Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Acta Biológica Colombiana
versão impressa ISSN 0120-548X
Acta biol.Colomb. v.15 n.2 Bogotá maio/ago. 2010
AN ASSESSMENT OF FISH COMMUNITIES ALONGA PIEDMONT RIVER RECEIVING ORGANIC POLLUTION(ACONQUIJA MOUNTAINS, ARGENTINA).
Evaluación de las comunidades de peces a lo largo de un ríopiedemonte que recibe contaminación orgánica(Montañas de Aconquija, Argentina).
LUIS FERNÁNDEZ1,2; JOSÉ A. BECHARA3 1 CONICET, Instituto Fundacion Miguel Lillo; Miguel Lillo 251, 4000 Tucumán, Argentina. 2 IBIGEO, Instituto de Bio y Geociencias Museo Ciencias Naturales de Salta, Mendoza 2, 4400 Salta, Argentina. luis1813@yahoo.com 3 Instituto de Ictiología del Nordeste. Facultad de Ciencias Veterinarias, Universidad Nacional del Nordeste y Consejo Nacional de Investigaciones Científicas y Técnicas. Sargento Cabral 2139. 3400 Corrientes. Argentina (deceased in March 8, 2008).
Presentado 12 de mayo de 2009, aceptado 17 de septiembre de 2009, correcciones 22 de octubre de 2009.
ABSTRACT
The relationships between fish assemblage structure and environmental variables along a pollution gradient in the Medina River were analyzed over a year in four sampling sites (S1-S4). The river flows in a mountain-plain transition and is affected by several small town wastewater and sugar cane industries effluents. Environmental variables were divided in two sets, hereafter named -pollution- and -natural-. The first set included water quality variables modified by anthropogenic activities such as D.O. (Dissolved Oxygen), C.O.D. (Chemical Demand Oxygen), and dissolved ion concentrations. Natural variables included altitude, position, and time of the year. The upstream site (S1) had the lowest species richness and C.P.U.E. (catchperunitofeffort). The number of species and density increased downriver(S2-S3). S1 was inhabited by an invertivor especies (Trichomycteruscorduvensis) that has low tolerance to adverse environmental conditions, and has high D.O. requirements. S4 sustained the most tolerant and abundant species (Otocinclus vittatus, Corydoras paleatus), which endure the lowest D.O. and the highest C.O.D. A Canonical Correspondence Analysis for natural variables showed a significant gradient of species composition related to altitude and discharge. Water quality degradation by sugar cane factories and urban development, coupled with natural climatic, topographic and hydrological factors explained a significant amount of spatial and temporal variation in fish community structure (48%). Natural and pollution variables shared about 15% of total variance. However, pollution variables were not significant after partitioning out the effects of natural variables. Natural variability remained significant after removal of pollution effects.
Key words: organic pollution, river gradients, Neotropical fish, canonical analyses, environmental factors.
RESUMEN
Las relaciones entre la estructura de una comunidad de peces y variables ambientales a lo largo de un gradiente de polución en el Río Medina fue analizado por un año en cuatro sitios muestreados (S1-S4). El río corre en una transición de montaña-llanura y es afectado por descargas de efluentes de pueblos e ingenios azucareros. Las variables ambientales fueron divididas en dos grupos, denominados -polución- y -natural-. El primer conjunto incluyó variables de calidad de agua modificadas por actividades antropogénicas tales como D.O. (Oxígeno Disuelto), C.O.D. (Demanda Química de Oxígeno), y concentración de iones disueltos. Las variables naturales incluidas fueron altitud, ubicación, y tiempo del año. Río arriba (S1) tuvo la más baja riqueza de especies y C.P.U.E. (Captura Por Unidad de Esfuerzo); pero río abajo incrementa el número de especies y densidad (S2-S3). S1 estaba habitado por especies invertívoras (Trichomycterus corduvensis), teniendo baja tolerancia a condiciones ambientales y altos requerimientos de D.O. S4 tuvo las especies más tolerantes y abundantes (Otocinclus vittatus, Corydoras paleatus), soportando los más bajos D.O. y los más elevados C.O.D. Un Análisis de Correspondencia Canónico mostró un gradiente en la composición de especies relacionado a altitud y descarga para las variables naturales. La degradación de la calidad del agua por ingenios azucareros y efluentes urbanos, con factores climáticos naturales, topográfico e hidrológico explicaron la variación espacial y temporal de la estructura de comunidad de peces (=48%). Variables naturales y contaminación compartieron el 15% de la varianza total. Sin embargo, las variables de contaminación no fueron significates después de la partición de los efectos de las variables natural, pero la variabilidad natural permaneció significativa después de remover los efectos de polución.
Palabras clave: contaminación orgánica, gradientes de río, peces neotropicales, análisis canónicos, factores ambientales.
INTRODUCTION
Urbanization often results in habitat degradation in streams and rivers, contributing to decreased ecological health in many watersheds around the globe. This is an accelerating process, because it is expected that by 2025, perhaps 60% of people globally and 83% in Europe and the Americas will live in cities (Morley and Karr, 2002). Overexploitation and environmental pollution of rivers, caused by increasing human populations, are among the main reasons of decline in fish assemblages (Mercado-Silva et al., 2003).
Beginning around 1900 and greatly increasing during the last 20 years, fish community structure has been employed to measure relative ecosystem health (Simon, 1998). A variety of quantitative indices can be used to define specific biological criteria, including indicator species or guilds, species richness, weighted diversity indexes, and similarity indices. In addition, the sentinel species approach has been used under the assumption that the morphological or life history characteristics of two species provide good indication of the condition of the whole environment (Kovacs et al., 2002; Davies and Jackson, 2006, Rodriguez-Olarte et al., 2006). Also useful are the Index of Well-Being, multivariate ordination and classification, and the Index of Biotic Integrity (Simon, 1998; Doberstein et al., 2000; Morley and Karr, 2002).
Several studies have documented changes in fish spatial distribution as a result of pollution plumes and sewage outfalls, as well as non-point source effects such as agricultural runoff, siltation, and cultural eutrophication (Allan etal., 2001, Meade, 2004; Mol and Ouboter, 2004; Wright and Flecker, 2004; Scott, 2006; Allan et al., 2006). A monitoring approach, integrating fish assemblage structure and other biological indicator information that reflect the integrity of the water resource directly, along with water chemistry, physical habitat, toxicity testing, landscape, as well as complementary monitoring is central to accurately defining today’s varied and complex environmental problems (Yoder and Smith, 1999).
Analyses of ecological patterns generally aim to understand the functioning of ecological systems and fish-habitat relationships (Schlosser, 1987; Poizat and Pont, 1996). Several ecologists have used canonical multivariate analyses to identify and interpret statistically significant patterns in fish assemblage structure as they relate to environmental conditions (Rodriguez and Lewis, 1997; Maret, 1999; Bechara et al., 2000; Winemiller et al., 2000; Petry et al., 2003; Vila-Gispert et al., 2003). In Argentina, few attempts have been made to quantify the impact of human activities on the biological status of fish resources in freshwaters, such as Menni et al., 1984, Menni et al., 1996, Miquelarena et al., 1990, Bistoni et al., 1999, Bechara et al., 2000 and Hued and Bistoni, 2005.
Moreover, longitudinal patterns in community structure are a trait common to all rivers and the relative role of upstream versus downstream process has important implications for the management and conservation of Andean rivers (Allan et al., 2006). Several rivers that drain the Andean region are affected by human pollution (Fernández, 2005). For example, Domínguez and Fernández, 1998, mentioned that the Medina and Chico rivers are polluted by sugar cane factory activity and sewage. According to those authors, in the Medina River, total and coliform bacteria had low values during spring and summer periods, while on autumn and winter they increased to levels that threatened human health (Domínguez and Fernández, 1998). As a consequence, anthropogenic and natural effects may be confounded along the watershed and it may be difficult to discriminate them when analyzing factors affecting fish communities. Partial Canonical Correspondence Analysis (PCCA) combined with variance partitioning techniques may be useful tool to generate hypotheses concerning the influence of different sets of variables (Borcard et al., 1992, ter Braak and Smilauer, 1998) on community structure. This technique has been applied with success in a large variety of environments and taxonomic groups (Magnan et al., 1994; Bechara et al., 2000; Cottenie et al., 2003; Halpern et al., 2006).
The objective of the present study was to assess the ecological conditions and main structural patterns of fish communities as related to natural spatio-temporal gradients and anthropogenic effects in the vicinity of industries (i.e., sugar cane factories) and small towns located along the Medina River in Tucumán Province, Argentina, by using three complementary approaches. First, the study focused on species richness, catch per unit of effort (C.P.U.E.), and guilds along the longitudinal gradient of the river. The second approach involved characterization of spatial and temporal variability of the fish community structure as related to pollution and natural physical variables. Finally, we deconstructed the relative contribution of natural variables respective to water quality variables anthropogenically altered in explaining fish community structure.
MATERIAL AND METHODS
STUDY AREA
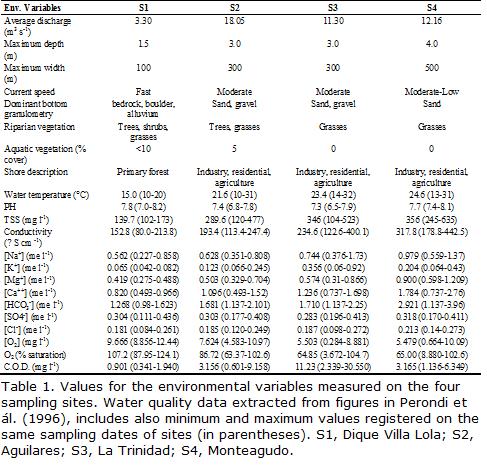
The Medina River (Fig. 1) originates in the Aconquija Mountains (Cochuna and Cañas rivers) at an altitude of 660 m above sea level (a.s.l.) and flows in a northwestern-southeastern direction into the Río Hondo Reservoir together with other three major rivers: Marapa, Gastona, and Salí. The upper portion of those rivers, up to about 400 m asl, runs along a subtropical montane cloud forest, known as Yungas. The Medina River is 123 km in length with a 2,348.3 km2 drainage area, and 18.05 m3/s mean flow at the mouth into the Río Hondo reservoir (Domínguez and Fernández, 1998). A marked seasonality in precipitation regime usually occurs. There is a strong contrast between the warmer half-year (October-March) with frequent thunderstorms, and the cooler half-year (April-September) when only 20 percent of the annual rainfall occurs (Hunzinger, 1995). During the winter, some months are completely rainless. Normally, short and intense rains (November and March) affect a limited area, while long duration and low intensity precipitation (May and September) affects a large area (Hunzinger, 1995). The mountain receives more than 1,500 mm of precipitation per year, in contrast with the 600 mm in the plains. As a consequence, the river usually supports heavy floods during the late spring and late summer, adding to the snow melt from the Aconquija Mountains in the spring. During the driest period in the rest of the years the watercourse usually flows at low levels. The main pollution inputs to the river include runoff from agricultural activities, sugar cane processing effluents, and sewage outflows from several small towns. The continuity of the river reach is not interrupted by dams permanently blocking the flow, but water diversion for agriculture and industry is a common practice, reducing annual average discharge downriver and probably affecting the natural thermal regime, particularly in sites S2-S3 (Table 1).
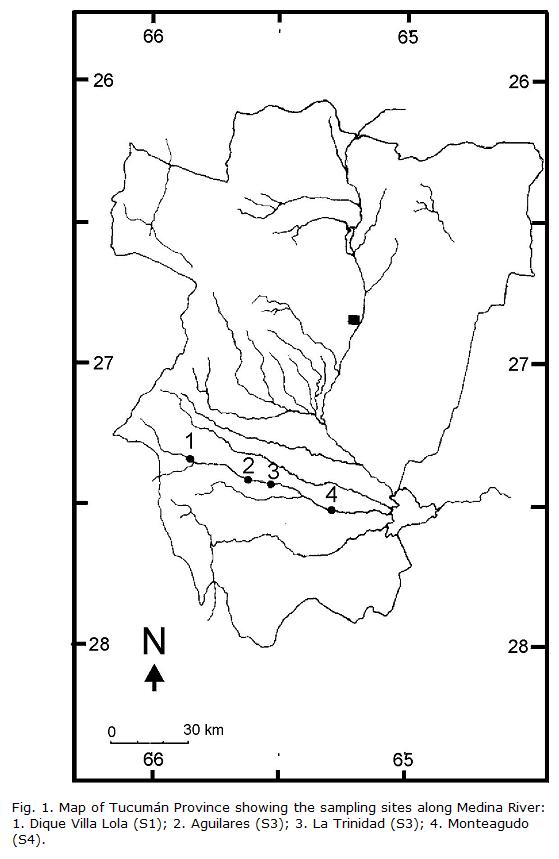
Location of the four sampling sites is shown in figure 1. They were situated along the Medina River at upstream (S1), midstream (S2 and S3), and downstream (S4) positions. The distances between sampling points respective to S1 are: 16 km to S2, 26 km to S3, and 52 km to S4. S1 (27°07’S, 65°28’W, 660 m a.s.l.) is located at the small town of Villa Lola. It is a river reach with little human influence and surrounded by dense riparian vegetation. An artificial dike 2 m high partially blocks upstream fish migrations during low water periods. S2 (27°26’S, 65°37’W, 372 m a.s.l.) is located at Aguilares, 500 m downstream of a sugar cane plant and the town of Aguilares (1,000 inhabitants). S3 (27°25’S, 65°37’W, 360 m a.s.l.) is located at the town of La Trinidad (3,830 inhabitants) and receives effluents from several sugar cane factories and the sewage outflow from the town. S4 (27°31’S, 65°17’W, 321 m a.s.l.), located at the town of Monteagudo (2,517 inhabitants), does not have sugar cane factory effluents but receives the remnants of those spilled from S2 and S3 (Perondi et al., 1996). None of these towns have sewage treatment systems.
Summarized water quality values from Perondi et al., 1996, are shown in Table 1. Some variables showed a tendency to increase downstream, such as water temperature, T.S.S. (Total Suspended Solids), conductivity, (Na+), (Ca++), (HCO3-) and (Mg-). Others decreased downriver, such as (SO4-) and D.O. (Dissolved Oxygen). In contrast, C.O.D. (Chemical Demand Oxygen) and (K-) rose steadily to S3, and then declined. pH was higher at S1, decreased at S2 and S3 and rose again at S4. At S3 and S4, very low values of D.O. occurred during winter or early spring months, periods which also exhibited the highest C.O.D. values. Oxygen supersaturation occurred in summer samples, mainly in S1 and S2.
DATA COLLECTION
Physical and chemical variables of the river. Monthly sampling was carried out between August 1992 and August 1993. Sampling was performed at three locations below (S2, S3 and S4) and one location above (S1) the discharge of sugar cane industries and sewage outflows from the small towns. Logistical problems, heavy rains and river floods precluded sampling during August and December 1992 and March and May 1993. Data on environmental variables were obtained from Perondi et al., 1996, who reported water quality values corresponding to the same sampling sites and dates (Table 1). Variables included water temperature, pH, Total Suspended Solids , conductivity, anion and cation concentrations (Na+, K+, Ca++, Mg++, HCO3-, Cl-, and SO4-), D.O. (in mg/l and % saturation) and C.O.D., the latter employed as an indicator of total dissolved organic matter. Other environmental variables such as depth, channel width, vegetation and bottom granulometry were obtained by the authors by direct measurement in the field or visual inspection. Average discharge values were obtained from -Estaciones de Aforo de Agua y Energía Eléctrica- from Provincial Government.
FISH SAMPLING
Each of the four sites was sampled by the same crew along a 200 m shoreline on one margin of the river using dip nets 60 cm long and 40 cm wide, and nylon beach seines 3 m length and 1 m high (2 mm mesh opening screen) at a maximum depth of 60 cm. The surveys, using all sampling gear, were conducted for approximately 50 minutes at each site. The techniques were slightly different according to the substrates. In the presence of large boulders such as at S1, the use of beach seines was restricted to open alluvium or bedrock sectors.
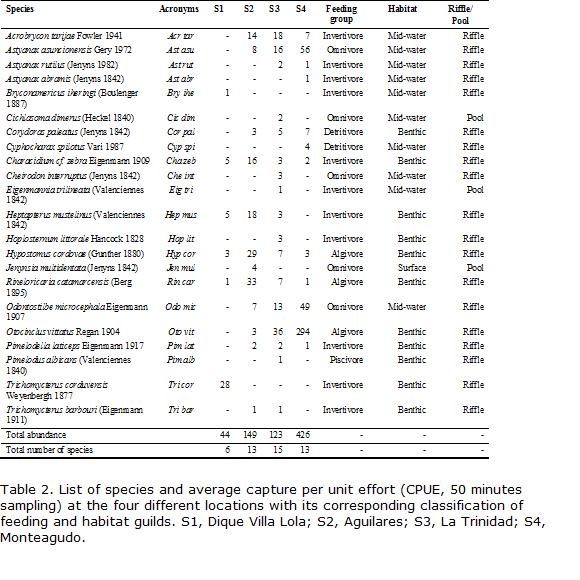
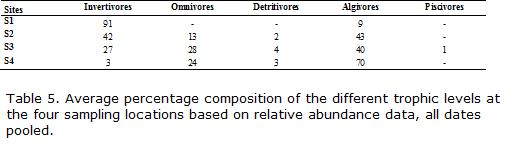
Captured fishes were fixed in formalin and later preserved in 70% ethanol for storage and identification. Results were recorded as presence-absence and number of specimens captured per unit effort (CPUE). This later variable was estimated as the total number of fish caught in 50 minutes, by pooling the two mentioned sampling devices. Voucher specimens of the 22 species found were preserved at Museo de Ciencias Naturales, Universidad Nacional de Salta, Argentina (MCNI 1351-1368, 1373-1379 and 1381-1388). Fish species were classified in feeding guilds and habitat types using available information. In some species (Heptapterus mustelinus, Trichomycterus barbouri, T. corduvensis and Characidium cf. zebra), between 10 and 25 stomach contents were analyzed, and fish species were classified in one of the following feeding guilds: algivore, herbivore, omnivore, piscivore, or invertivore. Data on the diet composition for others species were obtained from Casattiand Castro,1998, Castro and Casatti, 1997, Costa, 1987, Ferriz, 1998, Fernández, 2000, Horeau et al., 1998, Lima-Junior and Goitein, 2003, and Merona and Rankin-de-Merona, 2004. Fish were classified by habitat use according to different authors (Casatti et al., 2001; Peretti and Fatima Andrian, 2004; Pouilly et al., 2004). The following categories were established: benthic, mid-water, surface, riffle, and pool. The number of species and percentage contribution per feeding guild and habitat use was determined for every site by pooling all collections.
STATISTICAL ANALYSES
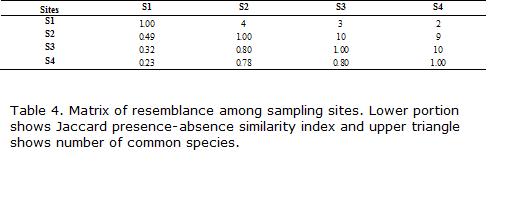
To quantify resemblance in species composition between sites, we employed the Jaccard index of similarity based on presence-absence data (Legendre and Legendre, 1998). Species from all sampling dates were pooled.
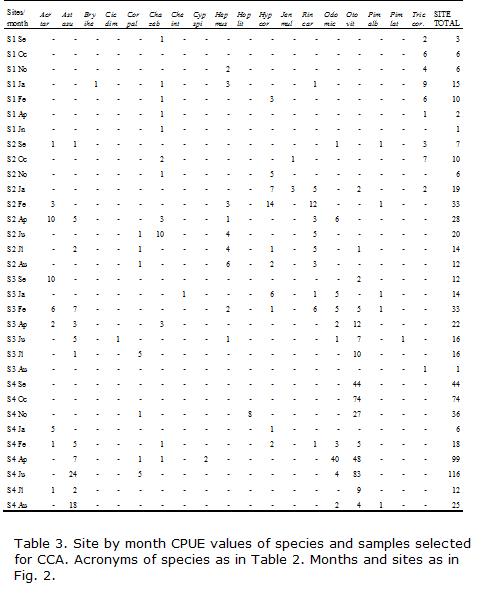
Canonical correspondence analysis (CCA; ter Braak, 1986) was carried out to detect the main ecological patterns of variation in species assemblages that can be significantly explained by the available environmental variables. This multivariate statistical approach always takes into account species relative abundance even if data are in absolute values. The species data matrix consisted in 31 sampling units and the CPUE of 12 selected species from the 22 recorded (Table 2). The following rare species, that appeared only once in the sampler, were not included in the analysis due to the sensitivity of the method to outliers: Astyanax rutilus, A. abramis, Bryconamericus iheringi, Cheirodon interruptus, Eigenmannia trilineata, Hoplosternum littorale, Cichlasoma dimerus, Cyphocharax spilotus, Pimelodella laticeps and Trichomycterus barbouri. This elimination of rare species resulted in five empty CPUE samples which had to be deleted from the matrix to perform CCA. As a result, data matrices consisted of 32 sampling units instead of the original 36.
In a first step, two different CCA were performed: (1) using the -pollution- data matrix, that consisted in 14 water quality variables as described above (Table 1). For the sake of simplicity we will refer to these data as -pollution variables- (P) in these data. To achieve a better fit of environmental variables and species data, the later were transformed to natural log (x+1). Some environmental variables that did not conform to normality were transformed to natural log (x+1) (C.O.D.), squared root (TSS) or exponential of 2.5 (D.O.); (2) another CCA was carried out employing -natural- variables of the river such as substrate, position in the longitudinal gradient, altitude, vegetation, width, depth, and current speed. For the sake of simplicity we will refer as natural variables (N) in these data. Vegetation cover was coded as a dummy variable for each category, coding them as -1- when present and -0- when absent (Legendre and Legendre, 1998). Sampling date in months was coded as an ordinal index from 1 (January) to 12 (December) and was included as a natural variable to account for temporal trends along the study period following Magnan et al., 1994. Substrate was also coded as an ordinal index, taking into account grain size, from the largest (boulders; coded 3) to the smallest (sand; coded 1). Statistical analyses were done with CANOCO Version 4.0 (ter Braak and Smilauer, 1998). Environmental variables were selected using forward stepwise procedures as implemented in CANOCO at P<0.15, so as to reduce the chances of artificial increase in explained variance as well as multicolinearity. This cut-off point allowed keeping variance inflation factor (VIF) for all selected environmental variables below 5. Downweighting of rare species as implemented in CANOCO 4.0 was also applied to reduce the excessive influence of species having high densities and low frequency of occurrence.
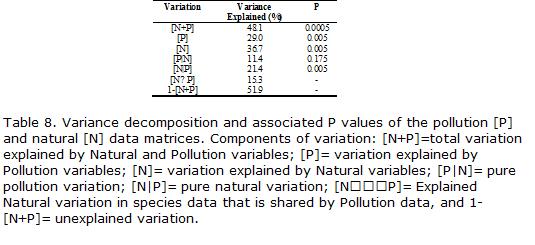
Since pollution variables may be confounded with natural variables, we decomposed the total variability in fish community structure explained by these two different sets of data using the technique of Borcard etal., 1992. The procedure consisted in the following steps:
(1) Compute the variation accounted for by pollution variables, (P) (CCAdescribed in step (1) above); (2) compute the variation accounted for by natural variables, (N) (CCA described in step (2) above); (3)compute the variation explained by the pollution variables after removal, by partial canonical correspondence analysis (PCCA), of the effect of -natural- variables, (P|N); (4) compute the variation explained by natural variables, after removal, by PCCA, of the effect of -pollution- variables, (N|P). The total explained variation (N+P) is the sum of the explained variation in (1) and (4) or in (2) and (3). The pollution (-pure-) environmental variation is given by step (3), and the natural (-pure-) environmental variation that is not related to pollution variables is given by step (4). The variation -shared- by pollution and natural variables, (N|P) is obtained by subtracting (3) from (1) or (2) from (4). The unexplained portion of variation is obtained by subtracting -pure- pollution and natural variables and shared variation from the total variation 1– (N+P). Overall significance of pollution and natural components was verified using Monte Carlo test after 2,000 random permutations, and the null hypothesis of no relationship betweenspeciesandenvironmentforthewholemodelwasrejectedwhenP<0.05(terBraak and Smilauer, 1998).
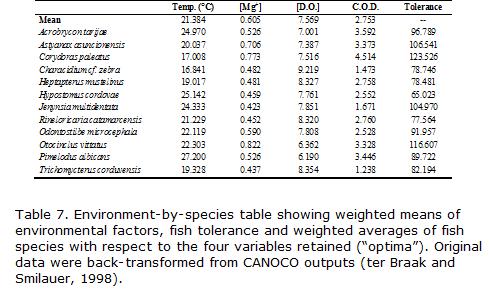
We also estimated the optimum condition and tolerance of the selected species with respect to the pollution variables, both indicators of species niche attributes (ter Braak and Smilauer, 1998). Due to the unimodal properties of the CCA, the optimum can be approximated by the CANOCO 4.0 output named -species-by-environment-table-, which are weighted averages of species with respect to standardized environmental variables. Since triplots with species data may be in error because they contain the main pattern only, this table is useful to verify that inferences drawn from the plots hold true in the actual data on weighted averages (ter Braak and Smilauer, 1998). These two species properties are derived from unimodal Gaussian curves relating environmental variables to abundance or frequency of occurrence. Optimum is defined as the value of a given environmental variable that corresponds to the maximum abundance of a species. Tolerance is a measure of ecological amplitude respective to the environmental variables (Jongman et al., 1995).
RESULTS
HABITAT DESCRIPTION
There were clear differences in habitat conditions between the upstream sampling point (S1) and the downstream sites (S2-S4) (Table 1). At the former, the water currents were faster than downstream and the substrate were mainly bedrock and boulders. Moreover, the riparian vegetation canopy covered completely the river preventing direct light penetration. The habitats of sampling sites S2 and S3 were fairly similar, and characterized by medium flow velocity, with the substrate composed mainly of sand and gravel. The riparian vegetation canopy only partially covered the river. Low water currents, higher depths,sandy-gravel substrate and restricted riparian vegetation cover characterized site S4.
FISH COMMUNITY ASSESSMENT
Information about fish captured at the four studied locations is summarized in Table 2. A total of 742 individuals were sampled, involving 22 species, 19 genera and 12 families. The greatest number of species (15) was collected in S3 and the fewest (6) in S1. They belong to different trophic guilds and had variable degree of tolerance to pollution. The genus Trichomycterus was only found in the two uppermost sites. Two species (Characidium cf. zebra and Hypostomus cordovae) were captured at all four sampling locations. Eight other species (Acrobrycon tarijae, Astyanax asuncionensis, Corydoras paleatus, Heptapterus mustelinus, Rineloricaria catamarcensis, Odontostilbe microcephala, Otocinclus vittatus and Pimelodella laticeps) were found at least at three of the four sites. In general, the species captured in most sites were also more abundant in number (CPUE, Tables 2 and 3). Maximum CPUE values occurred during summer months in S1, but during summer and autumn in S2 and S3 and spring and autumn in S4. High summer values in S1 were mainly due to increased densities of Trichomycterus corduvensis. At S3 and S4, high CPUE values were related to the growing downstream abundance of Astyanax asuncionensis, Odontostilbe microcephala and Otocinclus vittatus. High summer and autumn values at S2 were chiefly due to Hypostomus cordovae and Rineloricaria catamarcensis and in lesser extent to Acrobrycon tarijae and Characidium cf. zebra.
S1 had the highest similarity with S2, and shared Heptapterus, Cheirodon, and Trichomycterus (Table 4). Species in common and similarity with the other two sites declined markedly downstream. In contrast, the three downstream sites had much higher and comparable levels of similarity in species presence-absence. S2 differed from S3 (nearly 10 km below) by the presence of Jenynsia and the absence of Cichlasoma, Cheirodon, Eigenmannia and Pimelodus. Site S4 differed from S2 and S3 by the presence of Cyphocharax and Hoplosternum (Table 2).
The guilds with the greatest variation among sites included omnivores and invertivores, while piscivores were rare or absent (Table 5). The invertivores were indicators of more distant and less degraded environments, and were proportionally more important at S1. At S2 there were still more species of invertivores compared with S3 downstream. At S4 the percentage of omnivores was higher than the other three locations. Regarding habitat guilds, the number of benthic species was high in all sites (Table 2) and at S3 and S4 the midwater species raised in number, in relation to increasing depth and lower water speeds (Table 1).
CANONICAL CORRESPONDENCE ANALYSES (CCA)
The results of CCA involving to pollution data for all retained variables and sampling dates are shown in Fig. 2 and Table 6. Environmental variables accounted for 29.0% of total variation in species composition among sites and dates, and the overall Monte Carlo test showed significant species-environment relationship (P<0.0005). The first two axes encompassed the majority of the total explained variability (Table 6) and were used in environmental gradient interpretations.
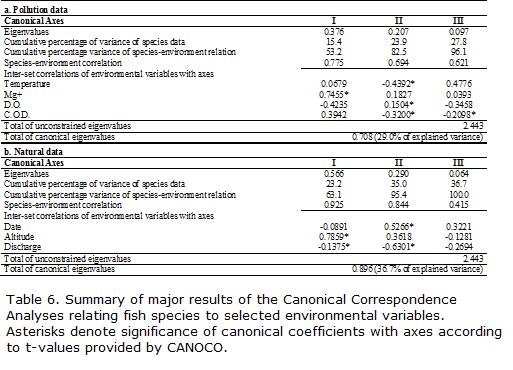
Gradient analysis indicates the possible influence of a small set of water quality variables on the spatial and temporal distribution of species assemblages along the Medina River. The first axis was negatively related to a gradient in dissolved oxygen and positively to a gradient of (Mg+) and C.O.D, with the latter employed as an indicator of dissolved organic matter. The second axis showed high negative correlations with C.O.D. and water temperature, but the remaining variables contributed little to the axis (Fig. 2, Table 6). Note that the vector length for a variable represents the relative importance of that variable for predicting (in the sense of multiple regression) fish habitat use (Copp, 1994).
Samples showed a general ordination pattern following upstream-downstream direction (Fig. 2). All samples from S1 were located at the upper-left quadrant of the plot and most samples of S4 on the upper-right side. Samples from S2 and S3 were placed in-between. However, temporal and spatial variability were confounded to a certain degree along the two canonical axes. Indeed, samples from all sites were closer in September, January and February, when they were located mostly toward the lower portion of the diagram, indicating higher temperatures and medium to high D.O. values. In contrast, most winter and spring samples from S1 were generally placed toward the upper-left side of the plot, while the winter and early-spring samples of the remaining sites tended to be placed toward the right side, indicating different seasonal responses of fish communities in relation to contrasted water quality conditions. Samples from this later site also exhibited a less marked temporal variability when compared to the remaining sites. Changes in (Mg+) similarly followed a wet-dry seasonal pattern from left to right along the first axis. Species locations in ordination diagrams represent the registered optima in relation to environmental variables along the first two axes. In addition, the species plotted near the center of diagram are more likely to be abundant in a wider range of habitat types (Fig. 2). Trichomycterus corduvensis and Characidium cf. zebra exhibited a preference for the highest D.O. levels, and the lowest temperatures and C.O.D. (Fig. 2, Table 6), being frequent and abundant in more pristine lotic habitats (S1-S2). As a consequence, these species were found to co-occur more often than expected simply by random process in both sites. Other species required high oxygen levels, but were also more tolerant to warmer temperatures or higher C.O.D. levels, such as Heptapterus mustelinus and Rineloricaria catamarcensis.
Some species (Otocinclus vittatus, Corydoras paleatus, Acrobrycon tarijae and Astyanax asuncionensis) were found in higher relative abundance in more polluted conditions. They inhabited waters having the lowest D.O. and the highest C.O.D. Other species might be considered intermediate in environmental preference, such as Jenynsia multidentata, Hypostomus cordovae, and Odontostilbe microcephala.
Species found more frequently in the more pristine habitats had generally the lowest tolerance, including Characidium cf. zebra, Heptapterus mustelinus, Trichomycterus corduvensis, and Rineloricaria catamarcensis. The most tolerant species were Otocinclus vittatus, Corydoras paleatus and Astyanax asuncionensis, which were found in a wide range of water quality conditions (Table 7). Some species exhibited intermediate tolerance in spite of being common at more degraded habitats. These include Acrobrycon tarijae and Pimelodus albicans which also seem to prefer higher water temperatures.
Results of the CCA involving natural data for all retained variables and sampling dates are shown in Table 6 and Figure 3. Selected environmental variables accounted for 36.7% of total variation in species composition among sites and dates, with the overall Monte Carlo test showed significant species-environment relationship (P<0.0005). The first two axes encompassed a large proportion of total explained variability (Fig. 3, Table 6) and were employed in interpretations of environmental gradients. The first axis was positively related to a gradient of altitude and negatively to a gradient of discharge. The second axis was negatively related with discharge and positively with time of the year; the remaining variable contributed little to the axis (Fig. 3, Table 6). In a manner similar to that of the previously shown pollution plot (Fig. 2), samples exhibited an ordination pattern in upstream-downstream sense along the first axis, but in this case it followed an altitudinal gradient, which also explained the influence of variables excluded from the model to avoid artificial over explanation, such as position along the river, decreasing current speed, decreasing bottom substrate size, decreasing riparian cover, among others (Table 1). In that manner, S1 samples appear grouped to the upper-right sector of the axis I and S3-S4 are placed closely on the opposite side, but with a larger occurrence in the discharge gradient. S2 appears intermediate at similar distances from the previous sites along this first Axis. Axis II explains mainly temporal variability, which is similar for all sites, with samples ordered nearly in parallel with that axis from spring (larger month numbers, 10-11) on the upper side, followed by autumn-winter samples (intermediate month numbers, 4-9), and finishing with summer samples (inferior month numbers, 1-2) in the lower portion.
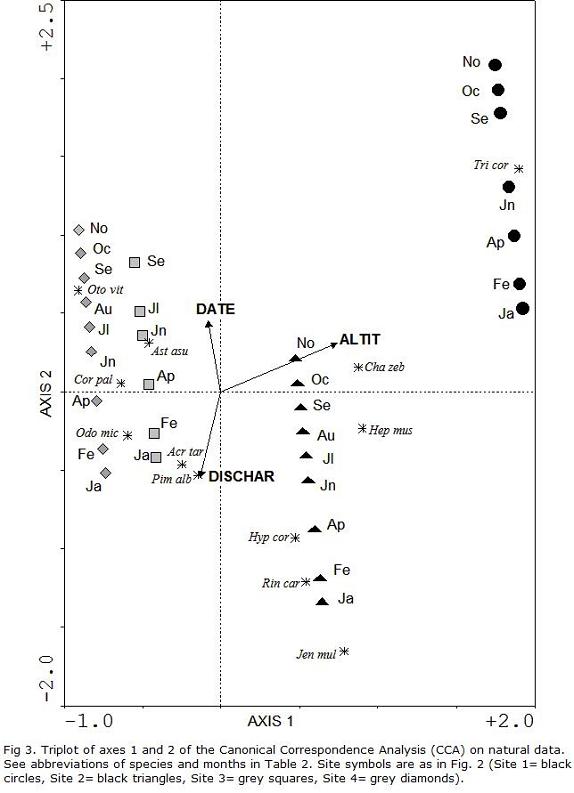
In terms of species locations along the altitudinal gradient, there was a prevalence of Trichomycterus corduvensis in the uppermost site (S1), followed by Characidium cf. zebra and Heptapterus mustelinus at lower altitudes (S1-S2) (Fig. 3). Hypostomus cordovae, Rineloricaria catamarcensis and Jenynsia multidentata were common in S2 at intermediate altitudinal levels. The remaining species were mainly related to S3 and S4 at the lowest altitudes. Along the second canonical axis, H. cordovae, R. catamarcensis and J. multidentata were common in summer samples of higher discharge sites. T. corduvensis and Otocinclus vittatus were located at opposite sides of the gradient, being typical of spring-autumn and low or intermediate discharge levels.
VARIANCE DECOMPOSITION
As depicted in Table 8, there was a significant relationship between pollution (P) and natural (N) variables within the fish community structure. However, the pollution component of the total variability was not significant after partialling-out the natural effects (P|N). In contrast, natural effects remained significant after the removal of pollution effects (N|P). Together, the two sets of variables explained a relatively large proportion of the total variability (48%), and also shared an important proportion of total variance (15%).
DISCUSSION
Based on our results, a seasonally variable longitudinal pattern of species composition occurs from the uppermost to the lowermost locations, as evidenced by the significance of -pure- natural variables in variance decomposition, suggesting the importance of altitude and its correlated variables, and to a lesser extent seasonal variation, in controlling fish assemblage structure. As showed by Hoeinghaus et al., 2004, compositional changes along a river continuum have been in general characterized as distinct faunal assemblages, with additions of species by environmental complexity and connectivity that increased downstream. Several studies conducted on Neotropical rivers and streams over the last two decades have found significant patterns of fish assemblage differentiation along longitudinal gradients (Ibarra and Stewart, 1989; da Silva Abes and Agostinho, 2001; Hoeinghaus et al., 2004; Gerhard et al., 2004; Fialho et al., 2007; Suarez and Petrere Junior, 2006, 2007).
Our analyses examined fish assemblage composition along the upper S1 (piedmont) and lower S2-S4 (llanos) sections. The upstream site (S1) had lower species richness, and species number increased downriver and that number remained fairly constant after S2. It may reflect in part the longest distance to the sources of colonization, and the increasing difficulty for upstream migration due to natural obstacles at higher altitudes, as well as artificial obstructions and pollution plumes. At the basin scale, Olden et al., 2001, found that connectivity was a major factor explaining species composition in fish communities along a network of lakes connected by streams on the Canadian Shield.
Suárez and Petrere Júnior, 2007),found that the most important variable explaining variation in fish community structure along a tributary of the Paraná River in Brazil was altitude, with little contribution of temporal variability.
We observed the highest species richness in the middle region (S3). These finding are in contrast to previous longitudinal gradient studies that generally have described patterns of increase in species richness in lower river stretches in Neotropical rivers (Videla and Bistoni, 1999; Bistoni and Hued, 2002; Hoeinghaus et al., 2004). The slight decrease in species richness in the lower most site (S4), contrary to the expected rise due to higher connectivity may reflect sampling error, but could also reflect an increasing effect of organic pollution downriver, as evidenced by lowest D.O. and highest C.O.D. values. The oxygen concentration decrease and organic matter increase during low water periods may explain part of the longitudinal differentiation, because they were variables with high correlations along the first and second axis of pollution CCA and have potentially a major influence on species composition, due to its physiological role on growth and survival. This assumption is however, tentative because the -pure- pollution effect was not significant, due in part to the large proportion of that effect shared with natural variability. For example, the first axis appears as a pollution gradient separating upstream from downstream sites, but in fact it is more likely an altitudinal gradient, to which pollution variables are correlated (Perondi et al., 1996). Similarly, the second axis in the pollution CCA shows temperature variations related mainly to water temperature that in the natural CCA is chiefly temporal variability. In fact, Pearson linear correlations between scorers of the first and second axes of the two CCA were both highly significant (r=0.76 and r=0.54; respectively, P<0.001; n=31).
The seasonal variability in longitudinal patterns was apparent when comparing winter against spring–summer samples. In this later seasons, when discharge increased, and sugar cane factories activity ceased, a recovery in river water quality was reflected by a larger resemblance among fish assemblages from different sites. Species that characterized the winter samples of the lower most site (S4, Astyanaxasuncionensis, Otocinclusvittatus, Corydoras paleatus) were also the most tolerant to a wide range of variation of pollution indicating variables (Fig. 2, Table 7). S3 and S4 also had the largest number of omnivorous species. In contrast, most samples from S1 and some months in S2 were characterized by species with lower tolerance, these being mostly benthic invertivores (Characidium cf. zebra, Trichomycterus corduvensis, and Heptapterus mustelinus). In addition, S1 had lower water temperature, which may have limited the range expansion of some Neotropical species upstream, a long with connectivity. This fact can also explain the presence of the three latter species which had the lowest optima for temperature (Table 7). In contrast, some species were more common during spring-summer at low altitude sites, preferring higher temperatures, such as Pimelodusalbicans, a migratory species probably moving downstream for reproduction, as well as Acrobrycon tarijae (Table 6).
In addition, some of the observed differences in species assemblage composition along the longitudinal gradient of the Medina River probably reflected local physical habitat features. Hoeinghaus et al., 2004, found that depth was the most important habitat variable related to fish assemblage composition in Neotropical streams from Venezuela. Several species of invertivore catfishes (e.g., genus Trichomycterus), are typical of the more rhytronic sectors, potentially due to their better adaptations to lotic habitats, but coupled with the supply of trophic resources according to their feeding habits, as well as the lower abundance of predators (Habit et al., 2005). Other authors also found a relationship between physical habitat features and fish community structure. Ibarra and Stewart, 1989, Da Silva Abes and Agostinho, 2001, found greatest species assemblage turnover along a longitudinal gradient in association with major changes in substrate composition (i.e., absence of rock/cobble in lower stretches). Also, the habitat complexity downstream supplies a wider variety of hiding places for fish.
In streams of the upper Paraguay River, Valerio et al., 2007, proposed that water velocity was the most important descriptor of community composition, followed by stream width, altitude, water conductivity, and stream depth. The water velocity of a stream may be also a factor hidden in our sample ordination, since a gradient of decreasing water velocity downstream was detected, with a concomitant increase of mid-water species. Hypostomus also occurred at higher densities over the predominant pebble and gravel substrates that are typical of fast-flowing lotic habitats. Although Trichomycterus was originally considered to be exclusively rheophilous, its juveniles were observed almost entirely in the absence of water currents and in shallow waters along marginal standing waters (LF, pers. obs.). In streams of the Colombian Andes, Chará et al., 2006, found only small individuals of Trichomycterus sp. in shallow waters during the day, areas normally avoided by juveniles and adults. According to Schlosser, 1987, environmental features of the dike fields (slow currents, high temperatures, and sand) make them suitable as nurseries for riverine fish and beaches may provide protection against swimming predators just as shallow raceways do in streams.
In summary, the main findings of the present study suggest that in spite of water quality degradation by sugar cane factories and urban development, natural variability still explained a major and significant amount of spatial and temporal variation in fish community along the river continuum. Organic pollution effects during droughts generated low oxygen concentrations, favoring the predominance of the most tolerant species in the two lower most sites and probably modified the expected increase in species richness down river. Summer and spring floods improved water quality, thereby allowing the recovery of the fish fauna in polluted reaches. The pristine upstream site had lesser environmental variability and supported lower species richness, with a community composed mainly by invertivores with reduced tolerance and with higher dissolved oxygen requirements. Therefore, a suitable management strategy for reducing pollution effects in rivers affected by sugar cane factories might be limiting waste discharge to high flow periods (December-March) when the natural purifying capacity of the rivers would be at its maximum.
ACKNOWLEDGEMENTS
We thank E. Domínguez, F. Cruz, and H. Fernández for comments on previous drafts of the manuscript. Funding for surveys were provided by Universidad Nacional de Tucumán: -Proyecto CIUNT 2005-2008, Estructura y funcionamiento de un río subtropical de montaña: Cuenca Río Lules, 26G/309-. L. Fernández was supported by a Kalbfleisch Pos tdoctoral Fellowship at the American Museum of Natural History, New York. Hospitality was provided by A. Bobadilla, S. Schneider, F. Lobo, G. Gonzo, and S. Valdecantos. This paper benefited from comments and suggestions of M. Stiassny and C. Ferraris, Jr.
BIBLIOGRAPHY
ALLAN JD, BRENNER AJ, ERAZO J, FERNANDEZ L, FLECKER AS, et al. Land use in water sheds of the Venezuelan Andes: a comparative analysis. Conserv Biol. 2001; 16:527-538.
[ Links ]ALLAN JD, FLECKER AS, SEGNINI S, TAPHORN DC, SOKOL E, KLING GW. Limnology of Andean piedmont rivers of Venezuela. J N Am Benthol Soc. 2006;25:66-81.
[ Links ]BECHARA JA, ROUX JP, SÁNCHEZ S, TERRAES JC, DOMITROVIC HA. Fish community variation below Yacyretá Dam (Paraná River, Argentina): the relative contribution of microhabitat, hydrology and limnology. Acta Limnol Bras. 2000;12:23-38.
[ Links ]BISTONI MA, HUED AC. Patterns of fish species richness in rivers of the central region of Argentina. Braz J Biol. 2002;62:753-764.
[ Links ]BISTONI MA, HUED AC, VIDELA MM, SAGREtti L. Efectos de la calidad del agua sobre las comunidades ícticas de la región central de Argentina. Rev Chil Hist Nat. 1999;72:325-335.
[ Links ]BORCARD DP, LEGENDRE P, DRAPEAU P. Partialling out the spatial component of ecological variation. Ecology. 1992;73:1045-1055.
[ Links ]CASATTI L, CASTRO RMC. A fish community of the São Francisco River headwaters riffles, southeastern Brazil. Ichthyol Explor Fres. 1998;9:229-242.
[ Links ]CASATTI L, LANGEANI F, CASTRO RMC. Peixes de riacho do parque estadual Morro Do Diabo Bacia do Alto Río Paraná, SP. Biota Neotropica. 2001;http://www.biotaneotropica.org.br/v2n2/pt/abstract/inventory+BN00201122001.
[ Links ]CASTRO RMC, CASATTI L. The fish fauna from a small forest stream of the upper Paraná River basin Southeastern Brasil. Ichthyol Explor Fres. 1997;7:337-352.
[ Links ]CHARÁ JD, BAIRD DJ, TELFER TC, RUBIO EA. Feeding ecology and habitat preferences of the catfish genus Trichomycterus in low-order streams of the Colombian Andes. J Fish Biol. 2006;68:1026-1040.
[ Links ]COPP GH, GUTI G, ROVNY B, CERNY J. Hierarchical analysis of habitat use by 0+ juvenile fish in Hungarian/Slovak flood plain of the Danube River. Env Biol Fish. 1994;40:329-348.
[ Links ]COSTA WJEM. Feeding habits of a fish community in a tropical coastal stream, Río Mato Grosso, Brazil. Stud Neotrop Fauna E. 1987;22:145-153.
[ Links ]COTTENIE K, MICHELS E, NUYTTEN N, DE MEESTEr L. Zooplankton Metacommunity Structure: Regional Vs. Local Processes in highly interconnected ponds. Ecology. 2003;84:991-1000.
[ Links ]DA SILVA ABES S, AGOSTINHO AA. Spatial patterns in fish distributions and structure of the ichthyocenosis in the Agua Nanci stream, upper Paraná River basin, Brazil. Hydrobiologia. 2001;445:217-227.
[ Links ]DAVIES SP, JACKSON SK. The biological condition gradient: a descriptive model for interpreting change in aquatic ecosystems. Ecol Appl. 2006;16:1251-1266.
[ Links ]DOMÍNGUEZ E, FERNÁNDEZ HR. Calidad de los ríos de la cuenca del Salí (Tucumán, Argentina) medida por un índice biótico. Fundación Miguel Lillo, serie con-servación de la naturaleza. Tucumán, Argentina; 1998.
[ Links ]DOBERSTEIN CP, KARR JR, CONQUEST LL. The effect of fixed-count subsampling on macroinvertebrate biomonitoring in small streams. Freshwater Biol. 2000;44:355-371.
[ Links ]FERNÁNDEZ L. Redescription of the teleost Trichomycterus barbouri (Eigenmann, 1911) occurrence in Argentina and comparison with related species (Ostariophysi: Siluriformes: Trichomycteridae). Stud Neotrop Fauna E. 2000;35:27-33.
[ Links ]FERNÁNDEZ L. Risk of extinction of a rare catfish of Andean groundwater and its priority for conservation. Ambio. 2005;34:69-70.
[ Links ]FERRIZ RA. Alimentación de Trichomycterus corduvense Weyenbergh, 1879 (Teleostei: Trichomycteridae) en dos ríos serranos de San Luis, Argentina. Revista Museo Argentino de Ciencias Naturales -Bernardino Rivadavia-. 1998;8:43-49.
[ Links ]FIALHO AP, OLIVEIRA LG, TEJERINA-GARRO FL, GOMES LC. Fish assemblage structure in tributaries of the Meia Ponte River Goias, Brazil. Neotrop Ichthyol. 2007;5:53-60.
[ Links ]GERHARD P, MORAES R, MOLANDER S. Stream fish communities and their associations to habitat variables in a rain forest reserve in southeastern Brazil. Env Biol Fish. 2004;71:321-340.
[ Links ]HABIT E, VICTORIANO P, CAMPOS H. Ecología trófica y aspectos reproductivos de Trichomycterus areolatus (Pisces, Trichomycteridae) en ambientes lóticos artificiales. Rev Biol Trop. 2005;53:195-210.
[ Links ]HALPERN B, COTTENIE K, BROITMAN B. Strong Top-Down control in Southern California kelp forest ecosystems. Science. 2006;312:1230-1232.
[ Links ]HOEINGHAUS D, WINEMILLER KO, TAPHORN DC. Compositional change in fish assemblages along the Andean piedmont-Llanos floodplain gradient of the río Portuguesa, Venezuela. Neotrop Ichthyol. 2004;2:85-92.
[ Links ]HOREAU V, CERDAN P, CHAMPEAU A, RICHARD S. Importance of aquatic invertebrates in the diet of rapids-dwelling fish in the Sinnamary river French Guiana. J Trop Ecol. 1998;14:851-864.
[ Links ]HUED AC, BISTONI MA. Development and validation of a Biotic Index for evaluation of environmental quality in the central region of Argentina. Hidrobiología. 2005;543:279-298.
[ Links ]HUNZINGER H. La precipitación Horizontal: su importancia para el bosque y a nivel de cuenca en la sierra de San Javier, Tucumán. Argentina. In: Brown A, Grau D, editors. Investigación, conservación, y desarrollo en las selvas subtropicales de montaña. Proyecto de Desarrollo Agroforestal / LIEY 1995. Universidad Nacional Tucumán, Tucumán, Argentina; 1995. p. 53-58.
[ Links ]IBARRA M, STEWART DJ. Longitudinal zonation of sandy beach fishes in the Napo River basin, eastern Ecuador. Copeia. 1989;(2):364-381.
[ Links ]JONGMAN RHG, TER BRAAK CJF, VAN TONGEREN OFR. Editors. Data analysis in community and landscape ecology. Cambridge University Press, UK; 1995. p. 299.
[ Links ]KOVACS TG, MARTEL PH, VOSS RH. Assessing the biological status of fish in a river receiving pulp and paper mill effluents. Environ Pollut. 2002;118:123-140.
[ Links ]LEGENDRE P, LEGENDRE L. Numerical Ecology. Second English Edition. Elsevier, Amsterdam, Holland; 1998.
[ Links ]LIMA-JUNIOR SE, GOITEIN R. Ontogenetic diet shifts of a Neotropical catfish, Pimelodus maculatus (Siluriformes, Pimelodidae): An ecomorphological approach. Env Biol Fish. 2003;68:73-79.
[ Links ]MAGNAN P, RODRIGUEZ MA, LEGENDRE P, LACASSE S. Dietary variation in a freshwater fish species: Relative contributions of biotic interactions, abiotic factors, and spatial structure. Can J Fish Aquat Sci. 1994;51:2856-2865.
[ Links ]MARET TR. Characteristics of fish assemblages and environmental conditions in streams of the upper Snake River basin in eastern Idaho and western Wyoming. In: Simon TP, editors. Assessing the sustainability and biological integrity of water resources using fish communities. CRC Press, Boca Raton Florida; 1999. p. 273-299.
[ Links ]MEADE R. Fish and invertebrate recolonization in a Missouri Prairie stream after an acute pollution event. N Am J Fish Manage. 2004;24:7-19.
[ Links ]MERCADO-SILVA N, LYONS JD, MALDONADO GS, NAVA MM. Validation of a fish-based index of biotic integrity for streams and rivers of central México. Rev Fish Biol Fisher. 2003;12:179-191.
[ Links ]MENNI RC, LOPEZ HL, CASCIOTTA JR, MIQUELARENA AM. Ictiología de las áreas serranas de Córdoba y San Luis (Argentina). Biología Acuática. 1984;5:1-63.
[ Links ]MENNI RC, GÓMEZ SE, ARMENGOL FL. Subtle relationships: freshwater fishes and water chemistry in southern South America. Hidrobiología. 1996;328:173-197.
[ Links ]MERCADO-SILVA N, LYONS JD, MALDONADO GS, MEDINA NAVA M. Validation of a fish-based index of biotic integrity for streams and rivers of central México. Fish Biology and Fisheries. 2003;12:179-191.
[ Links ]MERONA B, RANKIN-DE-MERONA J. Food resource partitioning in a fish community of the central Amazon floodplain. Neotrop Ichthyol. 2004;2:75-84.
[ Links ]MIQUELARENA AM, MENNI RC, LOPEZ HL, CASCIOTTA JR. Ichthyological and limnological observations on the Salí river basin (Tucumán, Argentina). Ichthyol Explor Fres. 1990;1:269-276.
[ Links ]MOL JH, OUBOTER PE. Downstream effects of erosion from small-scale gold mining on the instream habitat and fish community of a small Neotropical rainforest stream. Conser Biol. 2004;18:201-214.
[ Links ]MORLEY SA, KARR JR. Assessing and restoring the health of urban streams in the Puget Sound Basin. Conser Biol. 2002;16:1498-1509.
[ Links ]OLDEN JD, JACKSON DA, PERES-NETO PR. Spatial isolation and fish communities in drainage lakes. Oecologia. 2001;127:572-585.
[ Links ]PERETTI D, DE FATIMA ANDRIAN I. Trophic structure of fish assemblages in five permanent lagoons of the high Paraná River floodplain, Brazil. Env Biol Fish. 2004;71:95-103.
[ Links ]PERONDI ME, GALINDO MC, VECE MB, APELLA MC, HIDALGO M. Releva-miento de variables fisicoquímicas en el río Medina, Provincia de Tucumán. Serie mono-gráfica y didáctica n.° 31, Fac. Cs. Ns. e IML, UNT. Tucumán, Argentina. 1996. p. 25.
[ Links ]PETRY P, BAYLEY PB, MARKLE DF. Relationships between fish assemblages, macrophytes and environmental gradients in the Amazon River floodplain. J Fish Biol. 2003;63:547-579.
[ Links ]POIZAT G, PONT D. Multi-scale approach to species-habitat relationships: juvenile fish in a large river section. Freshwater Biol. 1996;36:611-622.
[ Links ]POUILLYM,YUNOKIT,ROSALESC,TORRESL.Trophic structure of fish assemblages from Mamore river floodplain lakes (Bolivia). Ecol Freshw Fish. 2004;13:245-257.
[ Links ]RODRIGUEZ MA, LEWIS Jr WM. Structure of fish assemblages along environmental gradients in floodplain lakes of the Orinoco river. Ecol Monogr. 1997;67:109-128.
[ Links ]RODRIGUEZ-OLARTE D, AMARO A, CORONEL J, TAPHORN BDC. Integrity of fluvial fish communities is subject to environmental gradients in mountain streams, Sierra de Aroa, north Caribbean coast, Venezuela. Neotrop Ichthyol. 2006;4:319-328.
[ Links ]SCHLOSSER IJ. The role of predation in age-and size-related habitat use by stream fishes. Ecology. 1987;68:651-659.
[ Links ]SCOTTMC.Winner sand losers among stream fishes in relation to land use legacies and urban development in the southeastern US. Biol Conserv. 2006;127:301-309.
[ Links ]SIMON TP. Modification of an index of biotic integrity and development of reference conditions expectations for dunal, palustrine wetland fish communities along the southern shore of Lake Michigan. Aquat Ecosyst Health. 1998;1:49-62.
[ Links ]SUAREZ YR, PETRERE JUNIOR M. Gradientes de diversidade nas comunidades de peixes da bacia do río Iguatemi, Mato Grosso do Sul, Brasil. Iheringia Ser Zool. 2006;96:197-204.
[ Links ]SUAREZ YR, PETRERE JUNIOR M. Environmental factors predicting fish community structure in two neotropical rivers in Brazil. Neotrop Ichthyol. 2007;5:61-68.
[ Links ]TER BRAAK CJF. Canonical correspondence analysis, a new eigenvector technique for multivariate direct gradient analysis. Ecology. 1986;67:60-72.
[ Links ]TER BRAAK CJF, SMILAUER P. CANOCO reference Manual and User s guide to Canoco for Windows. Software for Canonical Community Ordination (version 4). Microcomputer Power. Ithaca, NY, USA. 1998. p. 352.
[ Links ]VALERIO SB, SUAREZ YR, FELIPE TRA, TONDATO KK, XIMENES LQL. Organization patterns of headwaters-stream fish communities in the Upper Paraguay-Paraná basins. Hydrobiologia. 2007;583:241-250.
[ Links ]VIDELA MM, BISTONI MA. Composición y estructura de la comunidad íctica de un río serrano a lo largo de un gradiente altitudinal. Iheringia, Ser Zool. 1999;87:171-180.
[ Links ]VILA-GISPERT A, MORENO-AMICH R, GARCIA-BERTHOU E. Gradients of life-history variation: an intercontinental comparison of fishes. Rev Fish Biol Fisher. 2003;12:417-427.
[ Links ]WINEMILLER KO, TARIM S, SHORMANN D, COTNER JB. Fish assemblage structure in relation to environmental variation among Brazos river oxbow lakes. T Am Fish Soc. 2000;129:451-468.
[ Links ]WRIGHT JP, FLECKER AS. Deforesting the riverscape: the effects of wood on fish diversity in a Venezuelan piedmont stream. Biol Conserv. 2004;120:439-447.
[ Links ]YODER CO, SMITH MA. Using fish assemblages in a state biological assessment and criteria program: essential concepts and considerations. In: Simon TP editors. Assessing the sustainability and biological integrity of water resources using fish communities. Published CRC Press, Boca Raton Florida, US; 1999. p. 17-56.
[ Links ]













