Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Acta Biológica Colombiana
Print version ISSN 0120-548X
Acta biol.Colomb. vol.14 suppl.1 Bogotá Dec. 2009
SOME EVOLUTIONARY TENDENCIES OF NEOTROPICAL PRIMATES
Algunas tendencias evolutivas de los primates neotropicales
THOMAS R. DEFLER1, Ph. D. 1Profesor Asociado, Departamento de Biología. Universidad Nacional de Colombia. Bogotá, Colombia.thomasdefler@gmail.com
Presentado 11 de noviembre de 2009, aceptado 28 de febrero de 2010, correcciones 14 de mayo de 2010.
ABSTRACT
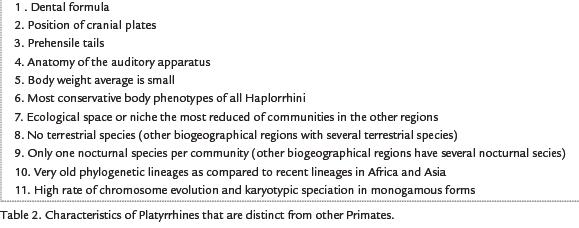
The evolution of neotropical primates has occurred isolated from other primates of the world, resulting in a distinct evolutionary history. Various characteristics of neotropical primates (Platyrrhini) are quite distinct from those of the Old World (Catarrhini), including the dental formula, the position of cranial plates, the anatomy of the auditory apparatus, much less average body weights, much less terrestrial adaptation, prehensile tails for some, and conservative phenotypes. Additionally monogamous forms of platyrrhini share a tendency for rapid chromosome evolution with one monogamous group of catarrhines (Hylobatids or gibbons). The phyletic history of the platyrrhine monkeys seems to contrast with that of the catarrhine inasmuch as there was a very early division (Miocene) of the New World monkeys into groups that exist today, whereas the appearance of Old World primate family groups seemed to have occurred much more recently in the Plio-Pleistocene. Some of these tendencies can be explained hypothetically, looking at ecological characteristics suggested for the new continent while other tendencies are perhaps the result of random evolutionary pathways taken during the course of evolution such as genetic drift and founder effect. Nevertheless there is still much work to be done to be able to recognize the singularities of the Platyrrines and to appreciate the details of their evolution.
Key words: primate evolution, platyrrhine evolution, neotropical primate evolution.
RESUMEN
La evolución de los primates neotropicales ha transcurrido aislada o de forma independiente a la de otros primates del mundo, porque poseen una historia evolutiva diferente. Hay varias características de los primates neotropicales (Platirrinos) que son bien distintas a las del viejo mundo (Catarrinos), incluyendo la fórmula dental, el arreglo de las placas craneales, la anatomía del aparato auditivo, pesos corporales menores, una menor adaptación a comportamientos terrestres, algunos poseen colas prensiles y baja diferenciación fenotípica. Formas monógamas de platirrinos comparten una tendencia de evolución cromosómica rápida con un grupo monógamo de Catarrinos (los Hilobátidos o gibones). La historia filogenética de platirrinos, contrasta con la de catarrinos debido a una división filética antigua (mioceno) de los primates del nuevo mundo en dos grupos, con características filogenéticas expresadas en las especies actuales. En contraste, la diferenciación de catarrinos con características que se pueden identificar en especies actuales no sucedió sino hasta el Plio-Pleistoceno. Algunas de estas tendencias, pueden ser explicadas hipotéticamente teniendo en cuenta las características ecológicas planteadas en el nuevo continente; otras tendencias tal vez son el resultado de caminos evolutivos tomados al azar durante la evolución del grupo o, como resultado tanto de deriva genética como de un efecto fundador. Sin embargo, queda mucho trabajo para reconocer la totalidad de las singularidades de los platirrinos y poder apreciar los detalles de su evolución.
Palabras clave: evolución de primates, evolución platirrinos, evolución primates neotropicales.
The neotropical (Platyrrhini), primates like the rest of the neotropical fauna evolved in isolation from the rest of the anthropoid primates (Catarrhini) for millions of years. This evolutionary development had few connections to other continents, except for an Antarctic link that lasted until its rupture by Drake’s passage around 30 mya (Lagabrielle et al., 2009). The isolated history of Platyrrhini primates and other neotropical autochthonous fauna resulted in the development of unique characteristics when compared to fauna in the rest of the world.
But, the primates of South and Central America did not have their origin in the Americas. Anatomical and fossil studies demonstrate a relationship to African primates, and we now appreciate that other autochthonous primate faunas developed in North America-Europe, Europe, Africa, Asia, and Madagascar (Martin, 1990; Fleagle, 1999; Houle, 1999).
Many anatomical characteristics of neotropical primates suggest that their origins come from an ancient African fauna: El Fayum, located in the now western deserts of Egypt. El Fayum is the first extensive ancient fauna known through fossils for that continent, tentatively dated to the Eocene-Oligocene boundary between 37-29 mya (Fleagle, 1999; Seiffert et al., 2008). El Fayum has produced a rich assemblage of fauna besides primates and has been studied for about a century. From these studies several groups of primates have been identified that are generally classified into three groups, Parapithecidae, Propliopithecidae and a single primate classified in the Oligopithecidae (Fleagle, 1999).
The parapithecidae are considered to be the most primitive of all known higher primates and have many similarities to living Platyrrhine monkeys:
- The parapithecids have the same dental formula as neotropical primates, which is 2/2, 1/1, 3/3, 3/3 = 36 teeth in totality (although the smallest callitrichids have lost their third molar for a total of 32 teeth – the Old World catarrhines have a dental formula of 2/2, 1/1, 2/2, 3/3 = 32).
- The parapithecids have an arrangement of cranial plates identical to the neotropical primates (and somewhat distinct from the living catarrhine primates of the Old World.
- The parapithecids have an auditory bulla that does not have an external bony canal from the middle ear to the exterior, exactly as do the Neotropical primates, in contrast to the living Old World catarrhines that have an external bony canal leading from the tympanum to the exterior world
- The parapìthecid genus Apidium has the fibula and tibia partially fused for about 40% of their length, just as Saimiri, a living platyrrhine genus.
- Proteopithecus (a late Eocene El Fayum parapithecid) has no characteristics that would exclude it from a basal platyrrhine ancestry.
(Miller and Simons, 1997; Simons, 1997; Fleagle, 1999; Fleagle and Gibert, 2006). It seems obvious that the neotropical primates or platyrrhines and the parapithecids of ancient Africa have some sort of phylogenetic relationship, unless all these shared characteristics are primitive (plesiomorphic) traits and as such would only prove that they came from a common ancestor (Hershkovitz, 1977; Fleagle, 1999). Additionally, Proteopithecus seems to be closest to a basal anthropoid as a possible ancestor of the platyrrhines. What needs to be identified without doubt, to prove a phylogenetic relationship, are some synapomorphic traits that define a sister group. Unfortunately there is not enough information about evolving primates and especially early anthropoids to be able to state that these shared characters between the catahrrines and platyrrines are plesiomorphic or synapomorphic, though the suite of characters is very suggestive of at least some of the shared characters being synapomorphies. Molecular genetics of course does not help, since it does not seem possible to retrieve organic material from the fossil parapithecids. Therefore, we have to wait for more evidence in the form of fossils, that can indicate to us more clearly how these two groups evolved. The evidence, however, is very suggestive, since are dealing with far more than one character in the two groups.
If it is true that the platyrrhines have come from the parapithecids, how did they arrive in South America? And why are there no other representatives of parapithecids in Africa or Asia? It seems that the parapithecids became extinct in the Old World during the Oligocene. There was a great deal of competition, not only from great apes which pre-empted many available niches during the Miocene, but also from other clades of primates that existed in the same El Fayum fauna as the parapithecids. Somehow, parapithecids or a related group seem to have made it to a South America where there was less competition, except from some forms of arboreal marsupials, perhaps early arboreal rodents, also apparently immigrants from Africa. Molecular studies suggest varied dates that the two primate clades separated about 39 mya in the late Eocene, though the most ancient platyrrhine fossils from La Salle, Bolivia have been dated to about 26 mya, so this gap must be filled by finding earlier primate fossils or by somehow reducing the time calculated on various molecular clocks (Schrago and Russo, 2003; Poux et al., 2006). The only living primates found in the Old World are groups that are so distinct from the platyrrhines that they have none of the characters mentioned above.
The crossing of the Atlantic Ocean by the platyrrhine forebears has always seemed to be a great difficulty for any possible ancestor coming from Africa. This biogeographic problem seemed so great that for many years it was argued that the ancestors came from North America, where more primitive strepsirrhine primates lived throughout the Eocene. But, despite evidence of a great diversity of primitive primates in North America, there is no evidence of more advanced primates there, such as parapithecids, and up to this point no primitive primates have been found in South America that could have been the ancestors of the playrrhines. There is just no support for a northern route for primates arriving in South America. It seems probable that the ancestors of the neotropical primates arrived on rafts of vegetation from Africa (Ciochon and Chiarelli, 1980; Takai et al., 2000; De Queiroz, 2005; Bandoni de Olivera et al., 2009).
Rosenberger (Rosenberger, 1979; Rosenberger and Strier, 1989; Rosenberger et al., 1991; Rosenberger et al., 2009) believes that the earliest radiations of modern species can be detected in the earliest neotropical fossils, first in the form of a major dichotomy that represents different clades that occupy different adaptive zones. One clade contains the origins of the smaller, insectivorous cebids, including Cebus, Saimiri and the callitrichines, while the other clade contains the origins of the seed-eating pithecines, including Pithecia, Cacajao, Chiropotes, Callicebus and including as well the large suspensory atelines (Alouatta, Lagothrix, Ateles, Brachyteles, as well as the subfossil Caipora and Protopithecus; Fleagle and Tejedor, 2002). According to this view, the origin of the most ancient ancestors of the Platyrrhines up to the Middle Miocene have been found in the southern cone (Fig. 1) and most of all in Argentina (principally), Bolivia but also Chile.
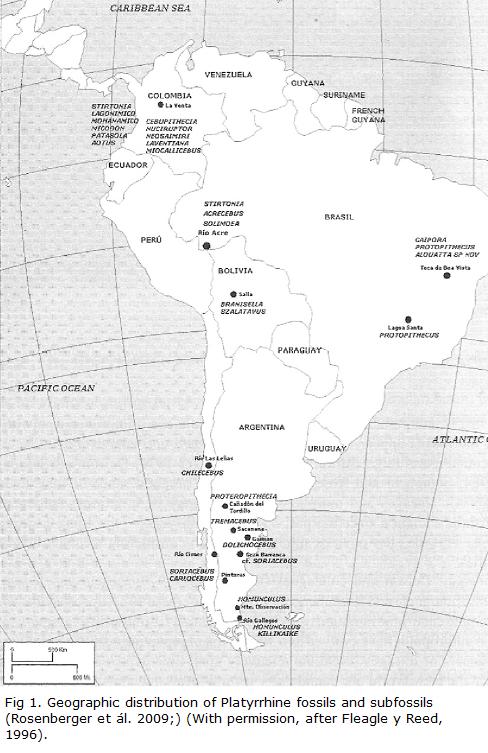
Up until the Middle Miocene there is no record of a primate in more northerly parts of South America. This of course may only be an artifact of collection sites, since the southern part of South America, and especially Patagonia, has been so important for the early history of mammals.
But an Amazonian forest as such is only a recent phenomenon created by the gradual uplift of the Amazonian watershed. This Amazonian uplift resulted in a damming of its western region by the growing Cordillera de los Andes and the concomitant formation of immense freshwater lakes and swamps overlying saltwater and mixed brackish environments throughout the low-lying basin (Pebas formation) and the eventual breach of the eastern barriers about 2.5 mya (Campbell et al., 2006). A peripheral primate paleo-environment to this great -Lago Amazonas- did exist, like La Venta, Colombia, that provides ample evidence of rich pre-Amazonian or proto-Amazonian primate communities (Kay et al., 1997).
The two ancient clades identified by Rosenberger, 2002, can be divided into four principal guilds that illustrate four evolutionary lines. These are evident in the La Venta fauna of the Middle Miocene in Colombia (about 13 mya):
- The callitrichines are small-bodied primates, predators of arthropods and fruits. These animals developed specialized locomotions and postures and the use of branch tips that sustain little weight. They exploit arthropods, although Leontopithecus specializes in larger sizes and some vertebrates while Callithrix and Cebuella exploit tree gums. The great majority are Amazonian, thought smaller radiations are found in the Atlantic forest of Brazil and the trans-Andean forests of Colombia. Their small size seems to be a recent secondary adaptation.
- The cebids are small to medium-sized primates, predators of invertebrates and small vertebrates as well as fruits. They utilize the canopy as their resource base for exploiting arthropods. Saimiri is basically Amazonian; the prehensile-tailed and widely distributed Cebus, a champion generalist, is a successful destructive forager.
- The pithecids have a small to medium-sized body and exploit hard, unripe Canopy fruits. Some get their protein from seeds (Pithecia, Chiropotes, Cacajao) or from arthropods (Aotus and Callicebus) and in the case of Callicebus also seeds (Palacios et al., 1997).
- The atelines are large-bodied primates (4-10 kg) that are frugivorous or Folivorous. They depend on trees for much of their food and exploit immature leaves for protein (Alouatta, Lagothrix, Brachyteles, Ateles). The exploitation of leaves permits the members of this guild to live in diverse habitats that are less fertile and even semi-deciduous; thus, Alouatta survives in conditions alongside rivers and lakes and areas with only gallery forests surrounded by savanna.
What can the fossil primates teach us about the evolution of these guilds? What can the living communities teach us about the paleobiology of these taxa?
We know that there were quite a few primates in the southern cone of the continent during the early part of the Miocene and from about 30 mya a progressive trend of cooling down of the southern part of the continent (Lewis et al., 2007; Lewis et al., 2008). What was the evolutionary context of these forms? During the early part of the Miocene, and against the predominant trend, the world experienced a warming that had profound effects on vegetation in Patagonia down to the tip of South America, since judging by the primates and other fauna found there, obviously trees and forest existed (Pascual and Ortiz-Jaurequizar, 1989; Blisniuk et al., 2005).
One primate (Killikaike blakei) from the early Miocene (Santacrucian, about 16.5 mya) was found at the southernmost point of South America (Tejedor et al., 2006). This is the southernmost primate ever found anywhere. It lived only 1.500 km from the northern point of the Antarctic Peninsula and lived during a time when East Antarctica retained some glaciers during the early to mid Miocene (23 – 15 mya). Oceans had cooled partly due to the formation of the Circumpolar Current and about 15 mya the ice cap in the southern hemisphere started to grow to its present form (Lagabrielle et al. 2009). Killikaike has been associated with the possible phylogeny of Cebus or Saimiri, because of its well-developed forebrain, clearly seen in the well-preserved skull. The authors of the description have placed this fossil unambiguously with the cebinae (Tejedor et al., 2006).
There were other Patagonian and Chilean primates whose fossils we have found living more or less during the same times as Killikaike although a bit further to the north (Fig. 1). What were the ecological conditions of these Miocene forests in the southern cone? With several species of Patagonian primates there had to be forests, despite a general increase of grassy savannas. Surely there were gallery forests along the rivers, although a climatic optimal from about 21 mya during the middle Miocene until 12-15 mya probably permitted denser forests during part of that time, especially during the height of the warming period between about 15-17 mya (Böhme, 2003; Lewis et al., 2008; Micheels et al., 2009). Patagonia was also a much more humid environment during the earlier Miocene, favoring denser forest growth. It was not until the Patagonian Andes formed a topographic barrier to atmospheric circulation that considerable aridification occurred eastward at around 16.5 mya, the time of Killikaike, though by then the forests must have been receeding (Blisniuk et al., 2005). Nevertheless, the discovery of the platyrrhine primate Propithecia neuquenensis somewhat further north and in sedimentary deposits just above consolidated volcanic tuff dated at 15.7 +/-0.07 mya shows that the region between 16-15 mya could still support primates (Kay et al., 1998; see Fig. 1).
Known Neotropical primate fossils fall into four regional provinces: Amazonia (very few), Atlantic, Caribbean and Patagonia/Bolivia. Since from Miocene times it is possible to recognize characteristics or radiations of living primate groups, recognized by known lineages from earliest times, many biologists postulate that an explosive radiation between 20 and 16 mya occurred, resulting in the lineages being equally distant from each other (Fleagle and Reed, 1999). This epitomizes the so-called -Long Lineage Hypothesis- (Rosenberger, 2002; Rosenberger et al., 2009). This long history of certain neotropical primate lineages contrasts with the primates of the Old World (Catarrhines) that show a pattern of evolution of adaptive radiations replacing other adaptive radiations (Fleagle and Tejedor, 2002).
These ancient neotropical lineages are reflected in the well-known mid-Miocene fossils of La Venta, Colombia (12 mya) which already in many cases reflect the phylogenies of many modern neotropical primates, of which I give a few examples here. The genus Aotus, well-represented with around 13 species (7 in Colombia) appears in the La Venta fauna with Aotus didensis (12.1-12.5 mya). The large orbit size in Tremacebus (Colhuehuapian, early Miocene) suggests an early relationship to Aotus, while the extremely broad molars of Chilecebus (Colhuehuapian, early Miocene, 20.9 +/-0.27 mya) would ally it with Saimiri and Cebus (Fleagle and Tejedor, 2002). The early Miocene (Colhuehapian) Dolichocebus has also been closely related to the Neosaimiri (La Venta, 12.1-12.5 mya) and Saimiri (Rosenberger, 1979; Fleagle and Kay, 1989). Killikaike itself is well-related to the cebid Cebus and Saimiri. Callicebus apparently is a very old lineage and has been related to Tremacebus, Carlocebus or Homunculus from the early Miocene at about 20 mya (Fleagle and Tejedor, 2002).
Ancient lineages illustrating adaptive zones are not typical for the Old World primates. These seem to exhibit lineages that become identifiable for a time, only to be replaced by other lineages. Modern groups are really not identifiable until the Pliocene and even the Pleistocene, much more recent than in the Platyrrhines (Jablonski, 2008).
The differences between Platyrrhine and Catarrhine primates may have a lot to do with the founder effect of a small population and the genetic variation representative of this small founder population that was probably distinctly different from the original African populations, although interestingly it seems that the phenotype of the new platyrrhines retained many features of the African founders. Nevertheless, the added effect of genetic drift has evolved a clade that is quite different from modern catarrhines, partially described here.
Though neotropical primates have been evolving in the Neotropical Realm for at least 26 million years and probably more than 30 million years according to the molecular evidence (Schrago and Russo, 2003), they seem to have ecological niches that are less diverse as a group than the Old World ones, again probably a founder effect. Fleagle and Reed, 1996, managed to compare the ecology of two communities in Africa, Asia, Madagascar and South America to assess the amplitude of the ecological niche for each region (Fig. 2), which they defined as Hutchinson, 1978, did, as a multivariate space defined by an array of ecological variables.
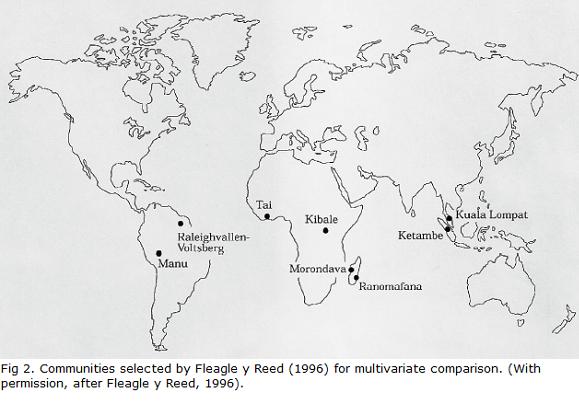
The questions that Fleagle and Reed, 1996, wished to address were: (1) are the primate communities in each region distinct in terms of their niche or are these niches biogeographically similar?, (2) is there a pattern of distribution in the ecological characteristics among the species of each community?; (3) is the combined ecological amplitude (space) of each community in the different regions distinct or similar?; (4) are the communities of species in a particular region more similar to each other than to other regions?; (5) are the ecological similarities of primate communities between some regions greater or smaller than differences between other regions?; and finally (6) what is the role of phylogeny in determining the ecological characteristics in these communities of primates?
Fleagle y Reed, 1996, chose two very diverse communities from each region and for each pairof communities they chose as distinct a habitat as possible. They characterized each species basedon ecological variables that are available in the primary literature with the objective of making the comparisons as precise as possible. They collected proportional time (expressed as percentage oftotal time) invested in the following behaviors and locomotion as ten ecological variables for eachof the 70 primate species as follow: (1) arboreal quadrupedalism; (2) terrestrial quadrupedalism;(3) leaping; (4) suspensory activities; (5) climbing; (6) fruits in diet; (7) foliage in diet; (8) fauna inthe diet; (9) cycles of activity (diurnal, nocturnal, cathemeral); and (10) body weight (table 1).

The authors created a matrix (10 variables X 70 species) and analyzed it by using PCA(principal components analysis) to better visualize species distribution in the multidimensionalmatrix. In order to compare the communities, the placement of each species and each communitywas diagrammed separately in the bidimensional space of each community; a polygon was used toenclose the limits of the space occupied by the species of each community. The analysis was basedon the first two factors in the PCA, since they explained 53% of the total variation. The figure belowcompares the data between communities and regions (Fleagle and Reed, 1996; )Figs. 3A y B.
The primate communities in Madagascar are characterized by two distinct groups of ecological types – small to medium-sized primates, folivores and leapers and small faunivores – arboreal quadrupedal frugivores. Madagascar has an abundance of folivores and folivorefrugivores, although any discussion of Madagascar must recognize the very recent (1000 years) extinction of very large, terrestrial lemurs (Archaeoindris at 160 kg and Archaeolemur 15-25 kg; Mittermeier et al. 2006). Adding the extinct lemurs increases the diversity of suspensory and terrestrial (already well-represented) primates. Nevertheless, frugivores are very common in Madagascar when compared to the other three regios.
There are very clear differences between the three continents and Madagascar. African primates are well-known for polyspecific groups that permit 4-6 species of cercopithecids to form feeding groups. The Africans are the most diverse in terms of the niche-space that they occupy and the least diverse in the polygons that define them, as they overlap almost completely with the polygons defining the niche-space of the other three regions. There is almost no ecological space that is unique to the African primate communities (that reach 16 sympatric species).
The communities of Asiatic primates are not as diverse in species as the other three, which are at their most diverse with 8 species. Asiatic communities tend to be groups of sister species of the same genus, probably a reflection of their evolution on the many islands of the region, according to the sea level. For this reason the groups are ecologically more similar. The characteristic that stands out for this region is the abundance of suspensory species (siamang, gibbon).
The ecological space represented by the Neotropical communities is the least extensive; the species are grouped close together. Among the neotropical primates, adaptations to folivory, suspensory activities, and nocturnal activities are moderately expressed so that the niche-space in this study is reduced in comparison with the other regions and species. Neotropical primates, in contrast with the communities in the other continents and Madagascar have only one nocturnal species per community, there are no terrestrial species; body sizes are moderate, the average size is well below that of the other primates, while the phenotypes are the most primitive when compared to the other three regions (Fleagle and Reed, 1996; Fleagle, 1999).
Terborgh and Van Schaik, 1987, suggested that the small size and limited folivory of the platyrrines is due to the high periodicity of the different phonological seasonality of leaves and fruit that create an intense pressure for primates during the dry season when there is a scarcity of food, obligating all to develop alternative strategies for finding resources. One strategy is not to be too large, the largest platryrrines known, found from Recent deposits in the Atlantic coast region of Brazil and now extinct only reached about 20 kg. Also, the radiation of the callitrichines resulted in a secondary reduction in size of the ancestors of Cebuella, Callimico, Callithrix and Saguinus so as to evolve specialized feeding, strategies that would not support large-bodied primates.
It is generally accepted that the uncertainty of the availability of resources is an important factor that affects the evolution of a community. Competition may be another factor affecting a communities’ evolution. In South America there are many animals (Rodentia, Marsupialia, Procyonidae) that are nocturnal and this may have left little eco-space for the nocturnal niche to be filled by primates. For example, members of the carnivorous family Procyonidae (raccoons) and especially Potos flavus are strong nocturnal competitors of the night monkeys (Aotus; Defler, 2004; Defler, 2010). But we know there are multiple factors that have influenced the trophic structure (pattern of movement of energy and resources in the system) of South American primates.
Neotropical primate social systems exhibit some distinctive evolutionary tendencies. Many species live in groups that are multi-male or age-graded and multi-female, the system most commonly shared by many primates in the rest of the world. Another system is that of rather rare monogamy, practiced by Callicebus (29 spp.), Aotus (10 spp.) and Pithecia (4 spp.; sensu Rylands and Mittermeier, 2009). Unlike primates from Africa, Asia or Madagascar, there are no solitary neotropical primates.
The only prehensile-tailed primates in the world are Neotropical. These are the atelids and Cebus monkeys (Rosenberger and Strier, 1989). There are also many other vertebrates in the neotropics with prehensile tails, certainly more than the rest of the world and thus showing another evolutionary tendency. One hypothesis is that increased fragility of branch tips and the great density of palm trees, which have mechanisms to discourage liana growth and the medium numbers of lianas in the neotropics (as compared to Africa which has many more, and Asia which has many less vines) confer an advantage on prehensile tails (Emmons and Gentry, 1983). This seems difficult to test and equally there are many animals in the neotropics that do not have a prehensile tail. Nevertheless, the Neotropics has far more animals with prehensile tails (including primates) than any other region in the world (Emmons and Gentry, 1983; Figs. 4).

And, what can be said about terrestrial primates in the Neotropics? In Africa there is a strong radiation of baboons, guenons and apes that are predominantly terrestrial. In Asia there are many species of essentially terrestrial macaques and langurs that spend the majority of their time on the ground. In Madagascar, many species of lemurs are largely terrestrial with a recently extinct huge 160 kg ground lemur (Archaeoindris) and the smaller 15 - 25 kg (Archaeolemur).
But in South America time spent on the ground is limited. Some species occasionally go to the ground for feeding; Cebus monkeys may be the most terrestrial, though they are usually seen in the trees. Two recently extinct atelids in the Atlantic coast region seem to have been at least semi-terrestrial, Protopithecus and Caipora. Why has terrestriality not evolved as a life-style like the several primates in the other three regions?
Ultimately it is important to recognize the possibility of an association between the phylogeny and ecology of primates, since neotropical primates began evolving from a very reduced population base, perhaps one small group, arrived from Africa. There is no doubt that the behavior and anatomy of species of primates are molded to the ecological conditions by natural selection. There is good evidence for variability in the behavior of any species, which can respond to local differences in habitat (Saimiri), but evolution is hierarchical and adaptations have evolved in a phylogenetic context; many believe that phylogenetic radiations reflect deep evolutionary history which must have been severely affected because of having passed through a bottleneck on arriving in South America. Any two groups in a single region (continental) are more similar to each other than they are to groups of other regions (or continents; Fleagle and Reed, 1999).
Chromosomes and molecular studies suggest much about the evolution of the platyrrhines. Work by various authors demonstrates that the platyrrhines are a very closely related group, consistent with the idea that the group was founded by very few animals (Schneider and Rosenberger, 1996; Schneider et al., 1993; Schneider et al., 2004; Ruiz-Garcia et al., 2004). What is more important, there is much evidence for the idea of an ancestral karyotype for the platyrrhines with a 2n=54; the putative ancestor would be almost identical to the capuchin karyotype (Cebus), congruent with a basal position among the platyrrhine families. All Platyrrhines are characterized by six fissions in four chromosomes (1, 3/21, 8 and 14/15) and by four fusions that form various synthetic associations (2/16, 5/7, 8/18 and 10/16). FISH (multi-color chromosome painting, -fluorescence in situ hybridization- for the detection and interpretation of chromosome structure) demonstrate that chromosome synthesis gives a diploid number of 2n=54 with an assemblage of chromosomes that still is present in Cebus. (Neusser et al., 2001; Stanyon et al., 2008)
It is well-known that the evolutionary rate of chromosome reshuffling is very high in both Aotus and Callicebus, and it would seem that the importance of chromosome speciation is very much underestimated in those genera (Seuánez et al., 2005): Aotus displays a variation from 46 to 58, while Callicebus varies from a diploid chromosome number of 16 (the lowest diploid primate chromosome number) to 59. Both genera have undergone intensive genomic shuffling with respect to the ancestral platyrrhine number of 2n=54 and both are extreme when compared to other neotropical genera (Defler, 2004; Defler and Bueno, 2007). Interestingly, the only catarrhine primates that come close to these two genera are the Hylobates or gibbons of the Old World, that possess highly variable chromosomes as well showing many polymorphisms (Jauch et al., 1992). Aotus, Callicebus and gibbons exhibit a monogamous social system, and that is probably the key to their highly evolved chromosomes, since monogamous animals are probably more often associated with small effective group sizes that upon becoming isolated through vicariant events (river cutoff etc.), enable genetic drift to result in new, fixed genotypes that may survive as new species (Wilson et al., 1975; Bush et al., 1977; Lande, 1979; Lande, 1985; Marks, 1987; Martin, 1990).
This type of speciation is distinct from the usual mechanism classically suggested for vertebrates where it is understood that there is a slow accumulation of genetic differences in an isolated population that finally constitutes a reproductive barrier (reproductive barrier is not the same as sterility in a hybrid). Chromosome speciation happens when some chromosome mutation results in a karyomorph or rearrangement that is distinct from the parental population and then is isolated in a small population where the mutation becomes fixed because of genetic drift. The new chromosome arrangement then acts as an isolating mechanism between it and the parent population, and if it is able to enter into a new habitat it may establish itself as a new species. This mechanism probably is much more rapid than the traditional idea of allopatric speciation, where the isolating mechanism(s) is thought of as being gradual change in successive loci, especially those that modify integrated development, physiology, behavior with epistatic interactions between genes (King, 1995).
In Colombia we have studied the effects of chromosome speciation in the evolution of Aotus (Defler and Bueno, 2007) and Callicebus (Bueno et al., 2006; Bueno and Defler, in press), a genus that seems quite ancient since it was present 13 mya in Middle Miocene La Venta (Colombia) deposits. Studies have demonstrated that the average age of a mammalian genus is generallyabout 3.5 million years old (Wilson et al., 1975; Bush et al., 1977). This antiquity of genera isduplicated as well in Saimiriat La Venta, which has been name Neosaimiri, but is evidently asynonym for Saimiri(Rosenberger et al., 1991).
Aotus, a nocturnal primate, was formerly classified as a single species throughout thecontinent and Panama, given the comparative uniformity of its phenotype. From the beginningof studies of Aotuskaryotypes it became obvious that the diversity of karyotypes defined severalspecies, since those differences represented reproductive barriers (Brumback, 1973; Brumback,1974). Now, most authors recognize ten species, all of them rearrangements of the chromosomecomplement. This genus is perhaps the most extreme example of extensive chromosome evolution.Earlier, primatologists did not imagine the number of species in this complex, because of theslight phenotypic variation.
Callicebushas not been easily understood as a complex of species instead of the one or twofirst recognized. Once again, it was the evidence of multiple karyotypes that permitted recognitionof more species in the genus, although in Callicebusmany new species are being identified with aminimum or lack of karyotypic differences, since the phylogenetic species concept has increasinglybeen used and despite great karyotypic differences between some species other species pairsexhibit minimum differences.
The various evolutionary tendencies of Neotropical primates discussed in this article arelisted in table 2. The list does not pretend to be inclusive but is merely the most obviouscharacteristics recognized at this time. These characters are diagnostic for the Plattyrrinhi andillustrate the ancient, separate evolution of this group of primates for the New World.
BIBLIOGRAPHY
BANDONI DE OLIVEIRA F, CASSOLA MOLINA E, MARROIG G. Paleogeography of theSouth Atlantic: a route for primates and rodents into the New World. En: Garber PA, Estrada A.,Bicca-Marques JC, Heymann EW, Strier KB, editors. South American Primates: ComparativePerspectives in the Study of Behavior, Ecology, and Conservation. New York: Springer; 2009. p.55-68.
[ Links ]BLISNIUK PM, STERN, CHAMBERLAIN CP, IDLEMAN B, ZEITLER PK. Climatic andecological changes during Miocene surface uplift in the southern Patagonian Andes. Earth PlanetSci Lett. 2005; 230: 125-142.410
[ Links ]BÖHME M. The Miocene climatic optimum: evidence from ectothermic vertebrates of Central Europe. Palaeogeogr Palaeoclimatol Palaeoecol. 2003;195(2002):389-401.
[ Links ]BUENO ML, DEFLER TR. ¿Está Callicebus lugens en Colombia? En: Monsalve V, editor. Primatología en Colombia. Congreso de Primatología, Bogotá. En prensa.
[ Links ]BUENO ML, RAMÍREZ-OJUELA C, LEIBOVICI M, TORRES O. 2006. Información cariológica del género Callicebus en Colombia. Rev Acad Colomb Cienc. 2006;XXX(114):109-116.
[ Links ]BRUMBACK RA. Two distinctive types of owl monkeys (Aotus). J Med Primatol. 1973;2: 284-289.
[ Links ]BRUMBACK RA. A third species of the owl monkey (Aotus). J Hered.1974;65:321-323.
[ Links ]BUSH GL, CASE SM, WILSON AC, PATTON JL. Rapid speciation and chromosomal evolution in mammals. Proc Natl Acad Sci U S A. 1977;74(9):3942-3946.
[ Links ]CAMPBELL KE, FRAILEY CD, ROMERO-PITTMAN L. The Pan-Amazonian Ucayali Peneplain, late Neogene sedimentation in Amazonia, and the birth of the modern Amazon River system. Palaeogeogr Palaeoclimatol Palaeoecol. 2006;239:166-219.
[ Links ]CIOCHON RL, CHIARELLI AB. Paleobiogeographic perspectives on the origin of the Platyrrhini. En: Ciochon RL, Chiarelli AB, editors. Evolutionary Biology of New World Monkeys and Continental Drift. New York: Plenum Press; 1980. p. 459-493.
[ Links ]DEFLER TR. Historia Natural de los Primates Colombianos. Universidad Nacional de Colombia; 2010.
[ Links ]DEFLER TR. Primates of Colombia. Bogotá: Conservación Internacional Colombia; 2004.
[ Links ]DEFLER TR, BUENO ML. Aotus diversity and the species problem. Primate Conserv. 2007;22:55-70.
[ Links ]DE QUEIROZ A. The resurrection of oceanic dispersal in historical biogeography. Trends Ecol Evol. 2005;20(2):68-73.
[ Links ]EMMONS LH, GENTRY AH. Tropical forest structure and the distribution of gliding and prehensile-tailed vertebrates. Am Nat. 1983;121:513-524.
[ Links ]FLEAGLE J. Primate Adaptation and Evolution. New York: Academic Press; 1999.
[ Links ]FLEAGLE J, GILBERT CC. The biogeography of primate evolution: The role of plate tectonics, climate and chance. En: Lehman SM, Fleagle JG, editors. Primate Biogeography. New York: Springer; 2006. p. 375-418.
[ Links ]FLEAGLE J, KAY RF. The dental morphology of Dolichocebus gaimanensis, a fossil monkey from Argentina. Am J Phys Anthropol. 1989;78:221.
[ Links ]FLEAGLE J, REED KE. Phylogenetic and temporal perspectives on primate ecology. En: Fleagle JG, Janson C., Reed KE. Primate Communities.Cambridge: Cambridge University Press; 1999. p. 92-115.
[ Links ]FLEAGLE J, REED KE. Comparing primate communities: a multivariate approach. J Hum Evol. 1996;30:489-510.
[ Links ]FLEAGLE J, TEJEDOR MF. Early platyrrhines of southern South America. En: Harwig WC, editor. The Primate Fossil Record. Cambridge: Cambridge University Press; 2002. p. 161-173.
[ Links ]HERSHKOVITZ P. Living New World Monkeys (Platyrrhini) With an Introduction to Primates. Vol.1. Chicago: The University of Chicago Press; 1977.
[ Links ]HOULE A. The origin of Platyrrhines: An evaluation of the Antarctic scenario and the floating island model. Am J Phys Anthropol. 1999;109:541-559
[ Links ]HUTCHINSON GE. An introduction to population ecology. New Haven: Yale University Press; 1978.
[ Links ]JABLONSKI NG. Fossil Old World monkeys: The late Neogene radiation. En: Hartwig WC, editor. The Primate Fossil Record. Cambridge: Cambridge University Press; 2008. p. 255-299.
[ Links ]JAUCH A, WIENBERG J, STANYON R, ARNOLD N, TOFANELLI S, ISHIDA T, CREMER, T. Reconstruction of genomic rearrangements in great apes and gibbons by chromosome painting. Proc Natl Acad Sci U S A. 1992;89:8611-8615.
[ Links ]KAY RF, MADDEN RH, CIFELLI RL, FLYNN JJ. Vertebrate paleontology in the Neotropics: The Miocene fauna of La Venta, Colombia. Washington, D.C.: Smithsonian Institution Press; 1997.
[ Links ]KAY RF, JOHNSON D, MELDRUM DJ. A new pitheciin primate from the middle Miocene of Argentina. Am J Primatol. 1998;45:317-336.
[ Links ]KING M. Species Evolution: the Role of Chromosome Change. Cambridge: Cambridge University Press; 1995.
[ Links ]LAGABRIELLE Y, GODDÉRIS Y, DONNADIEU Y, MALAVIEILLE J, SUAREZ M. The tectonic history of Drake Passage and its possible impacts on global climate. Earth Planet Sci Lett. 2009;279:197-211.
[ Links ]LANDE R. The fixation of chromosomal arrangements in a subdivided population with local extinction and recolonization. Heredity. 1985;54:323-332.
[ Links ]LANDE R. Effective deme sizes during long-term evolution estimated from rates of chromosomal rearrangement. Evolution. 1979;33(1):234-251.
[ Links ]LEWIS AR, MARCHANT DR, ASHWORTH AC, HEDENÄS L, HEMMING SR, et al. Mid-Miocene cooling and the extinction of tundra in continental Antarctica. Proc Natl Acad Sci U S A. 2008;105(31):10676-10680.
[ Links ]LEWIS AR, MARCHANT DR, ASHWORTH AC, HEMMING SR, MACHLUS ML. Major middle Miocene global climate change: Evidence from East Antarctica and the Transantarctic Mountains. Geoc Sol Am Bull. 2007;119(11/12):1449-1461.
[ Links ]MARKS J. Social and ecological aspects of primate cytogenetics. En: W. G. Kinzey, editor. Primate Models: The Evolution of Human Behavior. New York: State University of New York Press; 1987. p. 139-151.
[ Links ]MARTIN RD. Primate Origins and Evolution: A Phylogenetic Reconstruction. Princeton: Princeton University Press; 1990.
[ Links ]MICHEELS A, BRUCH A, MOSBRUGGER V. Miocene climate modeling sensitivity experiments for different C02 concentrations. Palaeontol Electronica. 2009;12(2):1-19. http://palaeo-electronica.org/2009_2/172/index.html
[ Links ]MILLER EP, SIMONS EL. Dentition of Proteopithecus sylviae, an archaic anthropoid from the Fayum, Egypt. Palaeogeogr Palaeoclimatol Palaeoecol. 1997;38:163-183.
[ Links ]MITTERMEIER RA, KONSTANT WR, HAWKINS F, LOUIS EE, LANGRAND O, et al. Lemurs of Madagascar. Second Edition. Washington, D.C.: Conservation International; 2006.
[ Links ]NEUSSER M, STANYON R, BIGONI F, WIENBERG J, MÜLLER S. Molecular cytotaxonomy of New World monkeys (Platyrrhini)-comparative analysis of five species by multicolor chromosome painting gives evidence for a classification of Callimico goeldii within the family of Callitrichidae. Cytogenet. Cell Genet. 2001;94:206-215.
[ Links ]PALACIOS E, RODRÍGUEZ A., DEFLER TR. Diet of a group of Callicebus torquatus lugens during the annual resource bottleneck. Int J Primatol. 1997;18(4):503-522.
[ Links ]PASCUAL R, ORTIZ-JAUREQUIZAR E. Evolving climates and mammal faunas in Cenezoic South America. J Hum Evol. 1989;19:23-60.
[ Links ]POUX C, CHEVRET P, HUCHON D, DE JONG WW, DOUZERY EJP 2006. Arrival and diversification of caviomorph rodents and platyrrhine primates in South America. Syst Biol. 2006;55(2):228-244.
[ Links ]ROSENBERGER AL. Platyrrhine paleontology and systematics: The paradigm shifts. En: Hartwig WC, editor. The Primate Fossil Record. Cambridge: Cambridge University Press; 2002. p. 151-159.
[ Links ]ROSENBERGER AL. Cranial anatomy and implications of Dolichocebus, a late Oligocene ceboid primate. Nature. 1979;279:416-418.
[ Links ]ROSENBERGER AL, HARTWIG WC, TAKAI M, SETOGUCHI T, SHIGEHARA N. Dental variability in Saimiri and the taxonomic status of Neosaimiri fieldsi, an early squirrel monkey from La Venta, Colombia. Int J Primatol. 1991;12(3):291-301.
[ Links ]ROSENBERGER AL, STRIER KB. Adaptive radiation of the ateline primates. J Human Evol. 1989;18:717-750.
[ Links ]ROSENBERGER AL, TEJEDOR MF, COOKE SB, PEKAR S. Platyrrhine ecophylogenetics in space and time. En: Garber PA, Bicca-Marques JC, Heymann EW, Strier KB, editors. South America Primates: Comparative Perspectives in the Study of Behavior, Ecology, and Conservation. New York: Springer; 2009. p. 69-113.
[ Links ]RUIZ-GARCIA M, CASTILLO MI, ALVAREZ D. Evolutionary trends of neotropical primates according to the AP68 and AP40 microsatellites. En: Mendes SL, Chiarello AG, editores. A Primatologia no Brasil. Volumen 8. Santa Teresa, Espirito, Brasil: Museu de Biologia Prof. Mello Leitão; 2004. p. 65-100.
[ Links ]RYLANDS AB, MITTERMEIER RA. The diversity of the New World primates (Platyrrhini): An annotated taxonomy. En: Garber PA, Estrada A, Bicca-Marques JC, Heymann EW, Strier KB, editors. South American Primates: Comparative Perspectives in the Study of Behavior, Ecology, and Conservation. New York: Springer; 2009. p. 23-54.
[ Links ]SCHNEIDER H, ROSENBERGER AL. Molecules, morphology, and Platyrrhine systematics. En: Norconk, MA, Rosenberger, AL, Garber, PA, editors. Adaptive Radiations of Neotropical Primates. New York: Plenum Press; 1996.
[ Links ]SCHNEIDER H, SCHNEIDER MPC, SAMPAIO M, HARADA ML, STANHOPE M, CZELUSNIAK J, GOODMAN M 1993. Molecular phylogeny of the New World monkeys (Platyrrhini, Primates). Mol Phylogenet Evol. 1993; 2(3):225-242.
[ Links ]SCHNEIDER I, SCHNEIDER H, SCHNEIDER MP, SILVA A. The prion protein and the New World primate phylogeny. Genet Mol Biol. 2004; 27(4):505-510.
[ Links ]SCHRAGO C, RUSSO CAM. Timing the origin of New World monkeys. Mol Biol Evol. 2003;20(10):1620-1625.
[ Links ]SEIFFERT ER, BOWN TM, CLYDE WC, SIMONS E. Geology, Paleoenvironment, and Age of Birket Qarun Locality 2 (BQ-2), Fayum Depression, Egypt. En: Fleagle JG, Gilbert CC, editors. Elwyn Simons: A Search for Origins. New York: Springer; 2008. p. 71-86.
[ Links ]SEUÁNEZ HN, BONVICINO CR, MOREIRA MAM. The primates of the Neotropics: genomes and chromosomes. Cytogenet. Genome Res. 2005;108:39-46-
[ Links ]SIMONS EL. Preliminary description of the cranium of Proteopithecus sylviae, an Egyptian late Eocene anthropoidean primates. Proc Natl Acad Sci U S A. 1997;94:14970-14975.
[ Links ]STANYON R, ROCCHI M, CAPOZZI O, ROBERTO R, MISCEO D, et al. Primate chromosome evolution: Ancestral karyotypes, marker order and neocentromeres. Chrom Res. 2008;16:17-39.
[ Links ]TAKAI A, ANAYA F, SHIGEHARA N, SEGOTUCHI T. New fóssil materials of the earliest New World monkey, Branisella boliviana, and the problem of platyrrhine origins. Am J Phys Anthropol. 2000;111:263-281.
[ Links ]TEJEDOR MF, TAUBER AA, ROSENBERGER AL, SWISHER III CC, PALACIOS ME. New primate genus from the Miocene of Argentina. Proc Natl Acad Sci U S A. 2006;103:5437-5441.
[ Links ]TERBORGH J, VAN SCHAIK CP. Convergence vs. noncovergence in primate communities. En: Gee JHR, Giller PS, editors. Organization of Communities, Past and Present, Oxford: Blackwell Scientific; 1987. p. 205-226. .
[ Links ]WILSON AC, BUSH GL, CASE SM, KING M-C. Social structuring of mammalian populations and rate of chromosomal evolution. Proc Natl Acad Sci U S A. 1975;72(12):5061-5065.
[ Links ]













