Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Acta Biológica Colombiana
Print version ISSN 0120-548X
Acta biol.Colomb. vol.17 no.2 Bogotá May/Aug. 2012
IMPROVING THE TOLERANCE OF Vicia faba AGAINST ENVIRONMENTAL SALINITY RESULTED FROM THE IRRIGATION WITH SEA WATER BY USING KNO3 AND (NH4)2SO4 AS CHEMICAL OSMOREGULATORS
Mejoramiento de la tolerancia de Vicia faba a salinidad ocasionada por irrigación con agua de mar usando KNO3 AND (NH4)2SO4 como osmoreguladores químicos
MOHAMED KAMEL1, Ph. D.
1Botany Department, Faculty of Sciences at Qena, South Valley University, BOX 83523, Qena, Egypt. mohamedkamelahmed@yahoo.com
Presentado el 30 de enero de 2012, aceptado el 2 de mayo de 2012, correcciones el 21 de junio de 2012.
ABSTRACT
The familiar solutes, Na+, K+, Ca2+, Mg2+, Cl-, PO43-, SO42-, soluble carbohydrates, amino acids and soluble proteins, which play a role in osmotic adjustment were estimated to investigate the role of potassium nitrate and ammonium sulphate as osmoregulators and their effects on the solutes composition. Vicia faba L. was cultivated and irrigated with 5, 10, 15 and 20 % (v:v) sea water. The plants were divided to three groups. The first was irrigated with sea water only. The second was treated with 5 mM KNO3 while the third was treated with 5mM (NH4)2SO4. The plants were left to grow until flowering stage. The results indicated that the non treated group increased the soluble carbohydrates in the roots to avoid the influx of sodium. The treatment with KNO3 decreased the sodicity (SAR) while (NH4)2SO4 treatment decreased the SK:Na value in the shoots at higher salinity. The availability of nitrogen as nitrate or ammonium ions enhances the accumulation of soluble carbohydrates in shoots. The plants of all groups were depended on Ca2+, as compatible solute more than Na+, and K+.
Key words: Osmoregulation, irrigation, salinity, sea water, Vicia faba.
RESUMEN
Los solutos Na+, K+, Ca2+, Mg2+, Cl-, PO43-, SO42-, carbohidratos y proteínas solubles, así como amino ácidos son importantes en ajuste osmótico y fueron estimados para determinar el papel de nitrato de potasio y sulfato de amonio como osmoreguladores y su efecto en la composición de solutos. Vicia faba L. fue cultivada e irrigada con 5, 10, 15 y 20 % (v:v) de agua de mar permitiendo crecimiento hasta el estado de floración. Las plantas fueron divididas en tres grupos. El primero fue irrigado con agua de mar solamente. El segundo fue tratado con 5 mM KNO3, mientras que el tercer grupo fue expuesto a 5mM (NH4)2SO4. Los resultados indican que las plantas del primer grupo (no tratado) incrementan carbohidratos solubles en sus raíces para evitar influjo de sodio. El tratamiento con KNO3 disminuye riqueza de sodio (SAR) mientras que la exposición a (NH4)2SO4 diminuye la de SK:Na en brotes a salinidad alta. La disponibilidad de nitrato o iones amonio aumenta acumulación en las raíces de carbohidratos solubles. Las plantas de todos los grupos dependen de Ca2+ como soluto compatible más que de Na+ o K+.
Palabras clave: osmoregulación, irrigación, salinidad, agua de mar, Vicia faba.
INTRODUCTION
The world now faces serious problems. The global warm is the greatest one; it will affect the rate and locations of rainfall (Xie et al., 2010). The population increasing is great problem too. Certainly, the arid and semiarid region will suffer strongly from both water deficit and soil salinity (Huq et al., 1999). Thus, the plants and crop production will be affected with drought and salinity (Ashraf et al., 2008; Kamal Uddin et al., 2009; Kamal Uddin et al., 2011). Therefore, several researchers have studied their effects on crop production (Mahajan and Tuteja, 2005; Jaleel et al., 2007) and the responses of plants to overcome these environmental stresses (Wyn Jones and Pritchard, 1989; Koyro, 2006; Heidari et al., 2008; Keshavarzi, 2012). One of the conclusions is that sea water or brackish water and salt tolerant plants should be considered for research (Breckle, 2009). Two main mechanisms are used by plants to overcome the drought and salinity stresses. The first is the quickly response through the re-osmotic adjustment depending upon inorganic solutes (Jacoby, 1999; Rubinigg et al., 2003; Neocleous and Vasilakakis, 2007; Hajlaouia et al., 2010; Aldesuquy et al., 2012). The solute particle number is the main reason which causes stress. The second way to overcome stress is depended mainly upon organic solutes (Wyn Jones and Pritchard, 1989). The later way need long time for compound synthesis and transformations to compatible solutes as proline, glycinebetaine, etc.
The most familiar inorganic solutes in nature are Na+, K+, Ca2+, Mg2+, and Cl-. These solutes when be available in excess in the medium will cause salt stress. The most harmful ions are Na+ and Cl- (Bergmann, 1992). The competition with K+ may decrease the high accumulation of Na+ (Kamel 2002; Kamel and El-Tayeb 2004). Calcium can also ameliorate sodium injury (Caines and Shennan, 1999; Hasegawa et al., 2000; Shabala et al., 2003; Arshi et al., 2005; Renault, 2005). NO3- ion can compete against Cl- and may lead to avoid chloride toxicity. Several physiological and eco-physiological studies were carried to study the effect of salinity on plants. Flowers and Flowers, 2005, suggested that domestication of halophytes is the best alternative to produce economical plants that could be grown in saline deserts. This suggestion can be useful in certain places around the world, where the saline deserts are found. But in the dry countries especially in North Africa, the agricultural lands suffer extremely from salinity as a result of high evaporation. Most of the crop plants are sensitive to moderately tolerant for salinity. Thus, it is necessary to find a suitable solution to avoid the salinity problems. Depending o the compatibility some ions as K+, Ca2+ and NO3-, etc., these ions cab be used as osmoregulators.
The current investigation is a complementary work to form chemical osmo-regulators. They can be used as fertilizers and as osmo-regulators which can help the plants to overcome the soil salinity under the effect of irrigation with saline waters.MATERIALS AND METHODS
Faba beans (Vicia faba L. cv. Giza 40) seeds were cultivated in 39 pots, eight seeds in each pot. Each pot (15 cm width x 12 cm height) contained 2 kg of sand: clay soil (2:1 respectively). They divided to four groups. First three groups were divided into four subgroups (3 replicates each) and irrigated with mixture of tap and sea water at 5, 10, 15 and 20 % sea water (v:v) respectively. Additionally to sea water treatments, the third group was treated with 5 mM KNO3 while the fourth group was treated with 5mM (NH4) SO4. The fourth group was a control irrigated with tap water, and contained three pots. The plants were grown till flowering stage in greenhouse. The temperature ranged between 33 °C and 5 °C day/night, humidity range 25 - 85 % and average day length about 13 h. The total dissolved salts in the soil solution were 215 mg.L-1 and electrical conductivity was 364 µS at 24 °C. At harvest, the plants were separated into roots and shoots. Roots were washed in the solution used for their irrigation and dried with a filter paper. All plant parts were dried at 70 °C for 48 h. The dried parts were ground and the solutes were extracted in distilled water according to El-Sharkawi and Michel, 1975. Sodium and potassium were measured by flame photometry (flame photometer, corning M410, UK) according to Williams and Twine, 1960. A volumetrical estimation of Cl-, Ca2+ and Mg+2 was performed according to Jackson, 1958 and Johnson and Ulrich, 1959. Sulphate, phosphate, soluble sugars, amino acids and soluble proteins were measured colorimetrically (spectrophotometer Jenway M6405 UK) according to Black et al., 1965, Woods and Mellon, 1941, Dubois et al., 1956, Lee and Takahashi, 1966 and Lawry et al., 1951, respectively. Potassium:sodium selectivity in the shoots was calculated as:
S K:Na (Shoot) = (Ksh * Nar) / (Nash * Kr) Equation 1
Where the subscripts r and sh indicate the ion concentration in the roots and shoots.
All experiments were performed with three replicates. Data were subjected to one way ANOVA, and significant differences between means were determined using the Statistical Package for Social Sciences (SPSS) for Windows (version 20.0). Results were expressed as means and their standard deviations. Significance was tested at the 5 % level.
RESULTS AND DISCUSSION
There were no significant differences in water content in the plants whether irrigated with sea water only or treated potassium nitrate or ammonium sulphate (Fig. 1). Certain crops may benefit from selection pressures, which improve their capacity to adjust osmotically or maintain more favorable water relations under salt stress (Tal and Gardi 1976; Shannon et al., 1987). This reflects the tendency of plants to maintain their water content at different stresses. The plants were depended on osmotic readjustment to maintain cell turgidity.
Sodium chloride is the most important constituent of sea water. Chloride represents only 55.03 % of the total salinity of sea water (Castro and Huber, 2003).
Plant adaptations to salinity are of three distinct types: osmotic stress tolerance, Na+ or Cl- exclusion, and the tolerance of tissue to accumulated Na+ or Cl- (Munns and Tester, 2008). Thus, plants irrigated with sea water increased their contents of chloride, sodium, potassium, calcium and magnesium (Fig. 2; Fig. 3) in their shoots gradually, to overcome the increase in external salinity. The osmotic readjustment is quickly induced by the changes in ion fluxes than the synthesis of compatible solutes (Wyn Jones and Pritchard, 1989; Lew, 1996). Generally, chloride content in the shoots of the plants irrigated with sea water was greater compared with the shoots of control plants. While chloride content was 33.7 mg.g-1 dry wt. in the control plants; was 38 mg.g-1 dry wt. in the plants it irrigated with 5 % sea water (Fig. 2). The shoots of all treated groups tended to increase their chloride concentration paralleled with the increasing of external salinity. Chloride is the micronutrient to be confirmed as generally most recent essential for the growth of higher plants (Broyer et al., 1954). All plants seem to be able to accumulate Cl- in the vacuoles of their cells, whereas many are deficient in the Na+/H+antiporter needed for Na+ occlusion in the vacuoles (Mennen et al., 1990). The minimal requirement of Cl- for crop growth of 1 g.kg-1 dry weight has been suggested (Marschner, 1995). The gradual increase of Cl- in the shoots will increase chloride toxicity. High tissue Cl-1 concentrations can be toxic to crop plants (Xu et al., 2000). Generally Cl- content was lower in roots than shoots (Fig. 2). Several species accumulate high concentrations of Cl- and not Na+ in leaves, such as soybean, woody perennials such as avocado (Munns and Tester, 2008). The low content of Cl- in roots may due to the exclusion of Cl- from roots. In most plants, Na+ and Cl- are effectively excluded by roots while water is taken up from the soil (Munns, 2005), or may due to the high rate of transport of chlorides to shoots to maintain turgidity. The treatment with 5 mM (NH4)2 SO4 enhanced the accumulation of Cl- in the shoots compared with roots which tended to decrease their content of Cl-. Martinez and Cerda, 1989, found that Cl- uptake was enhanced in cucumber when half the NO3- in the solution was replaced by NH4.
Plants tend to accumulate cations to eliminate the toxic effect resulted from chloride accumulation. Chloride is the prevalent anion accompanying Na+ and K+, hence its concentration in vacuoles, as well as cytoplasm, is usually in the same range as the sum of Na+ and K+. This concurrence of Na+ andCl- complicates the evaluation of Cl- specific toxicity (Pessarakli, 1999). As shown in figure 3, plants accumulated Na+ and K+ in roots more than shoots. The increase of external salinity increased the root content of sodium and potassium gradually in the plants which irrigated with seawater only or treated with KNO3. Shoots of first group, irrigated with seawater only, decreased their content of Na+ and K+. The treatment with KNO3 decreased Na+ content in the shoots compared with the control plants, except at 20 % seawater with KNO3. The low accumulation of Na+ in the shoots reflects the avoidance of sodium toxicity. It has long been known that NaCl toxicity is largely attributable to the effects of Na+, and only rarely those of Cl- (Tester and Davenport, 2003). This explains the high accumulation of Cl- in shoots compared with Na+. In most plants, Na+ and Cl- are effectively excluded by roots while water is taken up from the soil (Munns, 2005). Therefore, the increase of Na+ content in the shoots of the first group was opposite to the decrease in the roots. The treatment with KNO3 enhanced the transport of Na+ from roots to shoots. The treatment with (NH4)2 SO4 enhanced the transport of Na+ and K+ from roots to shoots.
At the low salinity (5 %), the roots content of Na+ was the highest than control and treated plants. The Na+ content decreased in the roots with increased external salinity, while shoots content of Na+ increased. The treatment with (NH4)2 SO4 increased the NH4+ concentration and consequently NH4+/NO3- ratio which increased the accumulation of Na+ in the leaves (Feigin, 1990). The availability of Ca+2 and Mg+2 in sea water enhanced the uptake of both (Fig. 3). The plants in the three groups accumulated Ca+2 more than Mg+2. Calcium has been extensively used to alleviate salt toxicity because of its crucial role in the maintenance of membrane processes, modulation of enzyme activities, and buffering of Na+-toxicity (Eschie 1995; Kurth et al., 1986; Bose et al., 1982). Magnesium was affected with external conditions. In group (1) plants, the roots content of Mg+2 decreased than control but increased gradually with increase of the external salinity to exceed the control at 20 % seawater with 5 mM (NH4)2 SO4. The shoots of second group increased their content of Mg+2 more than control and tended to decrease their content with increase of external salinity under the effect of KNO3 (Slavko et al., 1994). The salinized plant with the addition of 5 mM (NH4)2 SO4 increased their content of Mg+2 more than control; the increase was continued with the increased external salinity. The roots decreased Mg+2 content compared with control and increased gradually with external salinity (Alston, 1966).
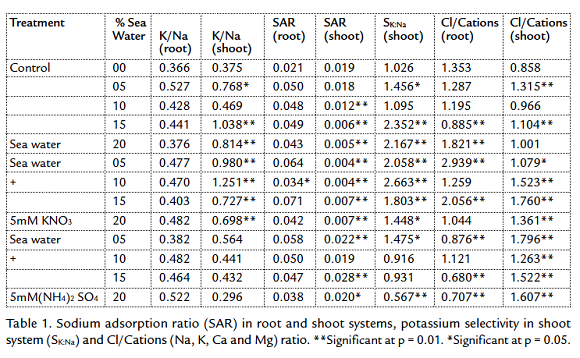
The transport of inorganic solutes inside the plants which survived under salt stress must be controlled to ensure the osmotic readjustment. So, some ratios of the main estimated ions were computed in table 1. K+/Na+ ratio was higher in the shoots of the first and second groups. The higher K+/N+ a ratio in shoots of barley cultivars compared with that in root medium solution indicated selective uptake of K+, which seems to be among the processes involved in tolerance of cultivars to salinity stress (Niazi et al., 1992). In the third group, treated with 5 mM (NH4)2 SO4, the ratio was lower than other groups. This may due to the high selectivity of potassium in the first and second groups, while the presence of (NH4)2 SO4 decreased the SK:Na value (Alder and Wilcox, 1995). The high accumulation of Ca+2 and Mg+2 decreased SAR ratio (Table 1). This ratio was high in roots due to the high accumulation of Na+, excluded from shoots (Drew and Lauchli, 1985) and selected K+. Potassium sodium selectivity highest values were recorded at the highest salinity in the first group (15 and 20 % seawater) and the lowest salinity in the second group (5, 10 and 15 % sea water with 5 mM KNO3). All plants avoided the accumulation of Na+ and K+ in the shoots to avoid the sodium toxicity. So, SAR values in shoots were lower than in roots (Table 1), this may due to the avoidance the transport of Na+ and K+ from roots to shoots. The first group plants tended to stabilize the sodicity in the roots, but in the same time the sodicity in the shoots decreased gradually with the increasing external salinity. This may due to the transport of Na+ and K+ from shoots to roots or decreasing the transport roots to shoots (Zepeda Jazo et al., 2008). The treatment with KNO3 helps the plants to stabilize the sodicity in the shoots. The treatment with ammonium sulphate in the third group affected the potassium sodium selectivity in the shoots. Potassium sodium selectivity decreased with increasing the external salinity. Sodicity values in the third group were the highest compared with the other two groups and control. The high accumulation of sulphates enhanced the accumulation of sodium more to neutralize the acidity resulted from SO4 in the plants.Cl/Cations ratio was higher in the roots of control plants than in shoot. In the first group, the ratio was 1.29 and 1.32 for the roots and shoots respectively. Except the ratio computed for roots of 20 % seawater irrigated plants; the plants tended to decrease and adjust the value of the ratio around one (Bear and Prince, 1945). The treatment with 5 mM KNO3 enhanced the accumulation of Cl- in the plants compared with control (Fig. 2). On another side, under the effect of external salinity, the chlorides decreased in root and this may due to the transport of Cl- to shoots (Maas and Ogata, 1972). So, the values of Cl/Cations were irregular, but the highest computed values were recorded in the roots of the plants irrigated with 5 % seawater with 5 mM KNO3 which reached up near three. The irrigation of plants with seawater and 5 mM (NH4)2 SO4 affected the distribution of chlorides inside plants. There was a tendency to decrease the Cl- in the roots may due to the low absorption of Cl- or the high rate of Cl- transport from roots to shoot, while chlorides increased gradually in the shoots. Thus, the Cl/Cations ratio was higher in all the levels of salinity in the shoots than roots.
Sodicity is the presence of a high proportion of sodium (Na+) ions relative to calcium (Ca+2) and magnesium (Mg+2) ions. The shoot in general decreased the sodicity (Table 1) inside plants by decreasing the Na+ concentration, avoiding the sodium toxicity. The studied plants tended to accumulate K+ more than Na+. So, the K/Na ratio was higher than that computed for roots of control, first and second groups. In the third group, the NH4+ decreased the accumulation on K+ in the shoots (Szczerba et al., 2008). Therefore, the K/Na ratio was lower than that computed for other groups (Table 1). With the high preference to accumulate K+, the plants tended to accumulate Ca2+ and Mg2+ in the shoots to ameliorate the toxic effect of sodium (Aldesuquy et al., 2012). Then, the Na+K/√Ca+Mg ratio was clearly lower than in roots. The computed potassium sodium selectivity (SK:Na) showed that the plants avoided the sodium toxicity by increasing their content of potassium at the highest salinity in the first group (Table 1). The treatment of plants with 5 mM KNO3 increased the K+ in the medium, so, the K+ compete the Na+ at the lower levels of salinity (Ashraf and Naqvi, 1991). The availability of NH4+ affect the absorption of potassium, so, the SK:Na decreased with increasing the external salinity. The preference of potassium accumulation in the shoots decreased the hazard producing from sodium accumulation. The high tendency of plants to accumulate calcium (Ca2+) and magnesium (Mg2+) ions decreased the sodicity (SAR) strongly in the shoots compared with roots. SAR decreased gradually with the increasing of salinity. This reflects the increased selectivity of divalent cations by plants (Obermeyer and Tyerman, 2005), with the increasing salinity. The availability of potassium ions with the accumulation of Ca2+ and Mg2+ is very helpful for plants to avoid the sodium toxicity. The presence of (NH4)2 SO4 in the external medium for third group enhanced the transport of Na+ and K+ from roots to shoots (Fig. 3), so, the SAR values were the highest in the shoots of third group (Table 1).
The low SAR in the shoots especially the plants that treated with KNO3 decreased the sodium toxicity and the increased potassium:sodium selectivity reflects the preference of K+ which plays important role in protein synthesis (Marschner, 1995). This decreased the concentration of amino acids and peptides (Fig. 4) and their role in osmotic adjustment, compared with non-treated plants. In another way, the treatment with potassium nitrate or ammonium sulphate enhanced the synthesis of carbohydrates which played the main role for osmotic adjustment in shoots to prevent the accumulation of inorganic solutes especially Na+ avoiding to injury. The plant treated with ammonium sulphate increased their content of amino acids and soluble proteins, where ammonium and sulphates plays a role in nitrogen assimilation in plants (Obermeyer and Tyerman, 2005). On the other hand, the non-treated plants accumulated more amounts of amino acids and soluble proteins to overcome the salinity hazard. This decreases the anabolism and growth (Munns 1988; Rodríguez et al., 1997). It was remarkable that the non-treated plants accumulated more soluble sugars in the roots (Marian et al., 2000) to increase the roots osmotic pressure, to prevent the influx of inorganic ions especially sodium. The high accumulation of soluble proteins in the non-treated plants shoots may be considered as a strategy to maintain water as bound water in the shoots (Frolow et al., 1996).
It can be concluded that the treatment with chemical compounds which contain compatible ions as K+, Ca2+ can be used as osmoregulators in the arid and semi-arid region where the salinity is high. The usage of potassium ions which can compete against the high concentration of Na+ in soil solution lead to decrease the effect of sodicity. Calcium also can ameliorate the harmful effects of salinity. Ammonium and nitrate, as a nitrogen sources, can be used as osmoregulators. The manufacturing of chemical osmoregulators is very useful to overcome the salinity in arid and semi-arid regions or to facilitate the usage of sea water in agriculture.
REFERENCES
ALDER RA, WILCOX GE. Ammonium increases the net rate of sodium influx and partitioning to the leaf of muskmelon. J Plant Nutr. 1995;18:1951-1962. [ Links ]
ALDESUQUY HS, BAKA ZA, EL-SHEHABY OA, GHANEM HE. Efficacy of seawater salinity on osmotic adjustment and solutes allocation in wheat (Triticum aestivum) flag leaf during grain filling. International Journal of Plant Physiology and Biochemistry. 2012;4:33-45. [ Links ]
ALSTON AM. The influence of N and Mg fertilizers and CaCO3 on the absorption of Mg by oats. J Agr Sci. 1966;66:61-66. [ Links ]
ARSHI A, ABDIN MZ, IQBAL M. Ameliorative effects of CaCl2 on growth, ionic relations, and proline content of Senna under salinity stress. J Plant Nutr. 2005;28:101-125. [ Links ]
ASHRAF M, ATHAR HR, HARRIS PJC, KWON TR. Some prospective strategies for improving crop salt tolerance. Adv Agron., 2008;97:45-110. [ Links ]
ASHRAF M, NAQVI MI. Responses of three arid zone grass species to varying Na/Ca ratios in saline sand culture. New Phytol. 1991;119:285-290. [ Links ]
BEAR FE, PRINCE AL. Cation equivalent constancy in alfalfa. Journal of the J. Amer. Soc. Agron, 1945;37:217-222. [ Links ]
BERGMANN W. In G Fisher, ed, nutritional disorders of plants development, visual and analytical diagnosis. Jena, Stuttgart, Germany. 1992. [ Links ]
BLACK CA, EVANS DD, ENSMINGER LE. Methods of Soil Analysis. Agronomy, 9 Amer Soc Agron Inc. Publisher, Madison, Wisconsin, USA; 1965. [ Links ]
BRECKLE SW. Is sustainable agriculture with seawater irrigation realistic? Salinity Water Stress. 2009;44:187-196. [ Links ]
BOSE B, SRIVASTAVA HS, MATHUR SN. Effect of some nitrogenous salts on nitrogen transfer and protease activity in germinating Zea mays L. seeds. Biol Plantarum. 1982;24:89-95. [ Links ]
BROYER TC, CARLTON AB, JOHNSON CM, STOUT PR. Chlorine: A micronutrient element for higher plants. Plant Physiol. 1954;29:526-532. [ Links ]
CAINES AM, SHENNAN C. Interactive effects of Ca2+ and NaCl salinity on the growth of two tomato genotypes differing in Ca2+ use efficiency. Plant Physiol Bioch . 1999; 37:569-576. [ Links ]
CASTRO P, HUBER ME. Marine Biology. New York: McGraw-Hill; 2003. [ Links ]
DREW MC, LAUCHLI A. Oxygen-Dependent Exclusion of Sodium Ions from Shoots by Roots of Zea mays (cv Pioneer 3906) in Relation to Salinity Damage. Plant Physiol. 1985;79:171-176. [ Links ]
DUBOIS M, GILLIES KA, HAMILTON JK, REBERS PA, SMITH F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350-356. [ Links ]
EL-SHARKAWI HM, MICHEL BE. Effects of soil water matric potential and air humidity on CO2 and water vapour exchange in two grasses. Photosynthetica 1975;11:176-182. [ Links ]
ESCHIE HA. Partitioning of chloride ions in the germinating seed of two forage legumes under varied salinity and temperature regimes. Commun Soil Sci Plan. 1995; 26: 3357-3370. [ Links ]
FEIGIN A. Interactive effects of salinity and ammonium/nitrate ratio on growth and chemical composition of melon plants. J Plant Nutr. 1990;13:1257-1269. [ Links ]
FLOWER TJ, FLOWERS SA. Why does salinity pose such a difficult problem for plant breeders? Agr Water Manage. 2005;78:15-24. [ Links ]
FROLOW F, HAREL M, SUSSMAN JL, MEVARECH M, SHOHAM M. Insights into protein adaptation to a saturated salt environment from the crystal structure of a halophilic 2Fe-2S ferredoxin. Nat Struct Biol. 1996;3:452-458. [ Links ]
HAJLAOUIA H, EL AYEBB N, GARRECC JP, DENDEND M. Differential effects of salt stress on osmotic adjustment and solutes allocation on the basis of root and leaf tissue senescence of two silage maize (Zea mays L.) varieties. Ind Crop Prod. 2010;31:122-130. [ Links ]
HASEGAWA PM, BRESSAN RA, ZHU JK, BOHNERT JH. Plant cellular and molecular responses to high salinity. Annu Rev Plant Phys. 2000;51:463-499. [ Links ]
HEIDARI F, ZEHTAB SALMASI S, JAVANSHIR A, ALIARI H, DADPOOR MR. The Effects of Application Microelements and Plant Density on Yield and Essential oil of Peppermint (Mentha piperita L.). Iranian Journal of Medicinal and Aromatic Plants. 2008;24:1-9. [ Links ]
HUQ S, KARIM Z, ASADUZZAMAN M, MAHTAB F. Ed. Vulnerability And Adaptation To Climate Change In Bangladesh. Dordrecht: Kluwer; 1999. [ Links ]
JACKSON ML. Soil Chemical Analysis. 1st edn. Prentice-Hall, Inc., Englewood Cliffs N.J; 1958. [ Links ]
JACOBY B. Mechanism involved in salt tolerance of plants. In M. Pessarakli (ed.) Handbook Of Plant And Crop Stress. Marcel Dekker, Inc., New York; 1999. p. 97-124. [ Links ]
JALEEL CA, GOPI R, MANIVANNAN P, PANNEERSELVAM R. Responses of antioxidant defense system of Catharanthus roseus (L.) G. Don. to paclobutrazol treatment under salinity. Acta PhysioL Plant. 2007;29:205-209. [ Links ]
JOHNSON CM, ULRISH A. Analytical methods for use in plant analysis. Bulletin 766, 26-78. Berkeley: University of California, Agricultural Experiment Station. [ Links ]
KAMAL UDDIN M, JURAIMI AS, ISMAIL MR, HOSSAIN A, RADZIAH O, RAHIM AA. Osmotic adjustment and plant adaptation to environmental changes related to drought and salinity. AJCS. 2011;5:620-629. [ Links ]
KAMAL UDDIN, M, JURAIMI AS, ISMAIL MR, RAHIM MA, RADZIAH O. Growth response of eight tropical turf grass to salinity. Afr J Biotechnol. 2009;8:5799-5806. [ Links ]
KAMEL M. The effect of sudden sodium chloride stress on the ion composition and the mechanism of osmotic adjustment in Vicia faba. Pak J Biol Sci. 2002;5:885-890. [ Links ]
KAMEL M, EL-TAYEB MA. K+/Na+ soil-plant interactions during low salt stress and their role in osmotic adjustment in faba beans. Span J Agric Res. 2004;2:257-265. [ Links ]
KESHAVARZI MHB. The Effect of Different NACL Concentration on Germination and Early Seedling Growth of Artemisia annua L. International Journal of Agriculture: Research and Review. 2012;2:135-140. [ Links ]
KOYRO HW. Effect of salinity on growth, photosynthesis, water relations and solute composition of the potential cash crop halophyte Plantago coronpus (L.). Environ Exp Bot. 2006;56:136-146. [ Links ]
KURTH E, JENSEN A, EPSTEIN E. Resistance of fully imbibed tomato seeds to very high salinities. Plant Cell Environ. 1986;9:667-676. [ Links ]
LAWRY CH, FARR AL, BUNDALL HJ. Protein measurement with the foline phenol reagent. J Biol Chem. 1951;193:265-275. [ Links ]
LEE YP, TAKAHASHI T. An improved colorimetric determination of amino acids with the use of ninhydrine. Analytical Biochemistry. 1966;14:71-77. [ Links ]
LEW RR. Pressure regulation of the electrical properties of growing Arabidopsis thaliana root hairs. Plant Physiol. 1996;112:1089-1100. [ Links ]
MAAS EV, OGATA G. Radial Transport of Sodium and Chloride into Tomato Root Xylem. Plant Physiol. 1972;50:64-68. [ Links ]
MAHAJAN S, TUTEJA N. Cold, salinity and drought stresses: An overview. Arch Biochem Biophys. 2005;444:139-158. [ Links ]
MARIAN EB, JOSE DA, FRANCISCO PA. Carbon partitioning and sucrose metabolism in tomato plants growing under salinity. Physiol Plantarum. 2000;110:503-511. [ Links ]
MARSCHNER H. Mineral Nutrition Of Higher Plants. 2nd Ed. Academic Press, San Diego, California, USA; 1995. [ Links ]
MARTINEZ V, CERDA A. Influence of N source on rate of Cl, N, Na, and K uptake by cucumber seedlings grown in saline conditions. J Plant Nutr. 1989;12:971-983. [ Links ]
MENNEN H, JACOBY B, MARSCHNNER H. Is sodium proton antiport ubiquitous in plant cells? J Plant Physiol. 1990;137:180-183. [ Links ]
MUNNS R, TESTER M. Mechanisms of salinity tolerance. Annu Rev Plant Biol. 2008;59:651-681. [ Links ]
MUNNS R. Genes and salt tolerance: bringing them together. New Phytol. 2005;167:645-663. [ Links ]
MUNNS R. Why measure osmotic adjustment? Aust J Plant Physiol. 1988;15:717-726. [ Links ]
NEOCLEOUS D, VASILAKAKIS M. Effects of NaCl stress on red raspberry (Rubus idaeus L. and Autumn bliss L.). Sci Hortic-Amsterdam. 2007;112:282-289. [ Links ]
NIAZI MLK, MOHMOOD K, MUJTABA SM, MALIK KA. Salinity tolerance in different cultivars of barley (Hordium vulgare L.). Biol Plantarum. 1992;34:465-469. [ Links ]
OBERMEYER G, TYERMAN SD. NH4+ Currents across the Peribacteroid Membrane of Soybean. Macroscopic and Microscopic Properties, Inhibition by Mg2+ and Temperature Dependence Indicate a Subpico Siemens Channel Finely Regulated by Divalent Cations. Plant Physiol. 2005;139:1015-1029. [ Links ]
PESSARAKLI M. Handbook of Plant And Crop Stresses. 2nd Ed. - Marcel Dekker, New York - Basel; 1999. [ Links ]
RENAULT S. Response of red-osier dogwood (Cornus stolonifera) seedlings to sodium sulphate salinity: effects of supplemental calcium. Physiol Plantarum. 2005;123:75-81. [ Links ]
RODRÍGUEZ HG, ROBERTS JKM, JORDAN WR, DREW MC. Growth, Water Relations, and Accumulation of Organic and lnorganic Solutes in Roots of Maize Seedlings during Salt Stress. Plant Physiol. 1997;113:881-893. [ Links ]
RUBINIGG M, POSTHUMUS F, FERSCHKE M, ELZENGA JTM, STULEN I. Effects of NaCl salinity on 15N-nitreate fluxes and specific root length in the halophyte Plantago martima L. Plant Soil. 2003;250:201-213. [ Links ]
SHABALA S, SHABALA L, VAN VOLKENBURGH E. Effect of calcium on root development and root ion fluxes in salinized barley seedlings. Funct Plant Biol. 2003;30:507-514. [ Links ]
SHANNON MC, GRONWALD JW, TAL M. Effects of salinity on growth and accumulation of organic ions in cultivated and wild tomato species. J Am Soc Hortic Sci. 1987;112:416-423. [ Links ]
SLAVKO P, ANDROULAKIS II, LOUPASSAKI MH. Effect of Summer Application of Nitrogen and Potassium on Mineral Composition of Olive Leaves. Acta Hortic. 1994;356:221-224. [ Links ]
SZCZERBA MW, BRITTO DT, ALI SA, BALKOS KD, KRONZUCKER HJ. NH4+- stimulated and -inhibited components of K+ transport in rice (Oryza sativa L.). J Exp Bot. 2008;59:3415-3423. [ Links ]
TAL M, GARDI I. Physiology of polyploid plants: Water balance in autotetraploid and diploid tomato under low and high salinity. Physiol Plantarum. 1976;38:257-261. [ Links ]
TESTER M, DAVENPORT R. Na+ tolerance and Na+ transport in higher plants. Ann Bot. 2003;91:503-527. [ Links ]
WILLIAMS CH, TWINE JR. Flame photometric method for sodium, potassium and calcium. In: Modern Methods of Plant Analysis (Eds K. Paech and M. V. Tracey). Springer-Verlag, Berlin; 1960:V. [ Links ]
WOODS JT, MELLON MA. Chlorostannous-molybdophosphoric blue colour in sulfuric acid system. In: Soil Chemical Analysis (Ed M. L. Jackson). Prentice-Hall International, Inc., London; 1941. [ Links ]
WYN JONES RG, PRITCHARD J. Stresses, membranes and cell wall. In: Plants Under Stress: Biochemistry, Physiology And Ecology And Their Application To Plant Improvement (Jones HG, TJ Flowers and Jones MB eds). Cambridge: University Press; 1989. p. 95-114. [ Links ]
XIE SP, CLARA D, GABRIEL AV, JIAN M, HAIYAN T, ANDREW TW. Global Warming Pattern Formation: Sea Surface Temperature and Rainfall. J Climate. 2010;23:966-986. [ Links ]
XU G, MAGEN H, TARCHITZKY J, KAFKAFI V. Advances in chloride nutrition. Adv Agron. 2000;68:96-150. [ Links ]
ZEPEDA JAZO I, SHABALA S, CHEN Z, POTTOSIN II. Na+-K+ transport in roots under salt stress. Plant Signal Behav. 2008;3:401-403. [ Links ]