Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Acta Biológica Colombiana
Print version ISSN 0120-548X
Acta biol.Colomb. vol.12 no.1 Bogotá Jan./June 2007
EFFECT OF SOME IONS ON SPERM ACTIVATION IN Brycon henni (EIGENMANN 1913)
Efecto de algunos iones sobre la activación espermática en Brycon henni (Eigenmann 1913)
JAVIER TABARES1, MV., M.Sc., TATIANA RUÍZ1, MV., Ph. D., LUCY ARBOLEDA2, Biólogo, M.Sc., MARTHA OLIVERA1*, MV., Ph. D. 1Fisiología y Biotecnología de la Reproducción, Facultad de Ciencias Agrarias, Universidad de Antioquia, Carrera 75 Nº 6587, AA. 1226. Tel: 5744 25 91 49, Medellín, Colombia. * molivera@catios.udea.edu.co 2Facultad de Ciencias Agropecuarias, Politécnico Jaime Isaza Cadavid. Carrera 48 N° 7151, AA. 4932. Medellín, Colombia. Tel: 5742 61 76 60.
Presentado 21 de noviembre 2006, aceptado 11 de diciembre de 2006, correcciones 31 de enero de 2007.
ABSTRACT
Spermatozoa in Characid fish remain immobile in seminal plasma and are activated when freed into water where the ionic balance seems to be the main factor starting the activation process. This process was the target of the present study with emphasis on the activation of motility and on motility maintenance over time. The effect of isosmotic solutions was analyzed taking into account the possible combinations of the following ions, Ca2+, K+, Mg2+ and Na+ as well as the effect of channel blocking agents. The parameters measured were cells with motility (%), duration of motility (s), plasma membrane potential, and the effect of channel blockers on activation time and on motility. There was an increased motility when the semen was incubated in solutions containing K+ (p<0.05) compared with the control (CaNaMgK solution); the longest duration of motility was attained when the incubation was performed in solutions containing Na+ and Mg2+ (p<0.05). All solutions induced a change in membrane potential detected after 15 s of activation. Blocking K+, Ca2+ and Na+ channels did not alter motility but decreased the activation time (p<0.05). Potassium induced activation at all concentrations up to 105 mM, but motility was drastically decreased at concentrations higher than 140 mM (p<0.05). The conclusion is that interaction of the ionic environment with the cell membrane leads to changes in membrane potential and intracellular signalling that trigger sperm motility in Brycon henni.
Key words: Ionic balance, channel blockers, membrane potential, Oxonol.
RESUMEN
Los espermatozoides en los carácidos se mantienen inmóviles en plasma seminal, activándose cuando se liberan en el agua. El objetivo de este estudio fue conocer algunos de los mecanismos que inducen la activación espermática (porcentaje de espermatozoides que adquieren movilidad y el tiempo que dura la misma). El efecto de las soluciones isosmóticas, se analizó considerando las combinaciones de los iones Ca2+, K+, Mg2+ y Na+, y el efecto de algunos bloqueadores de canales iónicos. Se evaluó el porcentaje y duración de la movilidad, así como el potencial de la membrana plasmática. La motilidad espermática fue mayor cuando el semen se incubó en las soluciones que contenían + (p<0,05) comparado con el control positivo. La mayor duración de la movilidad se obtuvo cuando se incubaron los espermatozoides en soluciones que contenían Na+ y 2+ (p<0,05). Todas las soluciones probadas indujeron un cambio en el potencial de membrana, detectado después de 15 segundos de la activación. Bloqueo de los canales de K+, Ca2+ y Na+ no alteró la movilidad, sino que disminuyó el tiempo de activación (p<0,05). Los iones potasio indujeron la activación en todas las concentraciones hasta 105 mM, pero la movilidad disminuyó drásticamente cuando las concentraciones fueron mayores de 140 mM (p<0,05). Se concluye que la interacción del ambiente iónico con la membrana de la célula conduce a cambios en el potencial de la membrana que conllevan a señales intracelulares que desencadenan la movilidad del esperma.
Palabras clave: balance iónico, bloqueadores de canales, potencial de membrana, Oxonol
INTRODUCTION
In Colombia the fish Brycon henni is a protected endemic species. It inhabits water bodies in coffee producing areas (700 1900 m.a.s.l.; 4º35’56’’N 74º04’51’’W; 1828°C). Insufficient knowledge of its basic biology and behavior prevent the commercial culture of this promising fish. Our interest is focused in sperm physiology of captive males, in order to improve fish biotechnologies.
Ionic balance in seminal plasma is important for the maturation of sperm (Ingerman et al., 2002), to keep spermatozoa immotile within the testis and in the spermatic duct (Müller et al., 1994), to develop the capacity of spermatozoa to move (Mochida et al., 1999; Ohta et al., 2001), and to maintain adequate motility when the sperm is delivere>d into the water (Morisawa and Suzuki, 1980; Scott and Baynes ,1980; Márián et al., 1993; Cosson et al., 1999). Activation of sperm and its relationship with ions has been demonstrated by inducing an osmotic shock (Krasznai et al., 1995; Márián et al., 1997), by changing the transmembrane potential (Emri et al., 1998), and through the regulation of interchangers and ionic channels for Na+, K+ and Ca2+ (Krasznai et al., 1995; Cosson et al., 1999; Vines et al., 2002; Krasznai et al., 2003; Felix et al., 2004). This ionic balance is believed to be related, among other factors, to the intracellular environment (Krasznai et al., 1995; Márián et al., 1997), the composition of the seminal plasma (Mochida et al., 1999; Ohta et al., 2001) and the ionic flow through the membrane that in turn is regulated by channels. Several types of channels have been described: 1) open pore channels: that function by gradients (Felix et al., 2004), 2) gate channels activated mechanically that open when the membrane expands due to turgency (Cosson et al., 1999; Krasznai et al., 2003), 3) voltagedependent channels that are activated when there is a change in membrane potential (Krasznai et al., 2000; Felix et al., 2004), 4) ligand channels working through specific proteins that interact with their receptors (Felix et al., 2004), and finally 5) interchanger channels in which the energy generated by the passageinvolving an ion gradientthrough the channel is used for the passage of another ion in the opposite direction (Vines et al., 2002). Mitochondria, having channels in both the internal and the external membranes, also regulate the intracellular ionic balance (Garlid and Paucek, 2003) allowing the flux of Ca2+, K+ and Mg2+ in the cell to flow in either direction (entryexit).
Cytosolic Ca2+ is a second messenger and a cofactor in the activation process: it activates adenyl cyclase to induce the production of cyclic AMP necessary for phosphorylation of tubulin and then for flagellar axoneme activation (Eddy and O’Brien, 1994; Suarez and Ho, 2003). Mg2+ and Ca2+ are necessary to produce the ATP required for oxidative phosphorylation (Sordahl and Silver, 1975; Barbiroli et al., 1999) that depends directly on the membrane potential and on the presence of metabolic precursors such as glucose (Lahnsteiner et al., 1998). The dinein ATPase of the axoneme utilizes ATP to bring about movement of the flagellum (Suarez and Ho, 2003). In teleosts, spermatozoa become activated when they are in contact with water: in the sea due to a hyperosmotic shock (Vines et al., 2002), and in fresh water due to a hyposmotic shock (Morisawa et al., 1983). The objective of this study was to expand our knowledge on the role of ions on the inhibition and activation of sperm function in Brycon henni.
MATERIALS AND METHODS
FISHES AND SAMPLES. Average fish body weight and size (total length) were 112 ± 7 g and 20.5 ± 0.7 cm respectively. Semen was collected by craneocaudal massage during the season of maximal sperm production from fish kept in captivity for one year. The samples were collected in 1.5 ml tubes and maintained at 4ºC for 57 h until used. Five sexual mature male fish were used, and four samples were collected in different dates during the reproductive season; thus, a total of twenty samples were tested with the ionic solutions (see next) at a 1:1000 dilution. The average semen concentration used with the various solutions was 2.3 ± 1.10 spermatozoids/ml.
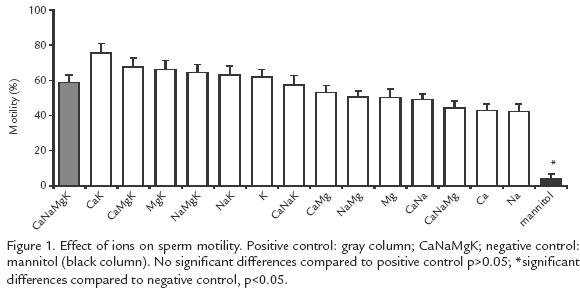
IONIC SOLUTIONS. Previous seminal plasma characterization was taken into account (Tabares et al., 2006). Fifteen different combinations of isosmotic solutions were prepared with Ca2+, K+, Mg2+ and/or Na+ (KCl: 37.3 mM, NaCl: 122.8 mM, CaCl2: 0.87 mM, MgCl2: 0.83 mM (see Fig. 1). Mannitol was used to replace the missing ion(s) to keep osmolality at 307 mOs/kg. The positive control was a solution with an ion concentration similar to that of seminal plasma (KCl: 37.3 mM, NaCl: 122.8 mM, CaCl2: 0.87 mM, MgCl2: 0.83 mM (Tabares et al., 2006). The negative control was an isosmotic solution of mannitol (63.63 mM). All solutions were buffered with 20 mM Hepes (pH 7.5).
EFFECT OF INCUBATION IN IONIC SOLUTIONS ON SPERM MOTILITY AND ACTIVATION TIME.
To determine the moment at which activation started and the activation time, the semen was diluted 1:1000 in each of the 15 solutions to be tested. The tubes were incubated for one hour at 20ºC and activated with a hyposmotic water solution containing 20 mM Hepes (pH 7.5; activator solution) at 20 mOs/kg. The gross motility and the activation time (in seconds) were measured (Tabares et al., 2006) using an inverted phase contrast microscope (Nikon TMSF 211571) at 40X.
EFFECT OF IONS ON PLASMA MEMBRANE POTENTIAL. The fluorochrome Oxonol (Molecular Probes, Ore,USA) (bisacid 1.3dibutylbarbituric) trimethine oxonol (DiBAC43) that has a high affinity for membrane lipids at rest, was used to detect changes in membrane potential. These changes induce the detachment of the fluorochrome and the consequent fading of the fluorescence. Nonmotile spermatozoa (5x106 spermatozoa/ml) were incubated with 1 µM oxonol for one hour at 20ºC. The mean fluorescence intensity (MFI) was measured at 15 s intervals for 2 min in a flow cytometer FACS SORT (Becton Dickinson, USA) at 488 nm between 200 and 400 mW.
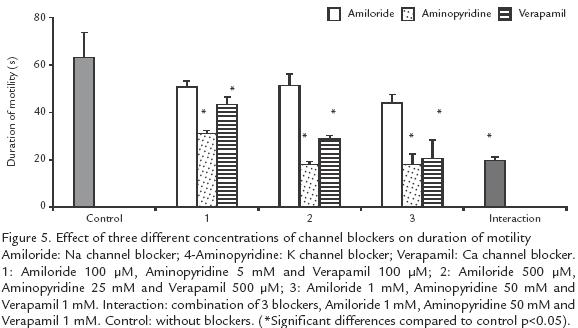
EFFECT OF CHANNEL BLOCKERS ON MOTILITY AND ACTIVATION TIME. To determine whether activation is due to the opening of ionic channels, competition blockers (Biomedicals, Germany) were used at different concentrations. Amiloride (100 µM, 500 µM and 1 mM) served as Na+ channel blocker; to block K+ channels 4aminopyridine (5 mM, 25 mM and 50 mM) was used; Verapamil (100 µM, 500 µM and 1 mM) was used to block Ca2+ channels. The blockers were evaluated individually at each concentration and in combination at the highest concentrations. Semen samples were diluted 1:1000 in an isosmotic solution and incubated at 20ºC for one hour, to allow ion interchange; then, the espermatozoa were activated with a solution containing the blockers. The gross motility and activation time were recorded. As negative control an activator solution without blockers was used.
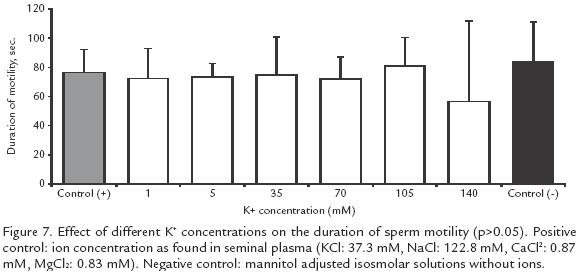
EFFECT OF K+ CONCENTRATIONS ON SPERM MOTILITY AND ACTIVATION TIME. Nine isosmotic + solutions were tested to determine the optimal conditions for sperm activation: 1, 5, 10, 15, 35, 70, 105 and 140 mM. Additionally, two other control solutions were included: one without K+ but isosmolar to seminal plasma and the other with Ca2+, K+, 2+ and Na+ at the same concentrations as in the seminal plasma.
STATISTICAL ANALYSIS. A General Lineal Model was used to study the quantitative stochastic relationship among the dependent (gross motility (%), activation time (s), average intensity of fluorescence (AIF) and the independent variables (ionic balance, channel blockers, K+ concentrations) using the Tukey and Kramer test (p<0.05). The variables, motility and AIF were transformed to log10 and log(100+X), respectively (Sas Institute, I., Cary, Nc 2002).
RESULTS
EFFECT OF IONS ON GROSS MOTILITY AND ACTIVATION TIME.
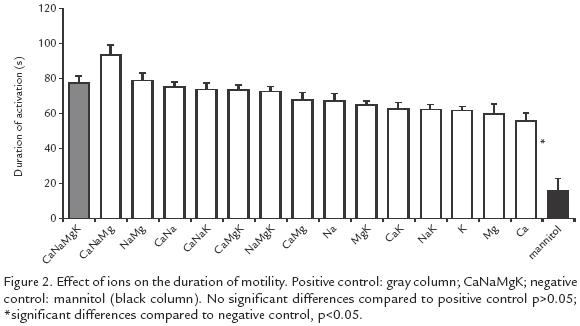
All the ionic solutions tested induced motility oscillating between 42 and 75% while in the negative control the motility was only 4% (p<0.005). The highest motility (57 75%) was obtained with solutions containing K+, but there was no difference with the positive control (58%) (Fig. 1). The activation time varied from 56 to 94 s and the difference was significant (p<0.05) compared to the negative control (16 s). The solutions yielding the longest activation time were: Na+Mg2+Ca2+ (94 s) and Na+Mg2+ (79 s) but no significant difference was found (p>0.05) with the positive control (78 s; Fig. 2).
EFFECT OF IONS ON PLASMA MEMBRANE POTENTIAL.
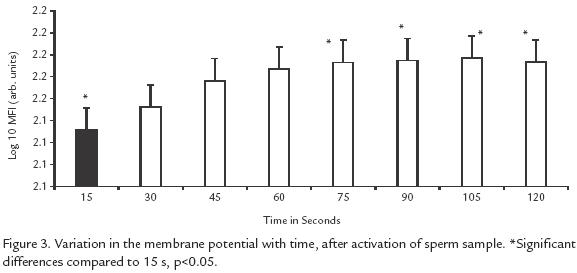
All of the solutions, including controls induced the same change in membrane potential (p> 0.5). This change was noticeable starting 15 s after activation, but the peak of sperms undergoing a change occurred at 30 s and remained steady up to 120 s (p<0.05; Fig. 3).
EFFECT OF CHANNEL BLOCKERS ON MOTILITY.
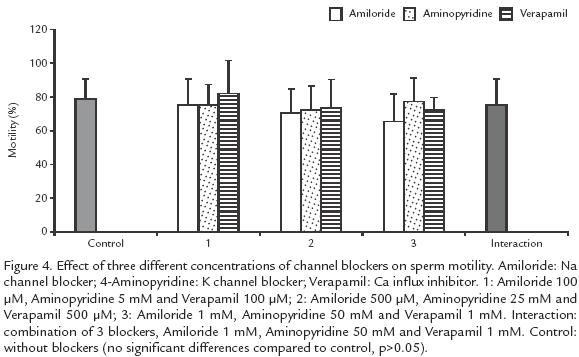
Blockers of K+, Ca2+ and Na+ channels, either individually or in combination did not affect motility but reduced the duration of activation, independently of the ionic concentration (p>0.05; Fig. 4). Ca2+ and K+ channel blockers shortened the duration of activation from 63 (control) to 18 s, when the highest blocker concentration was used (10 times concentrated; p>.05; Fig. 4). Blockers of Na+ channels showed a low effect, from 63 to 44 s (p<0.05; Fig. 5).
EFFECT OF K+ CONCENTRATION ON MOTILITU AND DURATION OF MOTILITY.
K+ between 1 and 70 mM had no effect on motility but there was a reduction from 7383% (positive control) to 2354% when the concentration was increased to 105 mM and to 011% when it was increased to 140 mM (p<0.05; Fig. 6). However, the duration of activation did not change significantly at different K+ concentrations (Fig. 7).

DISCUSSION
When an osmotic shock is produced to activate spermatozoa, there is a reduction of extracellular K+ probably inducing the mobilization of the ion from mitochondria through K+/H+ interchangers (Garlid, 1994) to the external milieu. The increase in positive charges induced by the efflux of K+ generates a change of membrane potential (Ohta and Shinriki, 1998) and this leads to an increase in cytosolic Ca2+ entering through voltage channels (Krasznai et al., 2000) and also possibly to the liberation of the Ca2+ reserves from mitochondria through the voltage or ligand channels (Carafoli, 2003). The increase of intracellular Ca2+ would thereby dissipate the signaling route by initiating motility.
It was found that extracellular K+ and Ca2+ are important for the initiation of motility (solutions with K+ and Ca2+, compared to the negative control, Fig. 1; p<0.05). On the other hand, Na+ and Mg2+ maintain this motility, but not by voltagedependent channels since blocking these ions did not affect the initiation of motility. The change in membrane potential is due in part to the low extracellular osmolality. However, this factor alone is not sufficient because in the absence of extracellular ions (negative control) the potential changed but not the initiation of motility. Therefore, initiation of motility in B. henni could be regulated by the ion flux through channels independently of changes in the membrane potential since it occurs with stretching, interchanging or ligand channels.
This study illustrates the importance of extracellular K+ for sperm motility in B. henni as opposed to results with other fresh water fishes such as Onchorhynchus mykss(Morisawa et al., 1983) and Clarias gariepinus (Mansour et al., 2002) in which K+ inhibits motility, or in Morone saxatilis (He et al., 2004) where this ion has no effect. When only K+ was tested, even up to twice the concentration found in normal seminal plasma, initiation of motility was observed, but at higher concentrations motility did not begin and this observation deserves further experimentation and analysis. As opposed to previous reports on salmonids (Billard and Coson, 1992) and the common carp Cyprinus carpio (Krasznai et al., 2000) demonstrating that extracellular Ca2+ is necessary to initiate motility, our results in Brycon indicate that Ca2+ is not necessary, and are in agreement with those of others (He et al., 2004) studying Morone saxatilis. Ca+ favors motility synergistically with + (Fig. 1), as proposed previously for C. carpio (Krasznai et al., 2000); the authors concluded that when K+ leaves the cells the consequent change in potential activates Ca2+ voltagedependent channels, as opposed to what occurs in B. henni, where the participation of other types of Ca2+ channels is suggested. The ionic solutions generating the highest percentages of motility are the same as those that generate the maximum number of sperms with a change of potential in their membranes (Fig. 1; Fig. 3). Interestingly, these solutions are not the same as those that induce the longest activation times (Fig. 2), suggesting that these two events use different mechanisms.
The longest motility times were observed with solutions in which Na+ and Mg2+ were combined (Fig. 2), in agreement with the report of others (Mansour et al., 2002) in Clarias gariepinus who suggest that the intracellular pH oscillates with the activation time and conclude that the antiporter Na+/H+ is involved in pH regulation depending on osmolality and extracelullar ions. Without extracellular Na+ the antiporter Na+/H+ cannot activate itself reducing the activation time. Mg2+ maintains motility for longer periods perhaps through the activation of adenyl cyclase to produce cAMP, that participates in the activation of protein movement through ATP hydrolysis with Mg2+ as a cofactor (Inaba et al., 1998; Barbiroli et al., 1999; Cosson et al., 1999). This mechanism is not completely understood but it is thought that Mg2+ modulates the opening and closing of mitochondrial channels thus creating the conditions for flagellar movement (Shi et al., 2002).
The duration of motility in B. henni (Fig. 5) may be associated to ionic regulation through different ion channels, contrary to what occurs in ciprinids that use a single type of channel (Krasznai et al., 2003). This parameter decreases significantly in B. henni whatever blocker is used suggesting that 1) the intervening channels are important for motility although other channels may be involved that are efficient in initiating rather than maintaining motility, and 2) the blockers require some time to saturate the channels before exerting their effect, such that motility is initiated but does In conclusion, activation of sperm motility in Brycon henni depends on a set of factors: 1) the ionic balance, with K+ playing a major role on the percentage of motile cells, and Na+ and Mg2+ on the duration of activation, and 2) the dynamics of the plasma membrane, indicated by the change in membrane potential, independently of the extracellular ionic balance.
AKNOWLEDGEMENTS
We are grateful to the Instituto Colombiano para el Fomento de la Ciencia y Tecnología, COLCIENCIASColombia, Project 11150912308, to the Universidad de Antioquia (CODI), and to Politécnico Jaime Isaza Cadavid, Medellín, Colombia for the financial support. We are also grateful to A.L. Haenni and J Ossa for their critical review of the manuscript.
REFERENCES
BARBIROLI B, IOTTI S, CORTELLI P, MARTINELLI P, LODI R, CARELLI V MONTAGNA P. Low Brain Intracellular Free Magnesium in Mitochondrial Cytopathies. J Cereb Blood Flow Metab. 1999;19:528532.
[ Links ]BILLARD R, COSON MP. Some Problems Related to the Assessment of Sperm Motility in Freshwater fish. J Exp Zool. 1992;262:122131.
[ Links ]CARAFOLI E. Historical Review: Mitochondria and Calcium: Ups and Downs of and Unusual Relationship. Trends Biochem Sci. 2003;28:175181.
[ Links ]COSSON J, BILLARD R, CIBERT C, DRÉANNO C. Ionic Factors Regulating the Motility of Fish Spermin. Edited by Claude Gagnon; 1999.
[ Links ]EDDY E, O’BRIEN D. The Espermatozoon. In: E.b.E.K.a.J.D.N. Second Edition, Raven Press, Ltd., New York (ed.) The Physiology of Reproduction. 1994. p. 2977.
EMRI M, BALKAY L, KRASZNAI Z, TRÓN L, MÁRIÁN T. Wide Applicability of a Flow Cytometric Assay to Measure Absolute Absolute Membrane Potentials on the Millivolt Scale. Eur Biophys J. 1998;28:7883.
[ Links ]FELIX R, LÓPEZGONZÁLEZ I, MUÑOZGARAY C, DARSZON A. Ion Channels and Sperm Function. Adv Mol Cell Biol. 2004;32:407431.
[ Links ]GARLID K. Mitochondrial Cation Transport: A progress report. J Bionerg Biomembr. 1994;26:537542.
[ Links ]GARLID K, PAUCEK P. Mitochondrial Potassium Transport: The K(+) Cycle. Acta Biochim Biophys. 2003;1606:2341.
[ Links ]HE S, JENKINSFEERAN K, CURRY W. Activation of Sperm Motility in Striped Bass Via a cAMPIndependent Pathway. Theriogenology. 2004;61:14871498.
[ Links ]INABA K, MORISAWA, S, MORISAWA M. Proteasomes Regulate the Motility of Salmonid Fish Sperm Through Modulation of AMPcDependent Phosphorylation of an Outer Arm Dynein Light Chain. J Cell Sci. 1998;111:11051115.
[ Links ]INGERMAN R, HOLCOMB M, ROBINSON M, CLOUD J. Carbon Dioxide and pH Affect Sperm Motility of White Sturgeon (Acipenser transmontanus). J Exp Biol. 2002;28852890.
[ Links ]KRASZNAI Z, MÁRIÁN T, BALKAY L, GÁSPÁR R. Potassium Chanels Regulate HypoOsmotic ShockInduced Motility of Common Carp (Cyprinus carpio) Sperm. Aquaculture. 1995;129:123128.
[ Links ]KRASZNAI Z, MÁRIÁN T, IZUMO H, DAMJANOVICH S, BALKAY L, LAJOS T. Morisawa Masaaki. Membrane Hiperpolarization Removes Inactivation of Ca2+ Channels, Leading to Ca Influx and Subsequent Initiation of Sperm Motility in the Common Carp. Proc Natl Acad Sci USA. 2000;97.
[ Links ]KRASZNAI Z, MASAAKI M, SACHIKO M, ZOÁRD TÍBOR K, LAJOS T. Role of Ion Channels and Membrane Potential in the Initiation of Carp Sperm Motility. Aquat Living Resour. 2003;16:445449.
[ Links ]LAHNSTEINER F, BERGER B, WEISMANN T, PATZNER R. Determination of Semen Quality of the Rainbow Trout, Oncorhynchus mykiss, by Sperm Motility, Seminal Plasma Parametres, and Spermatozoal Metabolism. Aquaculture. 1998;163:163181.
[ Links ]MANSOUR N, LAHNSTEINER F, PATZNER RA. The Spermatozoon of the African Catfish: Fine Structure, Motility, Viability and Its Behaviour in Seminal Vesicle Secretion. J Fish Biol. 2002;60:545560.
[ Links ]MÁRIÁN T, KRASZNAI Z, BALKAY L, BALÁZS M, EMRI M, BENE L. HypoOsmotic Shock Induces an OsmolalityDependent Permeabilization and Structural Changes in the Membrane of Carp Sperm. J Histochem Cytochem. 1993;41:291197.
[ Links ]MÁRIÁN T, KRASZNAI Z, BALKAY L, EMRI M, TRÓN L. Role of Extracellular and Intracellular pH in Carp Sperm Motility and Modifications by Hyperosmosis of Regulation of the Na+/H+ Exchanger. Cytometry. 1997;27:374382.
[ Links ]MOCHIDA K, KONDO T, MATSUBARA T, ADACHI S, YAMAUCHI K. A High Molecular Weight Glycoprotein in Seminal Plasma is a Sperm Immobilizing Factor in the Teleost Nile Tilapia, Oreochromis niloticus. Dev Growth Differ. 1999;41:619627.
[ Links ]MORISAWA M, SUZUKI K. Osmolality and Potassium Ion: Their Roles in Initiation of Sperm Motility in Teleosts. Science. 1980;210:11451147.
[ Links ]MORISAWA M, SUZUKI K, MORISAWA S. Effects of Potassium and Osmolality on Spermatozoan Motility of Salmonid Fishes. J Exp Biol. 1983;107:105113.
[ Links ]MÜLLER K, LABBÉ C, ZACHOWSKI A. Phospholipid Transverse Asymmetry in Trout Spermatozoa Plasma Membrane. Acta Biochim Biophys. 1994;192:2126.
[ Links ]OHTA H, SHINRIKI Y. Changes in Osmotic Pressure that Trigger the Initiation of Sperm Motility in the River Sculpin Cottus hangiongensis. Fish Physiol Biochem. 1998;18:2935.
[ Links ]OHTA H, UNUMA T, TSUJI M, YOSHIOKA M, KASHIWAGI M. Effects of Bicarbonate Ions and pH on Acquisition and Maintenance of Potential for Motility in Ayu, Plecoglossus altivelis Temminck et Schlegel (osmeridae), Spermatozoa. Aquac Res. 2001;32:385392.
[ Links ]SAS Institute. Statistical Analisis System. Introductory Grade for Personal Computers, Release Edition Bry, NicC. 2002.
[ Links ]SCOTT AP, BAYNES SM. Handling Storage of Samonid Spermatozoa. J Fish Biol. 1980;17:707739.
[ Links ]SHI J, KRISHNAMOORTHY G, YANG Y, HU L, CHATURVEDI N, HARILAL D. Mechanism of Magnesium Activation of CalciumActivated Potassium Channels. Nature. 2002;418:876880.
[ Links ]SORDAHL LA, SILVER BB. Pathological Accumulation of Calcium by Mitochondria: Modulation by Magnesium. Recent Adv Stud Cardiac Struct Metab. 1975;6:8593.
[ Links ]SUAREZ S, HO H. Hyperactivated Motility in Sperm. Reprod Dom Anim. 2003;38:119124.
[ Links ]TABARES CJ, MONTOYA AF, ARBOLEDA L, ECHEVERRI A, RESTREPO LF, OLIVERAANGEL M. Efecto de la pluviosidad y el brillo solar sobre la producción y características del semen en Brycon henni (Pisces: Characidae). Rev Biol Trop. 2006;54(1):179187.
[ Links ]VINES CA, YOSHIDA K, GRIFFIN FJ, PILLAI MC, MORISAWA M, YANAGIMACHI R. Motility Initiation in Herring Sperm is Regulated by Reverse SodiumCalcium Exchange. Proc Natl Acad Sci USA. 2002;99:20262031.
[ Links ]