Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Acta Biológica Colombiana
Print version ISSN 0120-548X
Acta biol.Colomb. vol.14 no.3 Bogotá Sep./Dec. 2009
STABILITY OF ANTHOCYANINS FROM Rubus glaucus AND Solanum betaceum Cav.dark-red strain AS AFFECTED BY TEMPERATURE, STORAGE AND WATER ACTIVITY
Efecto de la temperatura, almacenamiento y la actividad de agua sobre la estabilidad de antocianinas de Rubus glaucus y Solanum betaceum Cav.dark-red strain
C.M. OLAYA1, M.P. CASTAÑO2, G.A. GARZÓN1,*
1 Departamento de Química, Universidad Nacional de Colombia. A.A. 14490 Bogotá, Colombia.
2Frutar Ltda. Bogotá, Colombia.
*Person to whom correspondence should be addressed. Gloria Astrid Garzón, Departamento de Química, Universidad Nacional de Colombia. A.A. 14490 Bogotá, Colombia. Telephone (571) 316 50 00, ext. 14457. Fax (571) 316 52 20. agarzonmo@unal.edu.co
Received 5 September 2008, accepted 19 March 2009 and revised 27 July 2009.
ABSTRACT
The stability of sprayed-dried microencapsulated anthocyanins from Andes berry (Rubus glaucus) and Tamarillo (Solanum betaceum), as affected by storage time, water activity (Aw) and temperature was compared. The fruits were osmotically dehydrated with ethanol and the anthocyanin extract was microencapsulated with maltodextrin DE 20 by spray drying. Half life of the anthocyanins; changes in color, total phenolics, and antioxidant activity of the powders, were analyzed during storage at two different temperatures (25 °C and 40 °C) and two Aw levels (0.20 and 0.35). A decrease in monomeric anthocyanin was observed in both samples. The half life of the Andes berry pigments ranged between 11 and 32 days while the half life of the tamarillo pigments ranged between 9 and 21 days. A darkening effect occurred in both samples as a result of storage time. The antioxidant activity decreased while the phenolic content increased with time. Antioxidant activity of Andes berry samples was highly correlated with anthocyanin content and total phenolic content while the antioxidant activity of tamarillo samples was highly correlated with total phenolic content. These results would be useful in developing applications for spray-dried anthocyanins as powdered food-grade colorants.
Key words: Microencapsulation, anthocyanin, stability, antioxidant activity.
RESUMEN
Se comparó el efecto del tiempo de almacenamiento, la temperatura y la actividad de agua (Aw) sobre la estabilidad de antocianinas microencapsuladas de Mora de Castilla (Rubus glaucus) y tamarillo (Solanum betaceum). Las frutas se sometieron a deshidratación osmótica con etanol y el extracto antociánico se microencapsuló con maltodextrina ED 20 por atomización. La vida media de las antocianinas; los cambios en color, fenoles totales y actividad antioxidante se analizaron durante el almacenamiento a dos temperaturas (25 °C y 40 °C) y dos niveles de Aw (0,20 y 0,35). Se observó una disminución de la antocianina monomérica en las dos muestras. La vida media de los pigmentos de mora de Castilla varió entre 11 y 32 días, mientras que la vida media de los pigmentos del tamarillo varió entre 9 y 21 días. Hubo oscurecimiento de las muestras como resultado del tiempo de almacenamiento. La actividad antioxidante de las dos muestras dismi-nuyó, mientras que el contenido fenólico aumentó con el tiempo. La actividad antioxi-dante de las muestras de mora de Castilla presentó una alta correlación con los con-tenidos de antocianinas y fenoles totales mientras que la actividad antioxidante de las muestras de tamarillo se correlacionó con el contenido de fenoles totales. Estos resultados son útiles en el desarrollo de aplicaciones de antocianinas microencapsu-ladas como colorantes alimenticios.
Palabras clave: microencapsulación, antocianinas, estabilidad, actividad antioxidante.
INTRODUCTION
Color is an important quality factor when choosing a food product; for this reason, there is a high demand of colorants by the food industry. However, research has associated the consumption of synthetic food colorants with conditions such increasing levels of hyperactivity in children (McCann et ál., 2007). As a result, natural plant colorants have been in high demand for the past decade with the purpose of replacing synthetic dyes such as FD&C red 40 and the banned FD&C red No 2. (Fabre et ál.,1993).
Anthocyanins (ACNs) are highly colored plant pigments with high potential as natural colorants (Wrolstad, 2000). Besides the advantages ACNs present as food colorants, they are associated with health benefits such reduced risk of coronary heart disease, reduced risk of stroke, anticarcinogenic activity, anti-inflammatory effects, improved visual acuity, and improved cognitive behavior (Clifford, 2000; Prior, 2004). Nevertheless, limitations to the application of ACNs as natural colorants at a commercial scale are their low stability to processing, formulation and storage conditions (Cevallos-Casals and Cisneros-Zevallos, 2004) and the low availability of vegetable sources in seasonal locations. Concentration, temperature, pH, oxygen, enzymes, metal ions, ascorbic acid, and small changes in Aw contribute to destruction of the pigments (Mazza and Miniati, 1993; Garzón and Wrolstad, 2001). Microencapsulation of pigments with maltodextrins by spray drying is a technique currently investigated in the food industry with the aim to increase the shelf life of pigments (Dziezak, 1988; Dib Taxi et ál., 2003; Ersus and Yurdagel, 2007). This technique protects food pigments from destructive changes and allows controlled release of the substances under specific conditions.
Andes berry (Rubus glaucus Benth), and tamarillo (Solanum betaceum Cav. dark-red strain) are fruits native to high tropical areas such as the country of Colombia, where they are harvested throughout the year. Both fruits are promising sources of natural dyes and antioxidants due to their high ACN content. Cyanidin 3-rutinoside (Cy-3-rut) is the major ACN in Andes berry (Garzón, 2009) while delphinidin 3-rutinoside (Dp-3-rut) is the main ACN in tamarillo (Vera de Rosso and Mercadante, 2007).
During osmotic dehydration of Andes berry and tamarillo for production of healthy snacks, there is an important transfer of ACNs from the fruits to the osmotic solutions (Osorio et ál., 2007). As the solutions have been discarded so far, microencapsulation may be useful for utilization of this waste material and for obtaining ACNs as potential natural colorants. Accordingly, the main objective of this work was to find a potential added value for what currently constitutes a waste material. Andes berry and tamarillo ACNs were microencapsulated with maltodextrins by sprayed drying and their stability under two levels of water activity and temperature was compared. Further objectives of this work were to monitor changes in antioxidant activity and total phenolic content of the microencapsulated pigments and to determine the correlation between antioxidant activity and ACN concentration or phenolic content and during storage.
MATERIALS AND METHODS
REAGENTS
Sodium hydroxide, hydrochloric acid, sodium carbonate, calcium chloride, potassium persulfate, and Folin-Ciocalteau reagent were purchased from Merck® (Darmstadt, Germany). Trolox (6-hydroxy-2,5,7,8-tetramethychroman-2-carboxylic acid), 2,2 ;-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), and gallic acid were from Sigma-Aldrich (St. Louis, MO). Sodium metabisulphite was acquired from Carlo Erba® (Rodano, Italia) and corn maltodextrin with a dextrose equivalent (ED) of 20 was purchased from Corn Products, Brazil.
SOURCE OF ANTHOCYANIN PIGMENTS
Fresh Andes berries and tamarillo were acquired from the local market in Bogotá, Colombia. The fruits were characterized according to color, size, soluble solids content (°Brix) using a digital refractrometer Abbe II (Reichert-Jung, Leica Inc., Buffalo, N.Y., U.S.A.), and total titratable acidity (TA). TA was determined as g citric acid per 100 g fruit by titrating a sample of fruit extract with 0.1 N NaOH to a final pH of 8.2. Measurement of the pH was done with a Schott Gerate® pH meter, model CG820 (Mainz, Germany).
Monomeric ACN content was determined by the pH differential method (Giusti and Wrolstad, 2001) and expressed as mg of Cy-3-glu (molecular weight: 445.2 g/mol, ε: 26900) per 100 g of Andes berry and as mg of Dp-3-rut (molecular weight: 611.2g/mol, ε: 28800) per 100 g of tamarillo.
After characterization, the fruits were subjected to osmotic dehydration by mixing fruit and 96% ethanol in a 1:3 proportion in a sealed polyethylene to allow pigment extraction for 12 hours (Osorio et ál., 2007). Three consecutive cycles were applied by replacing the fruit.
PREPARATION OF MICROENCAPSULATED PIGMENT POWDERS BY SPRAY-DRYING
The ACNs obtained after ethanolic extractions were recovered and the ethanol was removed at 40 °C by using a Büchi rotoevaporator. Each pigment concentrate was mixed with corn maltodextrin ED 20 until reaching a 65 °Brix final solid content. The resultant slurries were spray-dried in a in a rotary spay dryer Mobile MinorTM (Niro Inc. Columbia, MD, USA) operated at 230 °C inlet temperature and 150 °C outlet temperature maintaining a feed flow of 10 mL/min. The moisture content of the microencapsulated pigments was determined on an Ohaus water were done on this stock solution.analyzer, model MB (New Jersey, USA) and the Aw was measured with a Novasina mJ1 water activity analyzer (Switzerland).
SCANNING ELECTRON MICROSCOPY (SEM)
The morphology of the microcapsules was evaluated by scanning electronic microscopy (SEM) using a QUANTA 200 FEI, (Ontario, Canada) electronic microscope. The microcapsules were attached to SEM stubs using a sputter Balzers SDC-050 and coated with gold-palladium (Plasma deposition method) and examined at 30 kV and 4 Torr.
STORAGE OF MICROENCAPSULATED PIGMENTS
Microencapsulated pigment (1.5 g) was evenly distributed on an open plastic Petri dish. To control Aw, the samples were placed on sealed desiccators containing saturated CH3COONa and CaCl2 solutions to obtain Aw levels of 0.20 and 0.35, respectively (Labuza, 1984). The desiccators were placed in separate incubators model HM-19 (Jaelsa, Madrid, Spain) at 25 ± 1 °C and 40 ± 1 °C for 35 days in the dark. The samples were allowed to equilibrate with the constant humidity environment before taking the first measurement. Afterwards, samples were retrieved at 7, 14, 21 and 35 days for analysis. At the same time, control samples were stored in close plastic Petri dishes at -14 °C in the dark.
COLOR EVALUATION
CIE L* a* b* values of the powders were measured directly in the Petri dishes using a Minolta CM 508I colorimeter (Osaka, Japan). The operating conditions were: reflectance mode, specular component included, illuminant D65 and a 10° observer angle. Hue angle (h0) was calculated as arc tan b*/a*, and chroma or color intensity (C*) as (a*2+ b*2)1/2.
CHANGES IN ANTHOCYANIN CONTENT, PHENOLIC CONTENT AND ANTIOXIDANT ACTIVITY
To determine changes of the microencapsulated samples, 500 mg of powder were resolubilized in 0.01% HCl, stirred for 10 min and taken to a 25-mL final volume. All analyses were done on this stock solution.
ACN content was determined as described. Determination of color density (A 420 nm -A 700 nm) + (A 520 nm-A 700 nm) and % polymeric color [(polymeric color/color density)] X 100 was done (Giusti and Wrolstad, 2001). Results are reported on a 100 g of microencapsulated pigment basis.
The antioxidant activity was measured by the Trolox Equivalent Antioxidant Capacity (TEAC) assay activity (Re et ál., 1999). Absorbance of the samples at 734 nm was compared to that one of Trolox standards and the results were expressed in terms of mmoles Trolox equivalents (TE)/100 g powder.
Total phenolic content was determined using the Folin-Ciocalteu method (Singleton and Rossi, 1965). Absorbance at 765 nm of the samples and standards was determined. Results are expressed as mg gallic acid equivalents (GAE) /100 g powder.
STATISTICAL ANALYSIS
The experiment was carried out in duplicate. Multifactor analysis of variance (ANOVA)was applied for each microencapsulated pigment. Significant (P<0.05) differencesbetween means were identified using the least significant difference procedure (LSD). Linear regression analysis was applied to determine adequacy of the model describingkinetics of ACN degradation. Rate constants were calculated from slopes of the linesplotted, and half lives (t1/2) were calculated from the equation: t1/2= Ln 0.5/k, where k= rate constant.
Significant correlations between antioxidant activity and ACN concentration or totalphenolic content were calculated by simple linear regression analysis. All analyses wereperformed with Statgraphics plus, version 2.1.
RESULTS
CHARACTERISTICS OF THE FRUIT
The compositional data of the fresh fruit is reported in table 1. Fresh Andes berry hada monomeric ACN content of 14.7 ± 0.89 mg/100 g fresh fruit, 15.2 % ± 0.02 solublesolids and a pH of 4.59 ± 0.01. Monomeric ACN content of tamarillo was 7.60 ± 1.23mg/100 g fresh fruit. This fruit had 9.82 % ± 0.02 soluble solids and a pH of 3.88 ± 0.01.
CHARACTERISTICS OF SPRAY DRIED MICROCAPSULES
The Andes berry spray dried ACNs had an average moisture content of 3.99 ± 0.43, aAw level of 0.35 ± 0.29 while the average moisture content and Aw of the tamarillopowder were 4.73 ± 0.27 and 0.45 ± 0.009 respectively.
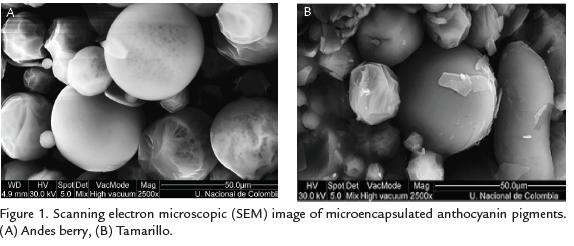
SEM microphotographs of Andes berry powder are presented in Figure. 1A. The microcap-sules showed spherical shapes with diameters ranging between 3.3 µm a 23.3 µm. Someof the particles exhibited smooth surface, while others presented either porous surface ofsurface dents. As for the tamarillo samples (Fig. 1B), the microcapsule diameter rangedbetween 2.5 µm a 30.2 µm and the surface was either smooth or presented surface dents.The ACN content, total phenolic content and antioxidant activity of microcapsulesimmediately after spray drying are shown in table 2. The average ACN in Andes berrypowder was 51.2 mg/100 g; this yield corresponded to approximately 3 times theconcentration of total ACNs in fresh fruit. The ACN yield for tamarillo powder was 39.4mg/100 g, which represents about 5 times the concentration of total ACNs in fresh fruit.The results indicated that the total phenolic content of Andes berry microcapsules (1271mg GAE/100 g) was about two-fold higher than the phenolic content in tamarillo microcapsules (602 mg GAE /100 g). The average antioxidant activity of Andes berrypowders (6.81 mmoles TE/100 g) was close to the average antioxidant activity of tamarillosamples (6.09 mmoles TE/100 g)..
CHANGES IN ANTHOCYANINS DURING STORAGE
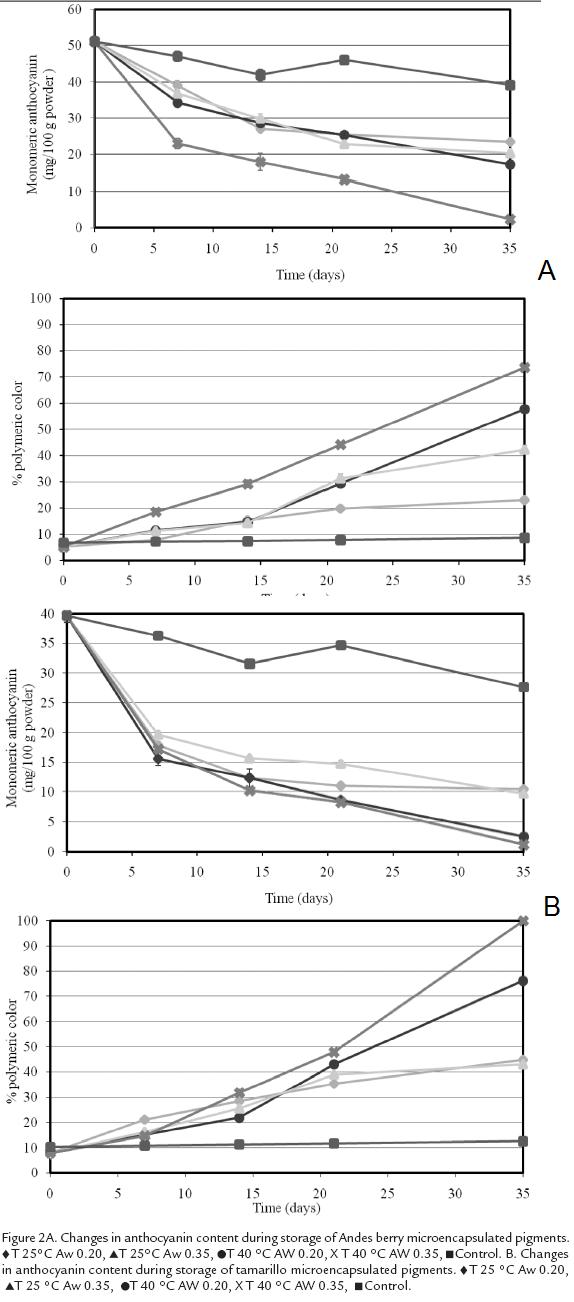
Figures 2A and 2Bshow changes in pigment concentration in Andes berry and tamarillomicrocapsules during storage. Temperature, storage time and Aw significantly affectedthe monomeric ACN content in Andes berry samples while only storage time andtemperature affected the monomeric ACN content in tamarillo samples (P<0.00001).At 25 °C Andes berry samples showed a decrease of 54 % at 0.20 Aw and of 60 % at 0.35Aw. The samples stored at 40 °C showed reductions of 66 % and 96 % for Aw levels of0.20 and 0.35, respectively. In contrast, the decrease in the control sample was of 24 %.The tamarillo systems stored at 25 °C and 0.20 Aw showed a decrease of 74 % in themonomeric pigment while the samples stored at 25 °C and 0.35 Aw showed a reductionof 76 %. At 40 °C the decrease in monomeric pigment was of 94 % and 97 % at Aw levelsof 0.20 and 0.35, respectively. The losses in the control samples were of 30.2%.
The degradation of the Andes berry and tamarillo pigments followed first-order kinetics,as confirmed by linear regression analysis (p-values<0.00001). As shown in table 3, Andesberry samples were significantly more stable than tamarillo samples (p-values< 0.05) atall storage conditions. The half life (t1/2) of the Andes berry samples was significantlyhigher than the t1/2of the tamarillo samples (p<0.05), and ranged between 11 and 32 days while the t1/2of the tamarillo microencapsulated samples ranged from 9 to 21. Due to thelittle degradation of the control samples stored at -14 °C, the correlation coeffi-cientsfound by linear regression were lower than those for the treatment samples. The estimatedt1/2of the controls was of 99 days and 77 days for Andes berry and tamarillo, respectively.
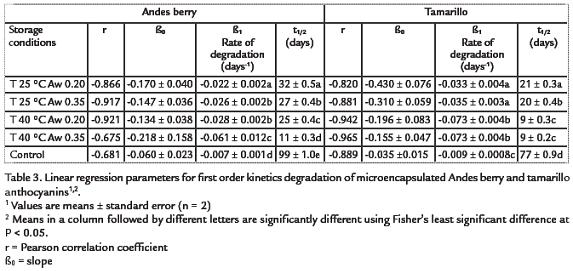
The degree of polymerization in the Andes berry powders was affected by storage time,temperature, and Aw (p<0.0001; Fig. 4A) with the samples stored at 40 °C and 0.35 Awhaving the highest polymeric color levels and the samples stored at 25 °C and 0.20 Awhaving the lowest. Similar trends of polymer formation were obtained with tamarillopowders, but these were significantly affected by storage time and temperature only (p < 0.0001). Control powders stored at -14 °C did not show significant increase in polymeric color.
CHANGES IN COLOR DURING STORAGE
Figure 3and Fig. 4 show variations in color parameters during storage of the microcapsules. Andes berry samples presented lower lightness (L*=45.7 ± 1.34) at the beginning of the storage than tamarillo samples (L*=58.7 ± 0.03). Storage time caused a significant decrease in L* value in powders from both fruits (P< 0.00001), which indicates darkening of the samples. There was a decrease of 18-23 units for Andes berry samples as compared to a decrease of 33-38 units for tamarillo samples. Aw level also had a significant effect on L* value of Andes berry pigments (p = 0.01). Samples stored at -14 °C did not present a significant change in L* value.
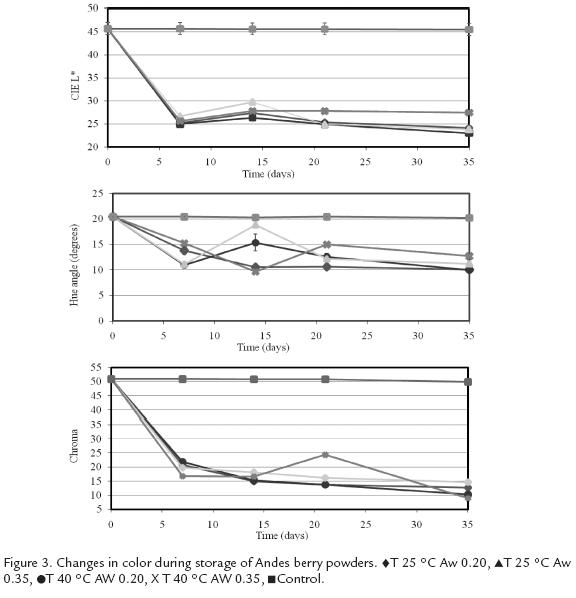
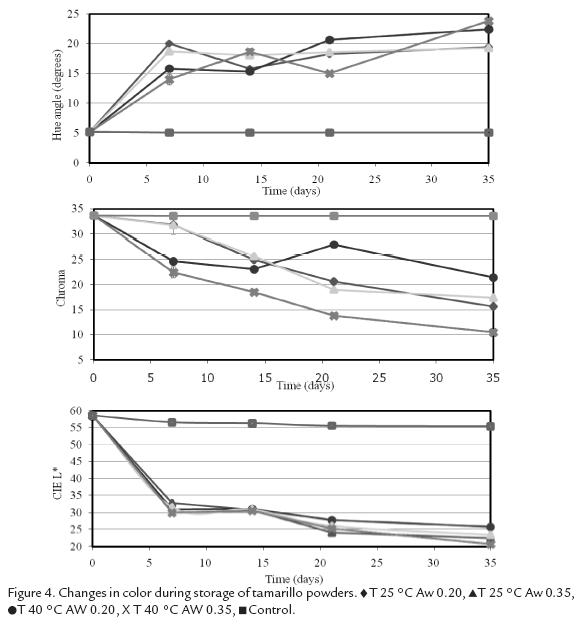
Microencapsulated Andes berry pigments showed higher hue angle (20.4° ± 0.05) than tamarillo powders (5.2° ± 0.10). Although a* and b* values showed a significant change in response to storage time for both pigments (p<0.0001), Andes berry samples showed a decrease in these parameters while tamarillo samples showed a decrease in a* value and an increase in and b* value. Therefore, the hue of Andes berry samples decreased indicating an overall shift towards red while the hue of tamarillo samples increased causing a shift towards orange red.
The initial color intensity of the Andes berry samples was higher (C* = 51 ± 0.50) than the tamarillo samples (C* = 34 ± 0.01). This color parameter was significantly affected by storage time (p < 0.00001) and Aw (p = 0.0001) in Andes berry powders and by storage time (p < 0.00001), temperature (p = 0.0009), and Aw (p = 0.029) in tamarillo systems. However, tamarillo microcapsules showed the lowest reduction in chroma during storage at all conditions. A maximum reduction of 23 units in chroma was observed at 40 °C and 0.35 Aw while Andes berry samples presented a maximum reduction of 42 units under the same conditions.
Low temperatures greatly improved color stability of the control samples, which did not present a significant change in any of the monitored color parameters.
CHANGES IN ANTIOXIDANT ACTIVITY AND PHENOLIC CONTENT
The antioxidant activity of the powders decreased significantly during storage (P< 0.05) (Table 4). The activity of tamarillo samples was affected only by storage time (p< 0.0001) and decreased between 63% and 70% with the higher decrease being for the samples stored at 40 °C and 0.35 Aw. The antioxidant activity of Andes berry samples decreased due to storage time (p < 0.0001) and Aw (p = 0.0314). The decrease ranged between 72 % and 85 % with the maximum decrease being for the samples stored at 25 °C and 0.20 Aw.
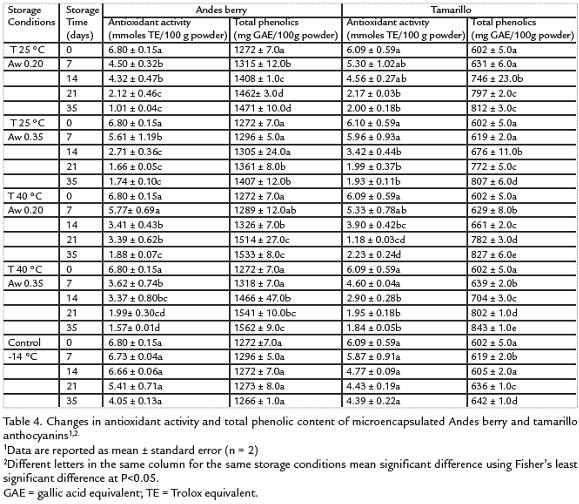
Microencapsulated Andes berry pigments showed higher total phenolic content (1272 ±7.0 mg GAE/100 g) than tamarillo (602 ± 5.0 mg GAE/100 g). Storage time caused asignificant increase on the total phenolic content in Andes berry systems (p = 0.027) andtamarillo systems (p<0.00001). Such change was more pronounced in the tamarillosystems which increased 35 % when stored at 25 °C and 0.20 Aw and a 34 % when storedat 25 °C and 0.35 Aw. At 40 °C the increase was 38 % when Aw was 0.20 Aw while theincrease was 40 % when the Aw was 0.35. Microencapsulated Andes berry pigments wereadditionally affected by temperature (p = 0.022); systems stored at 25 °C showed anincrease of 17 % at 0.20 Aw and of 12 % at 0.35 Aw. At 40 °C the increase was of 22 % at0.20 Aw and of 23 % at 0.35 Aw
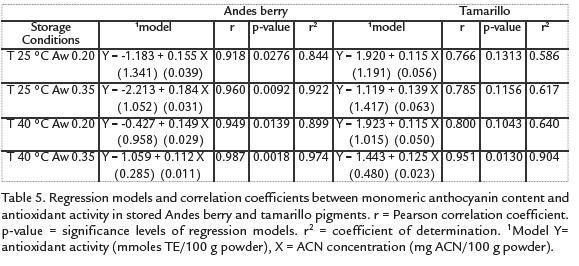
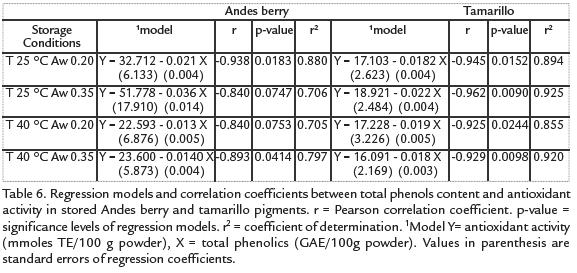
CORRELATIONS BETWEEN ACNS OR TOTAL PHENOLICS AND ANTIOXIDANT ACTIVITY
The relationship between antioxidant activity and total monomeric ACN or total phenoliccontent of the powders is described by the correlation parameters found by regressionanalysis (table 5and Tabla 6). There was a significant linear relationship between ACNconcentration (P<0.05), or total phenolic content (P<0.1), and antioxidant activity forthe Andes berry samples. The association between total phenolic content and antioxidantactivity was higher for the tamarillo samples than the one for Andes berry samples.
DISCUSSION
The monomeric ACN content of Andes berry was higher than the value reported by otherauthors (Osorio et ál., 2007), who found a concentration of 6.28 ± 0.01 mg of ACN/100g fresh fruit. Our experimental value is comparable to that one reported previously(Pantelidis et ál., 2007) for other four blackberry cultivars (Rubus fructicosus; 14.5-17.5 mgof ACNs per 100 g fresh fruit). Regarding tamarillo, the ACN experimental value is inaccordance with previous reports (Osorio et ál., 2007) of 7.41 ± 4.05 mg/100 g fresh fruit.
SEM analysis showed that both the Andes berry and tamarillo particles were among the size range for microcapsules (0.2 µm-5000 µm; King, 1995). The presence of surface dents can be attributed to the shrinkage at the cooling process during high drying rates which results in solidification of the wall prior to expansion of the microcapsules (Sheu and Rosenberg, 1995). This characteristic has also been related to wall materials with high proportions of carbohydrates such maltodextrins (Sheu and Rosenberg, 1995) while particle porosity has been explained as the result of fast evaporation of solvent during drying (Freitas et ál., 2004).
The total phenolic content of Andes berry powders is close to the one reported for evergreen blackberry seeds (1460 mg GAE/100g) while the content for tamarillo powder is lower than the one found for whole evergreen blackberries (822 mg GAE/100g) (Siriwoharn and Wrolstad, 2004).
Although the monomeric ACN and total phenolic contents of Andes berry were higher than the one for tamarillo, the antioxidant activity of the powders obtained from both fruits was comparable. Fruits are identified as rich sources of antioxidants including polyphenols, carotenoids, and vitamins (McCarty, 2004). Therefore, components other than ACNs and other phenolics must be contributing to the antioxidant activity of tamarillo powders. Comparison of our results with the antioxidant activity of several fruits showed that the obtained powders have similar values to those reported for red grapes (6.84 mmoles TE/100 g fw) and higher values than those for blueberry (3.95 mmol TE/100 g fw; Netzel, 2007).
Regarding changes in the microencapsulated ACNs during storage, other researchers observed similar kinetics during storage of microencapsulated purple carrot ACNs. Such pigments losses were of 30% at 25 °C and of 13 % at 4 °C after a storage period of 64 days (Ersus and Yurdagel, 2007). We hypothesize that one factor responsible for the higher stability of Andes berry powders is the higher ACN initial concentration. In experiments fortifying the ACN concentration in strawberry juice and concentrate with different pelargonidin derivatives, other authors confirmed that concentration of monomeric pigment increased stability regardless of the derivative (Garzón and Wrolstad, 2002). A shift in hue value represented the main changes observed in color. Decreases in this parameter have been observed during storage of processed cherries, grapes and berries (Garcia-Alonso et ál., 2003) while increases due to breakdown of ACNs have been reported during storage of strawberry juice and concentrate (Garzón and Wrolstad, 2002).
Although a decrease in antioxidant activity probably due to degradation of ACNs and vitamins was observed, there was an increase in total phenolic content. Such behavior has been reported for stored fruit products as the result of the compounds generated during oxidation that can react with the Folin Cioucalteau reagent (Klimczak et ál., 2007) and the result of hydrolytic reactions (Hatzidimitriou et ál., 2007; Zafrilla et ál., 2001). The significant linear relationship between ACN concentration (P < 0.05), or total phenolic content (P < 0.1), and antioxidant activity for the Andes berry samples suggest that both ACN content and phenolic content are good predictors of antioxidant activity of these microencapsulated pigments. This correlation between fruit ACNs and antioxidant activity has been observed by other researchers (Moyer et ál., 2002; Chaovanalikit and Wrolstad, 2004; Chaovanalikit et ál., 2004). The high association between total phenolic content and antioxidant activity of the tamarillo samples along with their lower and non-significant correlation coefficients between ACN concentration and antioxidant activity of tamarillo powders confirm our conclusion about components other than ACNs contributing to the antioxidant activity of the tamarillo microencapsulated pigments.
CONCLUSIONS
Microencapsulation of Andes berry and tamarillo anthocyanins by spray drying proved to be a useful technique for obtaining potential natural food colorants from waste material of fruit osmodehydration. Storage under 40 °C and 0.35 Aw caused the highest degradation rate in both systems under study. However, anthocyanins from Andes berry were more stable than anthocyanins from tamarillo. The antioxidant activity of the microencapsulated pigments was affected during storage and the decrease in the activity was higher for the Andes berry pigments. In these samples the antioxidant activity was highly related to both the ACN content and the total phenolic content while the antioxidant activity of the tamarillo powders was highly related to the phenolic content, which increased during storage. The color characteristics (hue angle shift towards red) and higher ACN stability of Andes berry powders during storage suggest that these microencapsulated ACNs have better potential as natural replacers of red artificial colorants as compared to tamarillo powders. These results would be useful in developing applications for spray-dried anthocyanin extracts as powdered food-grade colorants.
ACKNOWLEDGMENTS
Financial support from Colciencias is acknowledged. We thank Martha Quicazan from The Instituto de Ciencia y Tecnología de Alimentos ICTA, Universidad Nacional de Colombia, for kindly lending the Minolta colorimeter.
REFERENCES
CEVALLOS-CASALS B, CISNEROS-ZEVALLOS L. Stability of anthocyanin-based aqueous extracts of Andean purple corn and red-fleshed sweet potato compared to synthetic and natural colorants. Food Chem. 2004;86(1):69-77.
[ Links ]CHAOVANALIKIT A, WROLSTAD RE. Total anthocyanins and total phenolics of fresh and processed cherries and their antioxidant properties. J Food Sci. 2004;69(1):FCT67-FCT72.
[ Links ]CHAOVANALIKIT A, THOMPSON MM, WROLSTAD RE. Characterization and quantification of anthocyanins and polyphenolics in blue honeysuckle (Lonicera caerulera L). J Agric Food Chem. 2004;52(4):848-852.
[ Links ]CLIFFORD MN. Anthocyanins. Nature, occurrence and dietary burden. J Sci Food Agric. 2000;80:1063-72.
[ Links ]DIB TAXI C, DE MENEZES H, SANTOS A, GROSSO C. Study of the microencapsulation of camu-camu (Myrciaria dubia) juice. J microencapsulation. 2003;20:443-48.
[ Links ]DZIEZAK J. Microencapsulation and encapsulated ingredients. Food Technol. 1988;42:13-151.
[ Links ]ERSUS S, YURDAGEL U. Microencapsulation of anthocyanin pigments of black carrot (Daucuscarota L.) by spray drier. J Food Eng. 2007;80:805-812.
[ Links ]FABRE CE, SANTERRE AL, LORET MO, BABERIAN R, PAREILLEUX A, et ál. Production and food applications of the red pigments of Monascus rubber. J Food Sci. 1993;58:1099-111.
[ Links ]FREITAS S, MERKLE PH, GANDER B. Ultrasonic atomisation into reduced pressure atmosphere-envisaging aseptic spray-drying for microencapsulation. J Control Release. 2004;(95):185-195.
[ Links ]GARCIA-ALONSO FJ, PERIAGO ML, VIDAL-GUEVARA E, CANTOS E, ROS G, FERRERES F et ál. Assessment of the antioxidant properties during storage of a dessert made from grape, cherry, and berries. J Food Sci. 2003;68(4):1525-1530.
[ Links ]GARZÓN GA, RIEDL KH, SCHWARTZ SI. Determination of anthocyanins, total phenolic content, and antioxidant activity in Andes berry (Rubus glaucus Benth). J Food Sci. 2009;74(3):c227-c232
[ Links ]GARZÓN GA, WROLSTAD RE. Comparison of the stability of pelargonidin-based anthocyanins in strawberry juice and concentrate. J Food Sci. 2002;67(4):1288-1299.
[ Links ]GARZÓN GA, WROLSTAD RE. The stability of pelargonidin-based anthocyanins at varying water activity. Food Chem. 2001;75:185-96.
[ Links ]GIUSTI M, WROLSTAD RE. Characterization and measurement of anthocyanins by UV-visible spectroscopy. In: Handbook of food analytical chemistry. New Jersey: John Wiley & Sons; 2001. p. 19-31.
[ Links ]HATZIDIMITRIOU E, NENADIS N, TSIMIDOU M. Changes in the catechin and epicatechin content of grape seeds on storage under different water activity (aw) conditions. Food Chem. 2007;107(4):1504-1511.
[ Links ]KING A. Encapsulation of food ingredients. In: Encapsulation and controlled release of food ingredients. Am Chem Soc.Washington, DC (USA): American Chemical Society; 1995. p. 26-39
[ Links ]KLIMCZAK I, MALECKA M, SZLACHTA M, GLISZCZYNSKA-SWIGLO A. Effect of storage on the content of polyphenols, vitamin C and the antioxidant activity of orange juices. J Food Compos Anal. 2007;Vol 313-322.
[ Links ]LABUZA TP. Moisture sorption: Practical aspects of isotherm measurement and use. St. Paul, Minnesota: American Association of Cereal Chemists; 1984.
[ Links ]MAZZA G, MINIATI E. Anthocyanins in Fruits, Vegetables and Grains. Boca Raton, FL: CRC Press, Inc; 1993.
[ Links ]MCCANN D , BARRETT A, COOPER A, CRUMPLER D, DALEN L, GRIMSHAW K, et ál. Food additives and hyperactive behaviour in 3-year-old and 8/9-year-old children in the community: a randomised, double-blinded, placebo-controlled trial. Lancet. 2007;370(9598):1560-1567.
[ Links ]MCCARTY MF. Proposal for a dietary phytochemical index. Med Hypotheses. 2004;63:813-817.
[ Links ]MOYER RA, HUMMER KE, FINN CE, FREI B, WROLSTAD RE. Anthocyanins, phenolics and antioxidant capacity in diverse small fruits: Vaccinium Rubus and Ribes. J Agric Food Chem. 2002;50(3):519-525.
[ Links ]NETZEL MA, NETZEL G, TIAN Q, SCHWARTZ S, KONCZAK I. Native Australian fruits-a novel source of antioxidants for food. Innov Food Sci Emerg Tecnol. 2007;8:339-346.
[ Links ]OSORIO C, FRANCO MS, CASTAÑO MP, GONZÁLEZ-MIRET ML, HEREDIA FJ, MORALES AL. Colour and flavor changes during osmotic dehydration of fruits. Innov Food Sci Emerg Tecnol. 2007;8:353-59.
[ Links ]PANTELIDIS GE, VASILAKAKIS M, MANGANARIS GA, DIAMANTIDIS GR. Antioxidant capacity, phenol, anthocyanin and ascorbic acid contents in raspberries, blackberries, red currants, gooseberries and Cornelian cherries. Food Chem. 2007;102:777-783.
[ Links ]PRIOR RL. Absorption and metabolism of anthocyanins: Potential health effects. In: Phytochemicals: Mechanisms of action. Boca Raton, Fla.: CRC Press, Inc; 2004. p. 1-20.
[ Links ]RE R, PELLEGRINI N, PROTEGGENTE A, PANNALA A, YANG M, RICE-EVANS C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol Med. 1999;26:1231-123.
[ Links ]SHEU TY, ROSENBERG M. Microencapsulation by spray drying ethyl caprylate in whey protein and carbohydrate wall systems. J Food Sci. 1995;60:98-103.
[ Links ]SINGLETON VL, ROSSI JA. Colorimety of total phenolics with phosphomolybdic -phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144-158.
[ Links ]SIRIWOHARN T, WROLSTAD RE. Polyphenolic Composition of Marion and Evergreen Blackberries. J Food Sci. 2004;69( 4):FCT233-FCT240.
[ Links ]VERA DE ROSSO V, MERCADANTE AZ. HPLC-PDA-MS/MS of anthocyanins and carotenoids from Doyalis and Tamarillo fruits. J Agric Food Chem. 2007;55 (22):9135-9141.
[ Links ]WROLSTAD RE. Anthocyanins. In: Lauro GJ, Francis FJ, editors. Natural food colorants. New York, N.Y.: Marcel Dekker, Inc; 2000. p. 237-252.
[ Links ]ZAFRILLA P, FERRERES F, TOMÁS-BARBERÁN FA. Effect of processing and storage on the antioxidant Ellagic acid derivates and flavonoids of red raspberry (Rubus idaeus) jams. J Agric Food Chem. 2001;49(8):3651-3655.
[ Links ]













