Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Acta Biológica Colombiana
versão impressa ISSN 0120-548X
Acta biol.Colomb. v.13 n.1 Bogotá jan./abr. 2008
NOTES ON THE BIOLOGY AND ECOLOGY OF Sudanonautes floweri(DE MAN, 1901; CRUSTACEA: BRACHYURA: POTAMOIDEA: POTAMONAUTIDAE) IN RIVER OGBOMWEN, SOUTHERN NIGERIA
Notas de la biología y ecología de Sudanonautes floweri(De Man, 1901; Crustacea: Brachyura: Potamoidea: Potamonautidae) en el río Ogbomwen, sur de Nigeria
FRANCIS O. ARIMORO1, ENAKEME O. OROGUN1 1Department of Zoology, Delta State University, PMB 1, Abraka, Nigeria fransarimoro@yahoo.com
Submited 2 of October 2007, accepted 11 of December 2007, corrections 24 of January 2008.
ABSTRACT
Investigation into some aspects of the biology and ecology of the freshwater crab, Sudanonautes floweri(De Man, 1901) in River Ogbomwen, Edo State, southern, Nigeria was carried out between February and July 2006. The study revealed that the crab species were widespread and abundant in the river. Abundance in terms of number and biomass was more during the wet season with at a peak in the months of June and July. The crab grew allometrically attaining a maximum total length and weight of 11.5 cm and 65 g respectively. The condition factor ranged from 8.60-9.45. These values did not vary with size and sex of the crab but showed seasonal variations. Females of Sudanonautes floweriwere more abundant although not significantly different from the expected 1:1 ratio. There were some sexually matured females with stages III gonad development. Fecundity estimate ranged from 400 to 650 eggs. The gonadosomatic index varied between 14.97 and 24.11%. Feeding habits varied slightly with size with larger sized crabs feeding on more and varied food particles. Generally, Sudanonautes flowerifed predominantly on detritus, crustaceans, fish, algae, filaments, diatoms and sand grains.
Key words: Sudanonautes floweri, Condition factor, growth, gonadosomatic index, Fecundity, River Ogbomwen.
RESUMEN
Algunos aspectos de la biología y ecología del cangrejo de agua dulce Sudanonautes floweri(De Man, 1901) fueron estudiados en el río Ogbomwen, estado de Edo, al sur de Nigeria entre febrero y julio de 2006. Este estudio reveló que este cangrejo se encuentra en forma abundante y ampliamente distribuido en este río. Abundancia en términos de número y biomasa fue mayor en la estación lluviosa con un pico para los meses de junio y julio. El cangrejo tuvo un crecimiento alométrico alcanzando una longitud y peso máximos de 11,5 cm y 65 g respectivamente. El factor condicional varió de 8,6-9,45. Estos valores no variaron ni con el tamaño o el sexo del cangrejo pero mostraron variaciones estacionales. Las hembras de Sudanonautes flowerifueron más abundantes pero esto no fue significativo con respecto a la razón esperada de 1:1. Se encontraron algunas hembras sexualmente maduras con estado III de desarrollo gonadal. El estimado de fecundidad osciló entre 400 hasta 650 huevos. El índice gonadosomático varió entre 14,97 y 24,11%. Hábitos alimentarios variaron poco con el tamaño en donde cangrejos de mayor tamaño se alimentaron en forma más variada. En general Sudanonautes flowerise alimentaron de detritos, crustáceos, pescado, algas, filamentos, diatomeas y granos de arena.
Palabras clave: Sudanonautes floweri, factor condicional, crecimiento, índice gonadosomático, fecundidad, río Ogbomwen.
INTRODUCTION
Sudanonautes floweriis a common, brachyuran, freshwater crab that is widely distributed in Nigeria and Central Africa (Cumberlidge, 1999). Sudanonautes floweriare adapted to the freshwater by having lecitothrophic eggs, direct development (without larvae) and brood care (Gross and Kaus, 2005). Sudanonautes floweriare a strangely neglected component of the world's inland aquatic ecosystem (Cumberlidge, 1991). Despite their wide distribution throughout the tropical and warm temperate zones of the world and their great diversity, their role in the ecology of freshwater is very poorly understood. Sudanonautes floweriare distributed throughout humid tropical forest habitat, Nigeria, South Cameroun, Bioko, Central Africa Republic, Zaire, Congo and Gabon (Cumberlidge, 1999; Meye et al., 2003). Members of the group inhabit a variety of ecological niches ranging from high mountain streams, rivers, lakes, swamps, forest and dry savannah (Cumberlidge, 1999). They are also known to be vectors of parasitic disease, as the intermediate host of the flukeworm Paragonimus uterobilateralis and P. africanus that cause the disease paragonimiasis in humans (Sachs and Cumberlidge, 1991). Like their marine counterparts, Sudanonautes floweriexhibit heterochely; the state of one claw being larger than the other (Daniel, 2001). This occurs in both sexes and of the various African species examined, all have shown a tendency towards right-handedness but with a minority being left handed. The significance of this asymmetry may be related to sexual signaling and defense. The large claws of females may be an indication of reproductive vigour but equally they have an important role in defense. Females can develop up to three months towards the care of a brood, during which time the ability to defend their offspring may be highly adaptive (Daniel, 2001). Sudanonautes floweriare important prey items forming the dominant component of the diet of the otter and water mongoose (Purves et al., 1994) many bird species (Arkell, 1979) and fish species. The crabs are important detritivores, reducing the particles size of leaf litter and organic debris, presenting a source of nutrition to collector and filter feeding fauna, and enabling microbial activity (Hill et al., 1992). These crabs utilize energy from diverse tropic levels and contribute to energy and resource recycling within the river ecosystem (Hill et al., 1992). Elsewhere in Africa, they are valued as food source (Sachs and Cumberlidge, 1991; Cumberlidge and Clark, 1992; Meye et al., 2003). They can be collected using hand nets baited with ox-heart. Despite its importance in the river ecosystem, Sudanonautes flowerihas not been researched thoroughly, and the goal of this study is to report on its morphology, reproductive, growth characteristics and feeding habit.
MATERIALS AND METHODS
DESCRIPTION OF STUDY AREA
The study was carried out in the lower reaches of River Ogbomwen in South West of Egor Local Government Area, Edo state, Nigeria. River Ogbomwen lies between latitude 5º25 - 5º36'E and longitude 6º19 - 6º25'N (Figura 1). This River takes its source from "Egbaen" North East of Egor Local Government Area. Although the main source of the River (Egbaen) is dried, the River still flows continuously. This river flows into the Osse River (Ovia River) which in turn flows into the Benin River.
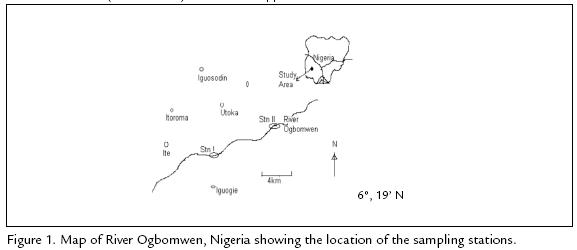
River Ogbomwen is principally fed by precipitation and surface runoff from the riparian communities. The climate of this area is characterized by the dry and wet season. The wet season extends from March to October with a short break during the month of August. The dry season occurs between October and March. During the wet season, the River experiences a rise in water level and flows more swiftly. The river substratum consists mainly of fine and coarse sand and also with pebbles. Decaying macrophytes debris also forms part of the substratum. The stream is affected by different human activities such as laundering, bathing, defecation, dumping of refuse, domestic waste etc.
STATION I.
The station is located along "Evbotubu" village. It has a substratum that consists of mainly silt, fine and coarse sand. Relevant human activities in this station are mainly subsistence farming, domestic washing and bathing. This station is well shaded with trees.
STATION II.
This station is located at about 5 km downstream of station I. It is located at "Ite" village. Major human activities include subsistence gaining, fishing and domestic washing. It has a substratum that consists of silt, fine and coarse sand. Vegetation is mainly grasses and shrubs.
MEASUREMENT OF SOME PHYSICOCHEMICAL PARAMETERS
The important physiochemical characteristics observed were pH, dissolved oxygen and flow velocity. pH was measured with the aid of a pH meter. Dissolved oxygen was measured titrimetrically by fixing with Winkler solution A and B and titrating with sodium thiosulphate (APHA 1985). Flow velocity was measured in the mid channel on three occasions by timing a float (average of three trials) as it moved over a distance of 10m.
COLLECTION OF CRABS
Crabs were collected from the two locations of the River Ogbomwen. The collection was made from the month of February to July 2006. The crabs were caught from the river using hand nets baited with ox-heart. Specimens collected in the field were preserved immediately in 10% formalin solution in the laboratory prior to analysis. The specimens were labeled according to sampling stations and date. Ovaries for studies were stored in small bottle containers with Gilson fluid for preservation.
IDENTIFICATION OF SPECIMENS
The striking taxonomic features of Sudanonautes floweriare the widened and highly arched carapace, and the lack of teeth on the anterolateral margins of the carapace. Also the small hand flap on the mandibular palp at the junction between the two segments and the conspicuous raised ridges on the sternum at the points where the chelipeds insert distinguish S. floweri from all other species of Sudanonautes which lack these features (Plate 1, 2, 3). Prof. Cumberlidge of Northern Michigan University confirmed the identification of the crab.
BIOMETRIC AND OTHER BIOLOGICAL DATA
In the laboratory, the biometric measurement taken for each specimen, were the carapace length (CL), carapace width (CW) and abdominal length (AL). They were measured on a board to the nearest 0.1 centimeter from the edge of the carapace back using a digital vernier calliper. The weight of the crab was determined using a top loading balance. Eviscerated weight was also measured. The carapace length/weight relationship was expressed by the regression equation
Log W = log a + b Log L
Were W = weight of the crab in gram, L=carapace length in cm, a=regression coefficient, b = regression constant.
The condition factor (k) of the crab was determined from the length and weight measured using the equation (Bagenal and Braun 1978).
K = 100W
L3
Where k = condition factor, L = carapace length, W =weight of the crab in grams.;
The male and female crabs were separated using the method described by Sachs and Cumberlidge (1991). Here the size of the abdomen distinguishes them. In males, it is triangular and inset into the underside. In females, it is broad and round and most obvious when the eggs are being carried. The gonad stages were observed and recorded. The maturation stage adopted were those used by Daniel (2001) with minor modification. The stages recorded are; 'Stage I, no gonad development; Stage II, partial gonad development; Stage III, gonad extending into carapace, orange in colour, testes formed with small stand; Stage IV, ripe carapace full of bright red gonad materials, testes are enlarged and thick'.
Fecundity estimates were obtained by weighing all the eggs from each specimen then the sub samples were weighed and the numbers of eggs there in were counted (Baganal and Braun 1978). The total numbers of eggs were calculated by direct proportionality. The gonad somatic index was calculated for each gonad weighed using the equation
GSI = Gonad Weight X 100 /Crab weight- Gonad weight
The stomach contents of the crabs were examined. The stomach lies on the anterior wall of the carapace. For each specimen, the cardiac stomach was removed and dissected. The stomach content was immediately transferred into a Petri dish, little water was added to it and viewed under a binocular microscope. The contents were identified as far as was practicable. It was analyzed using the numerical and occurrence method (Hyslops 1980). Visual estimate of gut fullness based on an index of 1-4, with 1 equal to 0 to 25% gut fullness and 4 equal to 75 to 100% gut fullness was assigned to the stomach of the crab, (Williams 1961). Gut contents were separated into four main categories; leaf detritus, algae, animal and inorganic materials using visual assessment. Numerical method was utilized. In this method, each food item in a grid was assigned a number 1-10 based on the percentage of the total area of the grid that the food type covered (Amundson et al. 1996). Another method, frequency of occurrence method was equally used. The numbers of stomachs that each food item occurred were recorded and this was expressed as the percentage of the total number of stomach examined.
RESULTS
SOME PHYSICOCHEMICAL PARAMETERS OF RIVER OGBOMWEN
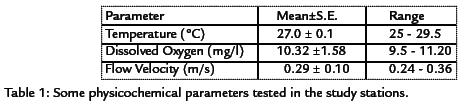
The amount of dissolved oxygen was in the range of 9.5 to 11.2 with a mean of 10.32 mg/L. Flow velocity in the study stations fluctuated between 0.25 and 0.36 ms-1 with a mean and standard error of 0.29 and 0.10 respectively (Table 1).
COMPOSITIONS, DISTRIBUTION AND ABUNDANCE OF Sudanonautes floweri
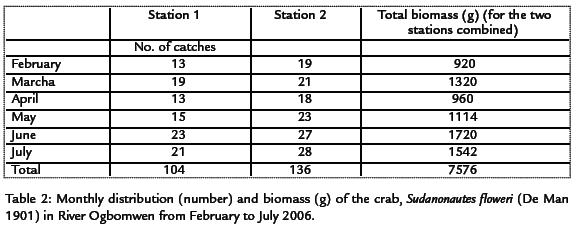
Sudanonautes floweri occurred throughout the months of February to July 2006 in River Ogbomwen. The males contributed 118 (49.2%) and females 122 (50.8%) of the total crabs caught. The monthly distribution of crabs and total biomass combined in the study stations is presented in Table 2. The total number of crabs caught fluctuated in the two stations in all the months. T-test analysis was used to compare the distribution of crab between the two sampling stations and it revealed that it was significant (p< 0.05) between the two sampling stations (T-calculated = 6.44, T-critical = 2.571 at 0.05 probability level.
CARAPACE LENGTH/ WIDTH FREQUENCY DISTRIBUTION
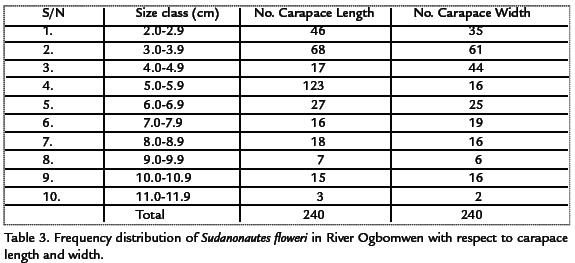
The carapace length frequency distribution of the crab Sudanonautes floweri is shown in Table 3. The carapace length ranged from 2.0 to 11.5 cm. In addition, a unimodal size distribution was indicated (3.0-3.9) with 68 individuals of the total population. The carapace-width frequency distribution of the crab, Sudanonautes floweri is also given in Table 3. The carapace width ranged from 2.0-11.5 cm. In addition, a unimodal-sized distribution was indicated (3.0-3.9) with 61 individuals of the total population. Only 3 individuals and 2 individuals recorded carapace length and width of above 11.0 cm respectively.
LENGTH - WEIGHT RELATIONSHIP
The weight of Sudanonautes floweri used for analyses ranged from 8 to 65 g. There was a linear relationship between the length and weight of the crabs. The log carapace length-log weight relationships is shown in Figura 2. The carapace length weight values for males, females and combined sexes are given below: Regression Equation:
For Males Log weight = log 592 + 3.71 log L (n=118, r = 0.54)
For Females Log weight = log 578.1 + 3.21 log L (n=122, r = 0.68)
For Combined Sexes Log weight = log 1170.1 + 5.641 log L (n=240, r = 0.71)

The values of regression coefficient "b" obtained for males, females and combined sexes were more than 3 which indicated that the crabs exhibited a positive, allometric growth. The correlation coefficient "r" was 0.71, showing a very high positive correlation between carapace length and total weight of the crab species.
CONDITION FACTOR (K)
The condition factor for MALES was higher in February and April than in other months, and was highest for FEMALES in February and May (Table 4). The values of the condition factor observed ranged between 15.2 and 45.6. It was also observed that there were variations in relation to size. The condition factor in relation to size and sex is given in Table 5. The condition factor showed significant difference with crab size. Crabs of size class 2.0-2.9 had the highest values than the others irrespective of sex. The crabs of size class 11.0-11.9 had the lowest condition factor values irrespective of sex.

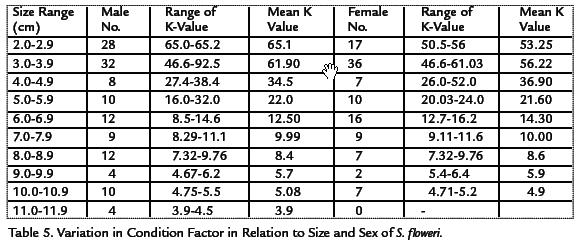
SEX RATIO
A total of 240 crabs were sampled out of which 118 were males and 122 were females giving a sex ratio of 1 male to 1.03 females. This was not significant (p>0.05). The females were predominant over the males in the months of February and April with sex ratio of 1:1.13 and 1:2.3. The monthly variation in sex ratio of Sudanonautes floweri is shown in Table 6. A chi-squared test value of 1.5148 at 0.05 level and 1df was obtained as compared with tabulated value of 3.841. This showed no significant difference between the males and females.
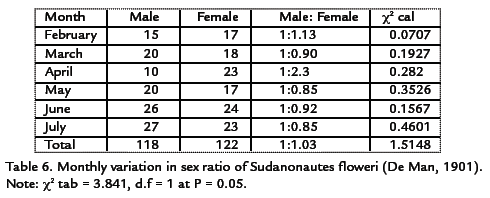
GONAD DEVELOPMENT
Specimens of Sudanonautes floweri observed in the months of February and March contained only gonad stage I and II (no gonad development and partial gonad development) respectively. Stages I and II were also observed in other months, stages III were observed in the months of May, June and July respectively. Sexual maturity was attained when the crabs were between 7.0-11.5 cm. Smallest male and female with stage V had carapace length of 2.0 and 3.5 cm respectively. The monthly occurrence of different stages of gonads in Sudanonautes floweri is shown in Table 7.
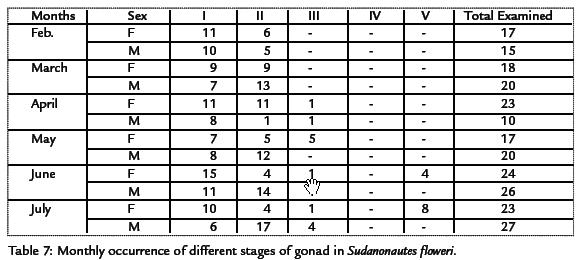
FECUNDITY
The fecundity estimate for Sudanonautes floweri is shown in Table 8. The carapace length of matured females of Sudanonautes floweri ranged from 7.0 to 11.5 cm with an average of 8.5 cm. The total body weight ranged from 10 to 65 g. The fecundity estimate ranged from 400 to 650 eggs. The egg weight ranged from 6.512 to 9.715 with an average of 7.779 g.

GONADOSOMATIC INDEX
Sudanonautes floweri (De Man, 1901) had a gonadosomatic index that varied from 14.97% (7.0 cm), 16.58% (10.7 cm), and 24.11% (11.0 cm) while the mean GSI was 18.55% (Table 9) The relationship of the gonad weight and body weight showed that as much as 18.55% of the weight of Sudanonautes floweri was put into the production of eggs.

FOOD AND FEEDING HABITS
Of the 240 crabs examined for stomach analysis, 60 had empty stomach. The frequency of empty stomach was also examined on monthly basis. February had the greatest number of empty stomach and this was 40.6%. The least number of empty stomachs was obtained in May with 2.7% of empty stomachs. The percentage of empty stomachs varied from month to month as shown in Table 10.
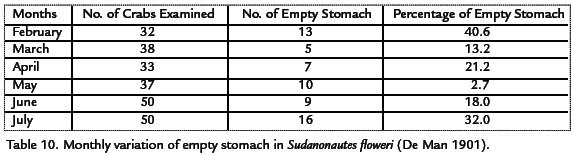
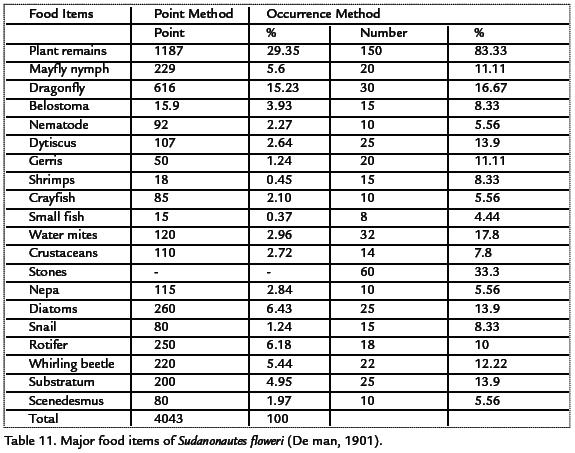
FOOD ITEMS OF Sudanonautes floweri (DE MAN, 1901)
One hundred and eighty specimens of the 240 of Sudanonautes floweri examined had food items in their stomachs. The species Sudanonautes floweri fed mainly on plants and other benthic macroinvertebrates such as dragon fly, mayfly nymph, Notonecta, Belostoma and also crustaceans among others. This is presented in Table 11. By point method, plant (detritus) was the most important consisting of 29.35%. By occurrence method, plant (detritus) contributed 83.33%. By point method, dragon fly contributed 15.23% while by occurrence method, dragon fly constituted (6.67%). All other macroinvertebrates also contributed significantly to the food items of Sudanonautes floweri.
DISCUSSION
The freshwater crab, Sudanonautes floweri was identified by the small hard flap on the mandibular palp at the junction between the two segments and the conspicuous raised ridges on the sternum at the point where the cheliped insert and these features distinguish Sudanonautes floweri from all other species of Sudanonautes (Bott 1955, Cumberlidge 1991). The results of this preliminary ecological study indicate that the freshwater crab, S. floweri showed a monthly fluctuation in abundance both in number and biomass of individuals. The highest number of crabs caught was recorded in June and July while the lowest number was recorded in February. Again, the highest biomass was recorded in June while the lowest biomass was recorded in February. Besides the changes in the total biomass, a seasonal or monthly fluctuation in the biomass of the different size classes was also reported. The different size classes showed variation in biomass in different months of the study period. A similar result was reported by Claridge et al. (1979), for the decapod, Cragon orangon, which showed differences in composition, biomass and density during the summer and winter, and for Potamonautes granularis by Daniel et al. (1998). The fluctuation in abundance in terms of number also revealed a seasonal trend with a peak towards the mid rainy season, which suggests that the rainy season changes in the habitat probably favoured the growth and development of the species hence the large number in the field at this period of the year. Similar monthly fluctuation in number or abundance has been reported for the species Sudanonautes africana by Okafor (1988) and for Sudanonautes orthostylis (Cumberlidge 1989). During the dry season months, most of the habitats will dry up and as such the samples are not easily collected at this period. The logarithmic plot of weight against carapace length indicated a linear relationship. This linear relationship indicates that an increase in carapace length led to increase in weight. The males, females and combined sexes of Sudanonautes floweri from River Ogbomwen exhibited a positive allometric growth because the regression coefficient "b" obtained was far greater than 3. The maximum length attained by S. floweri in this study was 11.5cm. There was a positive correlation between the length and weight for both sexes of the crabs. A similar relationship was reported for the crab Callinectes ornatus in Brazil (Mantellato and Fransozo 1999) and for Potamonautes species in South Africa by Gouws and Stewart (2001) and Daniel et al. (2003). The positive correlation between the length and weight reported here for S. floweri suggests that growth changes in these parameters are proportionally related. The condition factor (k) is used here to compare the condition or fatness or wellbeing of the individual of the species. The condition factor recorded for S. floweri in River Ogbomwen varied between 8.60 and 9.4. The k value varied seasonally with size of Sudanonautes floweri in River Ogbomwen. The condition factor in relation to sex does not show any significant difference. However, the male condition factor was higher. This agreed with what was reported by Warner (1977) who noted that in true crabs, the males were generally larger than females. The females were predominant over the males bringing an overall estimate of sex ratio of 1:1:03. The over all sex ratio was not significantly different from the expected 1:1 ratio. Meye et al. (2003) observed a male-female ratio of 1:1.2 of the fresh water crab, S. aubryi in Orogodo River, Delta state. Also, a similar sex ratio has been reported by Okafor (1988) for S. africanus. However, this result differs markedly from 4:1 ratio recorded by Ejike (1972) for S. africanus. This study reveals that sexually matured females and males are in the stage III of gonad development. The fecundity of S. floweri from River Ogbomwen range from 400 to 650 eggs with matured female of average carapace length and weight of 8.5 cm and 50 g respectively. Fecundity studies revealed that there was significant correlation between fecundity and length and also fecundity increases with increase in body weight. The Gonadosomatic Index (GSI) of S. floweri ranged from 14.97 to 24.11% with a mean of 18.55%. The GSI obtained indicated that 18.55% of its body weight is for egg production. The different variety of food items found in the individual stomach of S. floweri indicated that the crab was an omnivore and opportunistic feeder as the stomach content reveals the presence of detritus, Crustaceans, small fishes and other macroinvertebrates. Okafor (1988) in his observation found out that gut contents have demonstrated an essentially herbivorous and detritivorous diet among larger individuals whereas those with a carapace width less than 25mm although still largely herbivorous supplement their diet with aquatic invertebrates. There was no significant variation in comparison of food item in S. floweri in relation to size but small sized crabs fed on detritus mostly on small invertebrates while the larger crabs fed largely on detritus. This difference in diet may be related to the activity of the crabs: small and medium sized crabs actively pursue animal prey whereas larger ones presumably lacking adequate mobility have greater difficulty in capturing other animals.
CONCLUSION
From the results given on the general morphology, feeding habits, reproductive and growth characteristics of this crab, it is evident that this crab can be exploited domestically as potential species for aquaculture, both as food for humans and for formulating fish feed. In addition, studies of this nature are important in enabling us understand the biology of Sudanonautes species especially in tropical waters where they are endemic and where very little is known about their biology.
ACKNOWLEDGEMENTS
The authors are grateful to Prof. N. Cumberlidge of the Northern Michigan University for identifying the crab species collected for this study and the department of Zoology, Delta state University Abraka, Nigeria for the use of their laboratory space and assistance.
REFERENCES
AMUNDSON PA, GABLER HM, STALDVIK, FJ. (1996). A new approach to graphical analysis of feeding strategy from stomach contents data-medication of the Costello (1990) method. J Fish Biol. 48:607-614. [ Links ]
APHA - (AMERICAN PUBLIC HEALTH ASSOCIATION). Standard methods for the examination of water and wastewater. New York, APHA; 1985. [ Links ]
BAGENAL TB, BRAUN B. Egg and early life history. In T.B. Bagenal (ed). Methods of assessment of fish production in freshwater. Blackwell Sci Publ Oxford; 1978. p.165-201. [ Links ]
BOTT R. Die Suswassertbrabhan Von African (Crust, Decapoda), und thre stammesgeschchte. Anosles du muse royal du Congo B. Belge, Zoologies. Ser. 3.3 vol. 1 fase. 3;1955;295-306. [ Links ]
CLARIDGE PN, MOORE JW, MOORE IA. Seasonal changes in density, composition and reproductive biology of crustacean population in the server estuary. Crustaceana. 1979;36:113-121. [ Links ]
CUMBERLIDGE N. Redescription of Sudanonautes Orthostylis (Bott 1955), a freshwater crab from Nigeria, Cameroon and Ghana with notes on its ecology. Crustaceana. 1989;56(3):230-245. [ Links ]
CUMBERLIDGE N. The respiratory system of Globonautes macropus (Rathbun, 1898), a terrestrial freshwater crab from Liberia (Gecarcinucoidea, Gecarcinucidae). Cruscaceana. 1991;61:69-80. [ Links ]
CUMBERLIDGE N, CLARK PT. A new genus and species of freshwater crab from Cameroon, West Africa (Crustacea, Brachyma, Potamoidea, Potamonautidae). Bull Br Mus (Nat Hist.) Zool. 1992;58(2):149-156. [ Links ]
CUMBERLIDGE N, SACHS R. A key to the crabs of Liberian freshwater. Zeitschriftfur Angewandte Zoologie. 1989;76:221-229. [ Links ]
CUMBERLIDGE N. The freshwater crabs of West Africa. Family Potamonautidae. Paris, IRD. Collection; 1999. [ Links ]
DANIELS SR, STEWART BA, GILBBONS MJ. (1998). Potamonautes granuleris nov (Brachyura, Potamonautide). A new Cryptic species of river crab from the Olifants River system South Africa. Crustaceana. 1998;71(8):885-903. [ Links ]
DANIELS SR. Allometric growth, handedness, and morphological variation in potamonautes warreni (Calman, 1918) (Decapoda, Brachyura, Potamonautidae) with a redescription of the species. Crustaceana. 2001;74:237-253. [ Links ]
DANIELS SR, GOVWS G, STEWART BA, COKE M. Molecular and morphometric data demonstrate the presence of Cryptic lineages among freshwater crabs (Decapoda; Potamonautadae; Potamonautes) from the Drakensberg Mountains, South Africa. Biol J Human Soc. 2003;78:129-147. [ Links ]
EJIKE C. An analysis and sex of the amphibious freshwater Crab. Sudanonautes africanus (Crustacea, Decapoda). Nig J Scien. 1972;3:133-154. [ Links ]
GROSS M, KLAUS S. Upper Miocene freshwater crabs from the Northwestern margin of the Styrian Basin (Brachyura. Potamoidea). Ber Inst KF Univ Graz. 2005;10:21-23. [ Links ]
GOUWS G, STEWART BA. Potamonautid river crabs (Decapoda, Brachyura, Potamonautidae) of Kwa Zulu- Natal, South Africa. Water SA. 2001;27:85-98. [ Links ]
HILL MP, O KEEFE JH. Some aspects of the ecology of the freshwater crab (Potamonautes perlatus Milne Edwards) in the upper reaches of the Buffalo river, eastern cape province. South Africa. South Afri J Aqua Scien. 1992;18:42-50. [ Links ]
HYSLOP EJ. Stomach content analysis and a review of the methods and their application. J Fish Biol. 1980;17:411-429. [ Links ]
MANTELLATO FLM, FRANSOZO A. Reproductive biology and moulting cycle of the crab Callinectes ornatus (decapoda, portunidae) from the Ubatuba region, Sao Paulo, Brazil. Crustaceana. 1999;72(1):63-76. [ Links ]
MEYE JA, ARIMORO FO, EDOKPAYI CA. Observation on some aspects of the biology of S. aubryi. (H. Milne Edwards, 1886) in Orogodo river, Niger Delta, Nigeria. Trop Fresh Biol. 2003;12/13:105-118. [ Links ]
OKAFOR FC. The ecology of Sudanonautes africanus (H. Milne Edwards) (Crustacea: Decapoda) in Southeastern Nigeria. Tropical Ecol. 1988;29:89-97. [ Links ]
PURVES MG, KRUUK H, NEL JAJ. Crabs Potamonautes perlatus in the diet of Otter Aonyx capensis and water mongoose Atilax paludinosus in a freshwater habitat in South Africa. Zectschrift fur saugetier kunde. 1994;59:332-341. [ Links ]
SACHS R, CUMBERLIDGE N. The dwarf river crab Liberonautes (atidactylus manoides) Cumberlidge and Sachs, 1989, from Liberia-a new second intermediate host of Paragonimus uterobilateralis. Trop Med Parasitol. 1991;42:73-74. [ Links ]
WARNER GF. The Biology of crabs Paul Elek (Scientific Book). Ltd. London; 1977. [ Links ]
WILLIAMS TR. The diet of freshwater crabs associated with Similum neavei in East Africa. Crabs from West and East Uganda collected by the Cambridge East Africa expedition. 1959. Ann Trop Med Parasitol. 1961;55:128-131. [ Links ]














