62) and two high (IBPf.33, IBPf.60) antagonistic activity in vitro conditions against R. solani.
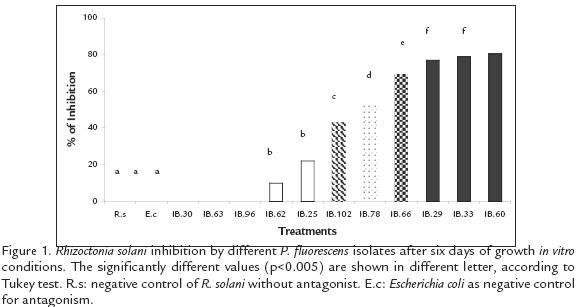
ROOT COLONIZATION DYNAMIC IN S. phureja BY P. fluorescens ISOLATES
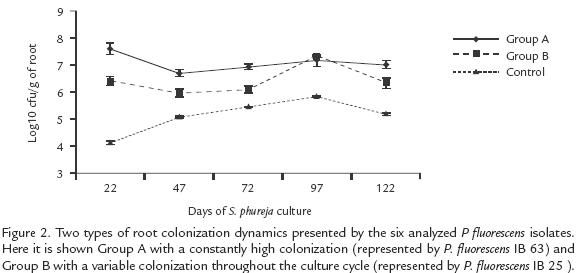
Pseudomonas fluorescens strains presented two main patterns in the dynamic of root colonization. In the first group from the seedling formation (day 22), up to the end (day 122), the cfu remained similar. Strains IBPf.33, IBPf.62 and IBPf.63 belong to this group represented by Group A (Fig. 2). The second pattern typified by Group B (Fig. 2), was featured by oscillations in the cfu throughout the potato culture cycle. The isolates of this group started with an intermediate cfu in the root system during the seedling formation. Then it was followed by a decrease in the fluorescent Pseudomonas population during flowering and tuber formation (days 47 and 72 respectively in Fig. 2), followed by an increase in cfu during tuber swelling (day 97) and ending in a decrease in cfu at harvesting time (day 122). Isolates IBPf.25, IBPf.60 and IBPf.96 belong to this group.
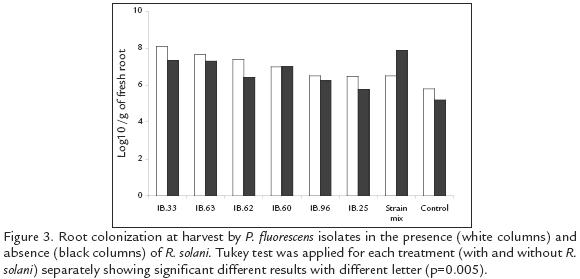
Pseudomonas fluorescens ROOT COLONIZATION IN THE PRESENCE AND ABSENCE OF R. solani
At the time of harvesting (day 122), all isolates presented higher cfu g1 of root in the presence of R. solani compared to the treatments without the pathogen (p=0.05*). The combined treatment showed a colonization pattern different to that expressed by the other treatments. It was best in absence of the pathogen, surpassing other treatments by at least 0.5 logarithmic units, whilst colonization diminished in the presence of the pathogen by 1.4 logarithmic units, placing it amongst those treatments having the least colonizing effect (Fig. 3). Statistically significant differences were found between isolates in terms of root colonization. In the presence and absence of R. solani, the IBPf.33 and IBPf.63 isolates presented the greatest root colonization, surpassing other isolates by a range of 0.5 to 1.5 logarithmic units.
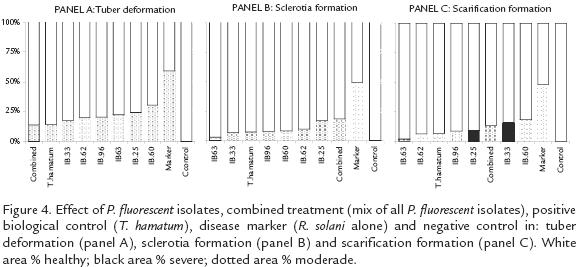
Pseudomonas fluorescens ANTAGONIST EFFECTIVITY AGAINST R. solani IN GREENHOUSE CONDITIONS
All tested strains of P. fluorescens significantly reduced (p=0.0001*) the severity of the disease in the plants grown in greenhouse conditions. The tubers collected from all the plants inoculated with Pseudomonas isolates presented at least 50% less incidence in all the symptoms at the moderate level (Fig. 4). Pseudomonas fluorescens application also prevented severe levels of disease by comparison with the disease marker, in which severe deformation, sclerotia and scarification were produced in 9.03%, 9.34% and 6.54% of the tubers respectively. In the same way, T. hamatum T21 application significantly reduced the symptoms of the disease and did not allow severe levels to be expressed (Fig. 4).
TUBER YIELD IN THE PRESENCE AND ABSENCE OF R. solani
Pseudomonas fluorescens inoculation into S. phureja plants in the absence of the pathogen, increased tuber number and weight significantly in comparison with the untreated control and the disease marker (Table 1). The IBPf.63 P. fluorescens strain and T. hamatum T21 positive control presented the greatest yield increase, with around two fold in comparison with both, the untreated control and the disease marker. The other strains although had lower tuber production increases (between 25% and 48%) and tuber weight gain (between 11% and 74%), were significantly greater than the control. It is important to mention that despite the differences in tuber number and weight between strains, the presence of R. solani did not affect those values within treatments (p=0.077). In the presence of the pathogen, the inoculation of P. fluorescens and T. hamatum T21, avoid the development of deformed small or large tubers, which were present in the disease marker (results not shown). This probably explains why a similar weight value in the disease marker, to that reached by tubers exposed to the Pseudomonas strains (p=0.506), was obtained.
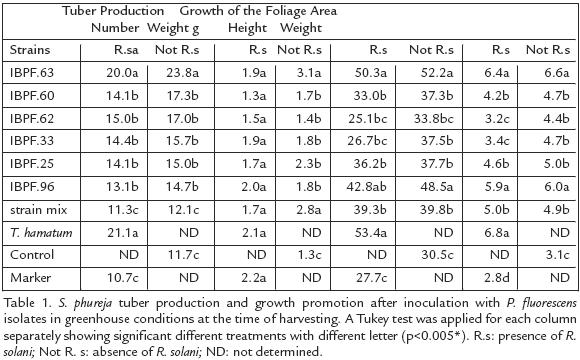
Solanum phureja GROWTH PROMOTION DUE TO THE ACTION OF P. fluorescens
The height of the plants inoculated with P. fluorescens was superior to that obtained by control plants (noninoculated) from day 72 onwards (results not shown). In the last stage of the cycle (day 122), P. fluorescens inoculants produced an increase in height of at least 11% and 40% in dry weight, in relation to the control. The isolates IBPF.63 and IBPF.96 produced the greatest increases, equivalent to 71% and 59% in height and 110% and 90% in dry weight, respectively (p=0.0001; Table 1). The growth promotion of the foliar area in the presence of the pathogen was also shown by the isolates IBPf.63 and IBPf.96 with increases of 128% and 106% in dry weight and 82% and 55% in plant height respectively. Those values were statistically different from the disease marker (2.77 g and 27.67 cm for weight and height, respectively).
DISCUSSION
One of the major goals in the study of soil microorganisms is to identify and manipulate natural microbial communities in the rhizosphere. In order to advance in this direction more studies need to be done in the structure and dynamics of microbial populations in the rhizosphere of plants (Nautiyal, 1997). With this idea in mind, our group started a research to identify the more representative species of fluorescent Pseudomonas in the potato rhizosphere, in order to improve the chances of selecting the most successfully established bacteria of this type, for the S. phureja rhizosphere (Uribe et al., 1999). We found P. fluorescens was the dominant species in the rhizosphere and rhizoplane of both S. tuberosumand S. phureja (Uribe et al., 1999). This finding allow us to design the current study where six strains of P. fluorescens were selected to identify their capacity of reduction of R. solani symptoms and the growth promotion in S. phureja plants. Despite of the lack of correlation between results of in vitro and in vivo antibiosis of fluorescent Pseudomonas against several soilborne pathogenic fungi (Wei et al., 1991; Reddy et al., 1994; Bagnasco et al., 1998), the in vitro antagonistic capacity of these bacteria has been continuously selected for screening purposes (Howell and Stipanovic, 1979; Mao et al., 1991; Wei et al., 1991; Reddy et al., 1994; Nautiyal, 1997; Mazzola, 1998; Nandakumar et al., 2001; Berg et al., 2002). The success of that strategy for the prediction of antagonistic activity against R. solani in the rhizosphere of S. phureja, was evaluated by application of P. fluorescens strains with none, moderate and high activity against R. solani under in vitro condition. No correlation of in vitro antibiosis with growth promotion or disease suppression under greenhouse condition was found. However, statistically significant correlation was found between colonization capacity and plant growth promotion (r2:77) and disease suppression (tuber deformation; sclerotia and scarfication formation reduction with r2:0.84, r2:0.89 and r2:0.81 respectively). These results suggest that the association of P. fluorescens isolates with the S. phureja rhizosphere and its antagonistic capacity against R. solani, is determined by the colonization capacity of the bacteria, more than the production of active compound against a pathogenic agent. The results obtained in this study show that P. fluorescens acts as a plant growth promoter of S. phureja by reducing the disease symptoms caused by R. solani, and increasing tuber production, plant weight and plant height. Although similar results have been reported for the related species S. tuberosum using P. fluorescens (Kloepper et al., 1980; Burr et al., 1978), this is to our knowledge, the first report of these kind of results for the Colombian native potato S. phureja. The dynamics of root colonization of six P. fluorescens isolates is presented here to understand the effect of this bacterium on S. phureja (Fig. 2). The population density obtained for each isolate varies differently throughout the crop cycle. Such variation is related with the phenologic changes of the plant as it is deduced from the relation between the tuber swelling phase (day 97), and the increase shown by the cfu of all Pseudomonas isolates. However, the variations found overall crop cycle between the different strains shows that the association dynamic is a strain specific phenomenon, which is not well understood within the current body of knowledge (Burr et al., 1978; Kloeper et al., 1980; Loper et al., 1984a; Mao et al.,1991, Nautiyal 1997).
Populations of the rifampicin resistant Pseudomonas for all the treatments (except the isolate IBPf.60 and the combined treatment), were greater on potato rhizosphere infected with R. solani than on roots without the pathogen (Fig. 3). This effect probably resulted from the increased availability of root exudates released through lesions incited by the root pathogen (Mazzola, 1998). Despite of the R. solani establishment, the increase of tuber production in all plants inoculated with a single strain and the increase of plant weight induced by all except IBPf33 and IBPf25 isolates, was not affected by the presence of the pathogen. The lower population obtained by the combined treatment in presence of the pathogen, suggests a decrease in the colonization capacity of the strain mix under these conditions. That effect was probably due to the expression of different antagonistic compounds by some or each isolates, affecting the colonization of the artificial community. Such compounds usually provides a selective advantage over rhizosphere colonist, even if they are from the same or related species (Mazzola et al., 1992; Raaijmakers et al., 1995; Mazzola, 1998). The above mentioned results also explain why the mixture of P. fluorescens strains tested in this study was not superior in diminishing the severity of the R. solani symptoms, in comparison to the results obtained for each strain alone. Other studies using combinations of fluorescent Pseudomonas have revealed the benefits of that strategy to provide greater control of different plant pests (Weller and Cook 1983; Pierson and Weller, 1994; Sindhu et al., 2002). This suggests to us, that other combinations of the evaluated Pseudomonas strains or even some strains with T. hamatum T21 isolate, can improve the results obtained in this study. Other strain combinations should improve results by expanding the espectrum of antifungal metabolites or mechanisms beyond those produced by P. fluorescens. Nevertheless, compatibility between these agents must first be evaluated (Mazzola, 1998).
CONCLUSIONS
The results presented through this manuscript suggested that the utilization of P. fluorescens for the control of R. solani is a promising strategy for the management of the disease in field conditions. This affirmation is supported by the fact that all tested P. fluorescens isolates, reduced the disease severity on the plants. Such reduction was evident due to the decrease in the number of affected tubers with deformation, decrease in the number of sclerotium formation and de abolishment of the presence of severe symptoms on the plant.
Under the conditions tested, the isolates IBUNPf 063 and IBUNPf 033 can be considered for a next level of evaluation as a promising isolate due to its good performance in terms of colonization, plant growth promotion and the reduction of the R. solani disease symptoms.
ACKNOWLEDMENTS
We thank COLCIENCIAS for its economical support for the development of this project; the Microbiology M.Sc. Program, the Facultad de Agronomía, The Instituto de Biotecnología, Universidad Nacional de Colombia, Sede Bogotá, for its technical and institutional support; Dr. Carlos Ñustes (Facultad de Agronomía, Universidad Nacional de Colombia, Sede Bogotá) for providing the S. phureja “clone one” seed and Dr. G. Khachatourians (Department of Applied Microbiology, University of Saskatchewan, Saskatoon, Canada) for his helpful comments and critical review of the manuscript.
REFERENCES
BAGNASCO P, DE LA FUENTE L, GUALTIERI G, NOYA F, ARIAS A. Fluorescent Pseudonomas spp. As a Biocontrol Agents Against Forage Legume Root Pathogenic Fungi. Soil Biol Biochem. 1998;30:13171322.
[ Links ]BAHME JB, SCHROTH MN. Spatial Temporal Colonization Patterns of a Rhizobacterium on Underground Organs of Potato. Phytophatology. 1987;77:10931100.
[ Links ]BENHAMOU N, BÉLANGER RL, TIMOTHY CP. Induction of Differential Host Responses by P. fluorescens in T DNA Transformed Pea Roots After Challenge with F. oxysporum f. sp. pisi and Pythium ultimum. Phytopathology. 1996;86:11741185.
[ Links ]BERG G, ROSKOT N, STEIDLE A, EBERL L, ZOCK A, SMALLA K. Plant Dependent Genotypic and Phenotypic Diversity of Antagonistic Rhizobacteria Isolated from Different Verticillium Host Plant. Appl Environ Microbiol. 2002;68:33283338.
[ Links ]BURR TJ, SCHROTH MN, SUSLOW T. Increased Potato Yields by Treatment of SeedPieces with Specific Strains of Pseudomonas Fluorescens and P. putida. Phytopathology. 1978;68:13771383.
[ Links ]CARRUTHERS FL, CONNER AJ, MAHANTY HK. Identification of a Genetic Locus in P. aureofaciens Involved in Fungal Inhibition. Appl Environ Microbiol. 1994;60:7177.
[ Links ]DE FREITAS JR, GERMIDA JJ. Pseudomonas cepacia and Pseudomonas putida as Winter Wheat Inoculants for Biocontrol of Rhizoctonia solani. Can J Microbiol. 1991;37:780784.
[ Links ]DELGADO MC, VARGAS M. Grupos de anastomosis del fungi Rhizoctonia solani (Kuhn) y su reacción en materiales de papa con diversos grados de resistencia [posgraduate Thesis] Bogotá: Universidad Distrital Francisco José de Caldas; 1989.
[ Links ]GAMALERO E, FRACCHIA L, CAVALETTO M, GARVAYE J, FREYKLETT P, VARESE GC, MARTINOTTI MG. Characterization of Functional Traits of Two Fluorescent Pseudomonads Isolated from Basidiomes of Ectomycorrhizal Fungi. Soil Biol Biochem. 2003;35:5565.
[ Links ]GHERNA J. Storage and Survival of Bacteria by UltraFree. Lett Appl Microbiol. 1986;3:127129.
[ Links ]HAMMER PE, HILL DS, LAM ST, PEE KHVAN, LIGON JM. Four Genes from Pseudomonas fluorescens that Encode the Biosynthesis of Pyrrolnitrin. Appl Environ Microbiol. 1997; 63:21472154.
[ Links ]HOWELL CR, STIPANOVIC RD. Control of Rhizoctonia solani on Cotton Seedlings with Pseudomonas fluorescens and with an Antibiotic Produced by the Bacterium. Phytopathology. 1979; 69:480482.
[ Links ]KLOEPPER JW, SCHROTH MN, MILLER TD. Effects of Rhizosphere Colonization by Plant Growth Promotion Rhizobacteria on Potato Plant Development and Yield. Phytopathology. 1980;70:10781082.
[ Links ]KRECHEL A, FAUPEL A, HALLMANN J, ULRICH A, BERG G. PotatoAssociated Bacteria and their Antagonistic Potential Towards PlantPathogenic Fungi and the PlantParasitic Nematode Meloidogyne incognita (Kofoid and Whithe) Chitwood. Can J Microbiol. 2002;48:772786.
[ Links ]LATOUR X, CORBERAND T, LAGUERRE G, ALLARD F, LEMANCEAU P. The Composition of Fluorescent Pseudomonad Populations Associated with Roots is Influenced by Plant and Soil Type. Appl Environ Microbiol. 1996;62:24492456.
[ Links ]LEAL ME, PLATA BY. Evaluación de cepas nativas de Trichoderma spp. en el control de Rhizoctonia solani Kuhn en fríjol (Phaseolus vulgaris L) [Masters Thesis] Bogotá: Facultad de Agronomía, Universidad Nacional de Colombia; 1996.
[ Links ]LEMANCEAU P, CORBERAND T, LATOUR X, LAGUERRE G, BOEUFGRA JM, ALABOUVETTE C. Effect of Two Plant Species, Flax and Tomato, on the Diversity of SoilBorn Populations of Fluorescent Pseudomonads. Appl Environ Microbiol. 1995;61:10041012.
[ Links ]MAO W, LEWIS JA, HEBBAR PK, LUMSDEN RD. Seed Treatment with a Fungal or a Bacterial Antagonist for Reducing Corn DampingOff Caused by Species of Phytium and Fusarium. Plant Dis. 1991;81:450454.
[ Links ]MAZZOLA M, COOK RJ, THOMASHOW LS, WELLER DM, PIERSON III LS. Contribution of Phenazine Antibiotic Biosynthesis to the Ecological Competence of Fluorescent Pseudomonads in Soil Habitats. Appl Environ Microbiol. 1992;58:26162624.
[ Links ]MAZZOLA M. The Potential of Natural and Genetically Engineered Fluorescent Pseudomonas spp. as Biological Control Agents. In. Microbial Interactions in Agriculture and Forestry. Volume I. Ed. Subba Rao N.S and Dommergues, Y.R. Science Publishers, Inc. Enfield, N.H. USA. 1998. p. 193217.
[ Links ]NANDAKUMAR R, BABU S, VISWANATHAN R, RAGUCHANDER T, SAMIYAPPAN R. Induction of Systemic Resistance in Rice Against Sheath Blight Disease by Pseudomonas fluorescens. Soil Biol Biochem. 2001;23:603612.
[ Links ]NAUTIYAL CS. Rhizosphere Competence of Pseudomonas sp. NBRI9926 and Rhizobiumsp. NBRI9513 Involved in the Suppression of Chickpea (Cicer arietinum L.) pathogenic fungi. FEMS Microbiol Ecol. 1997;23:145158.
[ Links ]NIELSEN TH, CHRISTOPHER C, ANTHONI U, SØRENSEN J. Viscosamide, a New Cyclic Depsipeptide with Surfactant and AntiFungal Properties Produced by Pseudomonas fluorescens DR54. J Appl Microbiol. 1999;87:8090.
[ Links ]NIELSEN TH, SØRENSEN D, TOBIASEN C, ANDERSEN JB, CHRISTOPHERSEN C, GIVSKOV M, SØRENSEN J. Antibiotic and Biosurfactant Properties of Cyclic Lipopeptides Produced by Fluorescent Pseudomonas spp. from the Sugar Beet Rhizosphere. Appl Environ Microbiol. 2002;68:34163423.
[ Links ]PARMAR N, DADARWALL KR. Stimulation of Nitrogen Fixation and Induction of Flavonoid Like Compounds by Rhizobacteria. J Appl Microbiol. 1999;86:3644.
[ Links ]PIERSON EA, WELLER DM. Use of Mixtures of Fluorescent Pseudomonads to Suppress TakeAll and Improve the Growth of Wheat. Phytopathology. 1994;84:940947.
[ Links ]POPLAWSKY AR, ELLINGBOE AH. TakeAll Suppressive Properties of Bacterial Mutants Affected in Antibiosis. Phytopathology. 1989;79:143146.
[ Links ]RAAIJMAKERS JM, I VAN DER SLUIS, MOSTWER M, BAKER PA, SCHIPPERS B. Utilization of Heterologous Siderophores and Rhizosphere Competence of Fluorescent Pseudomonas spp. Can J Microbiol. 1995;41:126135.
[ Links ]RAAIJMAKERS JM, WELLER DM, THOMASHOW LS. Frequency of Antibiotics Producing Pseudomonas spp. in Natural Environments. Appl Environ Microbiol. 1997;63:881887.
[ Links ]REDDY MS, INÉS RK, LAZAROVITS G. Relationship Between in vitro Growth Inhibition of Pathogens and Suppression of Preemergence CampingOff and Postemergence Root Rot of White Bean Seedlings in the Greenhouse by Bacteria. Can J Microbiol. 1994;40:113119.
[ Links ]SANDS DC, HANKIN L. Ecology and Physiology of Fluorescent Pectolytic Pseudomonads [Pseudomonads fluorescens, Pseudomonas putida, Soft Rot, Plants]. Phytopathology. 1975;65:921924.
[ Links ]SAS-Institute. SasStat User’s Guide. Aceclusfrq. Version 6. Volume 1. Forth Edition. Cary, North Carolina, USA; 1994. p. 890.
SINDHU SS, SUNEJA S, GOEL AK, PARMAR N, DADARWAL KR. Plant Growth Promoting Effects of Pseudomonas sp. on Coinoculation with Mesorhizobium sp. Cicer Strain Under Sterile and “wilt sick” Soil Conditions. Appl Soil Ecol. 2002;19:5764.
SLINNINGER PJ, VAN CAEWENBERGE JE, BOTHAST RJ, WELLER DM, THOMASHOW LS, COOK RJ. Effect of Growth Culture Physiological State, Metabolites and Formulation on the Viability, Phytotoxicity and Efficacy of the TakeAll Biocontrol Agent Pseudomonas fluorescens 279 Stored Encapsulated on Wheat Seeds. Appl Microbiol Biotechnol. 1996;45:391398.
[ Links ]SNEH B, BURPEE L, OGOSHI A. Identification of Rhizoctonia Species. Academic Press. New York; 1991.
[ Links ]SUSLOW TV, SCHROTH MN. Rhizobacteria of Sugarbeets: Effects of Seed Application and Root Colonization on Yield Fungal and Bacterial Phytopathogens. Phytopathology. 1982;72:199206.
[ Links ]URIBE D, ORTIZ E, PORTILLO M, BAUTISTA G, CERÓN J. Diversidad de pseudomonas fluorescentes en cultivos de papa de la region cundiboyacense y su actividad antagonista in vitro sobre Rhizoctonia solani. Rev Colomb Biotecnol. 1999;2:5058.
[ Links ]VAN PEER R, PUNTE HL, WEGER LA, SCHIPPERS B. Characterization of Root Surface and Endorhizosphere Pseudomonads in Relation to their Colonization of Roots. Appl Environ Microbiol. 1990;56:24622470.
[ Links ]WEI G, KLOEPER JW, TUZUN S. Induction of Systemic Resistance of Cucumber to Colletotrichum orbiculare by Select Strains of Plant Growth Promoting Rhizobacteria. Phytopathology. 1991;81:15081512.
[ Links ]WELLER DM, COOK RJ. Suppression of TakeAll of Wheat by Seed Treatments with Fluorescent Pseudomonads. Phytopathology. 1983;78:463469.
[ Links ]











