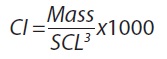Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Acta Biológica Colombiana
Print version ISSN 0120-548X
Acta biol.Colomb. vol.19 no.3 Bogotá Sept./Dec. 2014
https://doi.org/10.15446/abc.v19n3.42615
Artículo de investigación
http://dx.doi.org/10.15446/abc.v19n3.42615
INTRASPECIFIC VARIATION OF THE GREEN TURTLE, Chelonia mydas (CHELONIIDAE), IN THE FORAGING AREA OF GORGONA NATURAL NATIONAL PARK (COLOMBIAN PACIFIC)
Variación intraespecífica de la tortuga verde Chelonia mydas (Cheloniidae) en el área de forrajeo del Parque Nacional Natural Gorgona (Pacífico colombiano)
LAURA SAMPSON1; Ph. D (c); LUIS FERNANDO PAYÁN2, Biólogo; DIEGO FERNANDO AMOROCHO3, Ph. D.; JEFFREY A. SEMINOFF4, Ph. D.; ALAN GIRALDO5, Ph. D.
1 Universidad del Valle. Calle 13 N.#° 100-00. Cali, Colombia. lausamps@gmail.com
2 Parques Nacionales Naturales de Colombia. Cali, Colombia. lucho_payan@hotmail.com
3 WWF Latino América y el Caribe. Cali, Colombia. dfamorocho@wwf.org.co
4 NMFS-Southwest Fisheries Science Center. Jolla, United States. jeffrey.seminoff@noaa.gov
5 Grupo de Investigación en Ecología Animal, Universidad del Valle. Cali, Colombia. alan.giraldo@correounivalle.edu.co
Autor de correspondencia: Laura Sampson, lausamps@gmail.com
Received April 1st 2014, first decision May 5th 2014, accepted May 28th 2014.
Citation / Citar este artículo como: SAMPSON L, PAYÁN LF, AMOROCHO DF, SEMINOFF JA, GIRALDO A. Intraspecific variation of the green turtle, Chelonia mydas (Cheloniidae), in the foraging area of Gorgona Natural National Park (Colombian Pacific). Acta biol. Colomb. 2014;19(3):461-470.
ABSTRACT
The size distribution and body condition of the two morphotypes of green turtle (Chelonia mydas) foraging in Gorgona Natural National Park (GNNP) in the Colombian Pacific was assessed from 2003 to 2012. Measurements of straight carapace length (SCL), curved carapace length (CCL), weight, and body condition of 1,023 turtles captured on the GNNP reefs were recorded. More black turtles (n = 747) than yellow turtles (n = 276) were captured, all of them juveniles. Black turtles were significantly larger and heavier than yellow turtles. The size of recruitment to the neritic zone was 40.0-49.9 cm SCL for both morphotypes, but there were more yellow than black turtles in this size class, indicating a difference in the recruitment pattern. The body condition index of yellow turtles was significantly higher than that of black turtles, which could indicate differences in resource use. Based on our results, we suggest that GNNP might function as a recruitment area for yellow turtles, which arrive at smaller sizes, and as part of a coastal migratory route for black turtles, which arrive at larger sizes and maintain residence at this location for an unknown period of time.
Keywords: black morphotype, condition index, foraging ground, size-frequency distribution, yellow morphotype.
RESUMEN
Se comparó la distribución de tallas y condición corporal de los dos morfotipos conocidos de tortuga verde (Chelonia mydas) en el área de forrajeo del Parque Nacional Natural Gorgona (PNNG) en el Pacífico colombiano entre 2003 y 2012. Se tomaron medidas de largo recto de caparazón (LRC), largo curvo de caparazón (LCC), peso y condición corporal de 1.023 tortugas capturadas en los arrecifes del PNNG. Se capturaron más tortugas negras (n = 747) que amarillas (n = 276), todas juveniles. Las tortugas negras fueron significativamente más grandes y pesadas que las amarillas. El tamaño de reclutamiento a la zona nerítica fue de 40,0-49,9 cm para ambos morfotipos, pero hubo más tortugas amarillas que negras en este intervalo de tamaños, lo cual sugiere una variación en el patrón de reclutamiento. El índice de condición corporal de las tortugas amarillas fue significativamente más alto que el de las tortugas negras, lo cual podría indicar diferencias en la utilización de recursos. Con base en los resultados obtenidos, se sugiere que el PNNG podría funcionar como un área de reclutamiento para las tortugas amarillas, las cuales llegan más pequeñas a esta zona; y como parte de la ruta migratoria costera de las tortugas negras, las cuales llegan más grandes e incluso residen en esta localidad durante un lapso de tiempo desconocido.
Palabras clave: área de forrajeo, distribución de frecuencia de tamaño, índice de condición corporal, morfotipo amarillo, morfotipo negro.
INTRODUCTION
As they go through their different life stages, Chelonia mydas individuals, like those of other sea turtle species, inhabit widely different environments. After hatching, the young sea turtles move to epipelagic areas, where they spend several years, migrating then to neritic foraging areas where they remain until reaching sexual maturity. Adults migrate between breeding and foraging areas (Meylan et al., 2011). Since each life stage is subject to different pressures and threats, management and conservation strategies need to be geared toward the specific challenges that each life stage confronts, helping generate effective conservation plans (Heppell et al., 2003). Determining the size of recruitment to neritic areas (size at arrival), the time of residency and the size at maturity (size at departure) is important for implementation of conservation measures (Heppell et al., 2003; Meylan et al., 2011).
The protection of sea turtles during their different life stages is critical, particularly for areas that include animals from different stocks, such as foraging areas where animals share the same resources and face the same threats (Bass et al., 2004). The environmental conditions at a given foraging ground affect the health of individuals from different stocks that will migrate to different breeding areas, so that conditions at the foraging ground will ultimately have an effect on the conservation status of geographically distant breeding populations (Lahanas et al., 1998; Engstrom et al., 2002; Heppell et al., 2003).
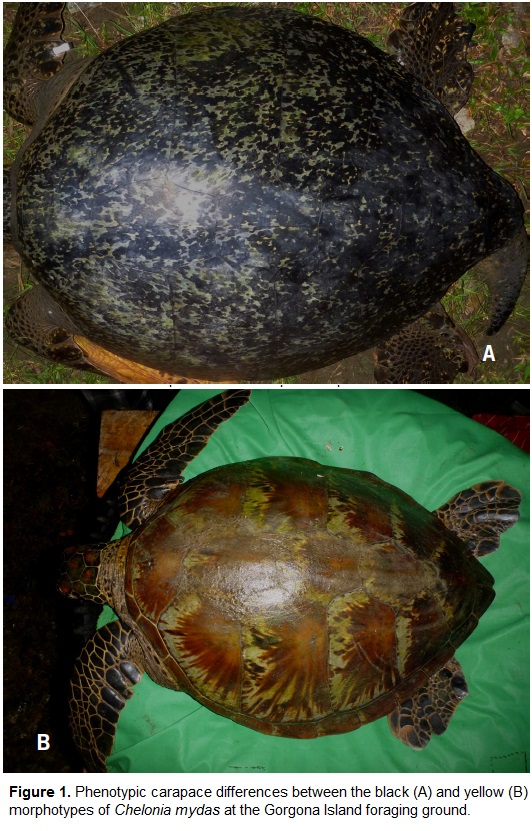
Chelonia mydas has a worldwide distribution. In the Eastern Pacific, however, C. mydas individuals have a distinct coloration and shape that sets them apart from the all other populations (Parker et al., 2011). The black morphotype (known locally as the black turtle or East Pacific green turtle) can be found along the eastern margin of the Pacific Ocean, from Chile to California, USA (Wallace et al., 2010). It has a dark grey to black heart-shaped carapace, sometimes with lighter dots (Fig. 1A; Pritchard, 1998; Amorocho and Reina, 2007). The yellow morphotype, which can be found over the remaining distribution of C. mydas, has a distinctive yellow to light brown coloration with black streaks (Fig. 1B; Hirth, 1997). The distribution of this morphotype in the Eastern Pacific is limited, with published reports only from the Galapagos Islands, Ecuador (Pritchard, 1971; Green, 1981) and Gorgona National Park (Amorocho et al., 2012).
The black morphotype is known to forage in neritic and offshore waters of many areas from Chile to the United States (Seminoff et al., 2012). Gorgona National Park (GNP) is the only known foraging ground for C. mydas in the Colombian Pacific. This area is important regionally because it protects one of the most developed coral reef formations in the Eastern Tropical Pacific (Cortés et al., 1997; Zapata, 2001), and is also one of the few places in the world where both morphotypes of C. mydas co-exist (Pritchard, 1971; Márquez, 1990; Amorocho et al., 2012). Black turtles have only been reported to nest in the Eastern Pacific, where yellow turtles have not been seen nesting (Pritchard, 1971; Márquez, 1990).
The fact that these two morphotypes coexist year-round in this foraging area provides a unique opportunity to compare intraspecific phenotypic variations and body condition of organisms that share the same habitat and are probably consuming the same resources.
The present study was undertaken to describe and compare the size structure and the body condition of the two C. mydas morphotypes present at GNP, a mixed stock foraging ground. This information is vital to develop efficient conservation strategies, geared towards a specific life stage whose conservation could have repercussions on the survival of other life stages.
MATERIALS AND METHODS
Study area
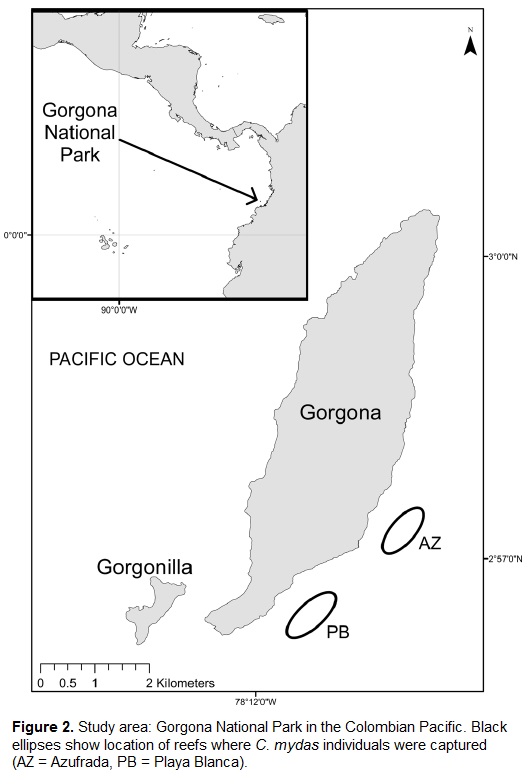
Gorgona National Park (GNP) is located off the southwestern coast of Colombia (between 2#°49´ and 3#°06´N, and between 78#°06´ and 78#°18´W), approximately 30 km from the mainland. GNP measures 616.8 km2, comprising the islands of Gorgona and Gorgonilla, and a marine area that makes up 97.5 % of the park (Fig. 2). Fringing reefs occur on the eastern side of Gorgona Island. The two main reefs, La Azufrada (11.2 ha), and Playa Blanca (10.86 ha) are composed mainly of corals of the genus Pocillopora (Glynn et al., 1982; Zapata et al., 2001). These reefs provide shelter for several species of fish, invertebrates, and algae, and offer resources for large vertebrates such as manta rays and whale sharks (Acevedo-Bueno et al., 2004; Sampson and Giraldo, 2014).
Turtle capture and measurements
Since 2003, a monitoring study of C. mydas has been carried out at GNP. Between 2003 and 2006, surveys were carried out by the National Park service in conjunction with CIMAD (Centro de Investigación para el Manejo Ambiental y el Desarrollo); between 2006 and 2008, the Colombian National Park service and WWF Colombia were in charge; finally, between 2008 and 2012, surveys were carried out solely by the Colombian National Park service. Turtles were captured by hand at night while two to five people searched the reef area using snorkelling gear. When a turtle was sighted, it was grabbed by the carapace and transferred to an outboard motor boat. The searching continued for one hour (8 to 9 hours) or until five turtles had been captured. The same monitoring protocol has been followed during all years of this study; however, due to the variations in personnel involved over the years, and the lack of data on time effort, no catch per unit effort data were calculated.
The turtles were brought to shore, where straight carapace length (SCL) and curved carapace length (CCL) were measured from the nuchal notch to the posterior notch, and mass was recorded. Straight measurements were taken using forestry callipers with 0.05 cm accuracy; curved measurements were taken with a flexible tape with 0.05 cm accuracy; mass was measured using a spring scale with 0.5 kg accuracy. The carapace color was noted at the time of capture. Turtles were tagged on the trailing edge of the right or left front flipper, using numbered Inconel tags (No. 681, National Band and Tag Co., Newport, Kentucky, USA). Data obtained from tagged turtles (recaptures and growth) will be published separately.
Body condition index
A condition index (CI), which can be used as a proxy for individual health and can be used to infer quality of available resources (Bolger et al., 1989), was calculated for each morphotype, year, and size class as:
Where mass is given in kg and SCL, in cm (Bjorndal et al., 2000). The condition index (CI) was calculated for each morphotype separately; data from 745 black turtles and 270 yellow turtles was used to calculate CI.
STATISTICAL ANALYSES
Measurement data (SCL, CCL and mass) and CI data were checked for normality using Lilliefor´s test, and for homogeneity of variances using Levene´s test. To compare CI between color morphs, Welch´s t tests were used when data failed the normality assumption. To compare size measurements between morphs and years, and CI values between morphs and years, Kruskal-Wallis tests were used when data failed the normality assumption. Post-hoc multiple comparisons with Bonferroni corrections were carried out to detect differences between samples. All analyses were carried out using Statsoft v. 8.0.
RESULTS
TURTLE CAPTURES
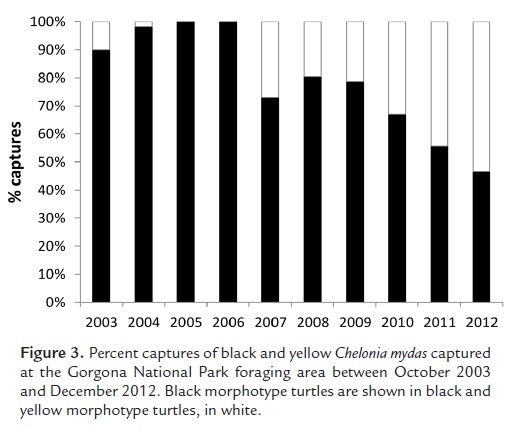
Measurements of 1,023 C. mydas turtles were taken from 2003 to 2012; 747 were black and 276 were yellow. There were only five yellow morphotype turtles caught at GNP before 2007; four were caught in 2003 and one in 2004. After 2007, the number of yellow morphotype turtles caught has increased from 30 turtles in 2007 to 79 in 2012 (Fig. 3).
TURTLE MEASUREMENTS
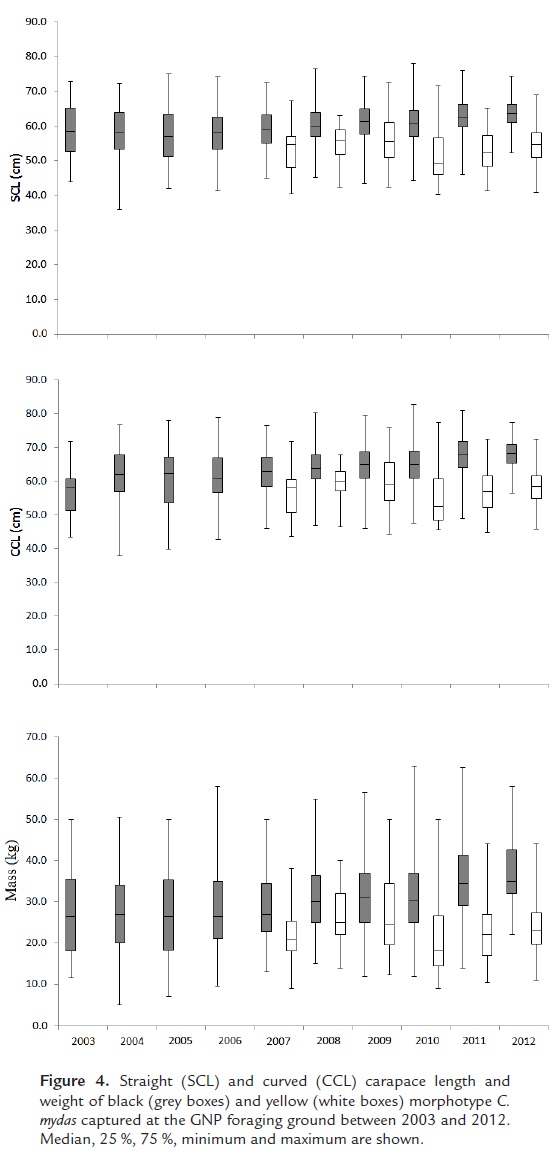
Black morphotype turtles were significantly larger (SCL and CCL) and heavier than yellow turtles (Kruskal-Wallis, H =191.89, p<0.01; Fig.4). Mean SCL of black turtles was 60.2 cm (min = 36.0, max = 78.0 cm), mean CCL was 64.0 cm (min = 37.9, max = 82.7), and mean weight was 31.1 kg (min = 5, max = 63). Mean SCL of yellow turtles was 53.6 cm (min= 40.2, max= 72.5 cm), mean CCL was 57.5 cm (min = 43.7, max = 77.3), and mean weight was 23.7 kg (min = 9, max = 50).
There were significant differences in the size and mass of black turtles among years (SCL: Kruskal-Wallis, H=9, p<0.01; CCL: Kruskal-Wallis H=9, p<0.01; mass: H=9, p<0.01). The size of black turtles in the last two years of sampling was statistically different from size during 2004-2008, indicating an increase in the size of sampled turtles (Multiple comparisons of mean ranks, p<0.01). Yellow turtle size and weight also varied significantly among years (SCL: Kruskal-Wallis, H=5, p=0.190; CCL: Kruskwal Wallis H=5, p=0.0076; mass: H=5, p=0.0033). For yellow turtles, 2010 was statistically different, indicating a smaller size of turtles in that year (multiple comparisons of mean ranks, p<0.01).
The size at maturity (SM) of C. mydas individuals was calculated as a weighted mean of the SM at the rookeries from which turtles at GNP originate. Approximately 88.5 % of black turtles come from the Galapagos Islands (SM = 79.7 cm SCL) and 11.5 % come from Michoacan (SM = 77.3 cm SCL), while approximately 1.7 % of yellow turtles come from French Frigate Shoals (SM = 80.0 cm SCL) and 96.3 % come from French Polynesia (SM = 96.3 cm SCL) (Márquez 1990; Hirth, 1997; Balazs and Chaloupka 2004; Zárate 2004; Amorocho et al,. 2012; Correa-Cárdenas, 2013). The estimated SM of black turtles at GNP was 79.4 cm SCL and that of yellow turtles was 96.0 cm SCL. All captured turtles in this study measured less than SM, indicating that they were all at the juvenile or sub-adult stage and were likely not sexually mature.
SIZE-FREQUENCY DISTRIBUTION
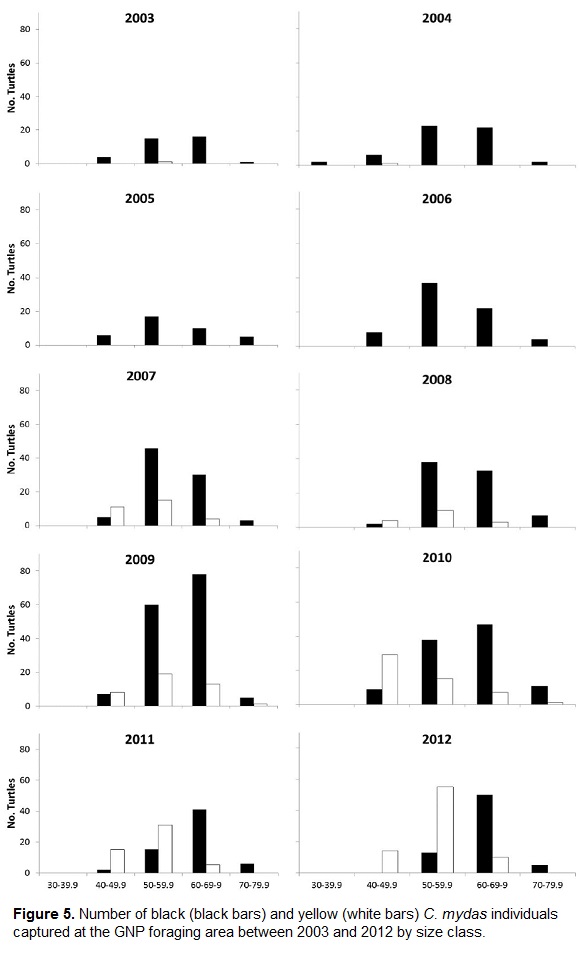
Only two turtles measuring between 30 and 39.99 cm SCL were caught, indicating that the size of recruitment to the neritic zone of GNP was 40 to 49.9 cm SCL for both morphotypes (Fig. 5). There were no significant differences in size distribution between the two morphotypes (Kolgomorov-Smirnov test, p>0.10). However, most black turtles (86.1 %) measured between 50 and 69.9 cm SCL, whereas most yellow turtles (83.1 %) were smaller, measuring between 40 and 59.9 cm SCL (Table 1).
There were more yellow turtles recruiting at the 40-49.9 cm SCL size class (29.8 % of all individuals captured), while only 6.3 % of all black turtles captured belonged to this size class (Table 1). The number of black turtle recruits ranged from 0 in 2012 to 9 in 2010, while yellow turtles recruits ranged from 1 in 2004 to 28 in 2010.
BODY CONDITION INDEX
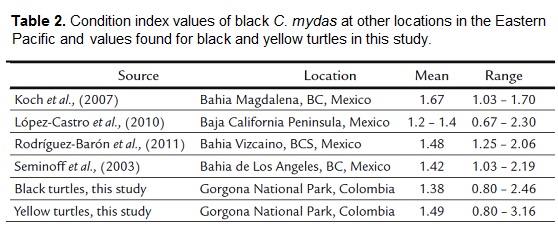
The overall CI (all years and size classes combined) of black morph turtles had a mean value of 1.38 (SD = 0.15), and ranged from 0.80 to 2.46, while that of yellow morph turtles had a mean value of 1.49 (SD=0.22), and ranged from 0.80 to 3.16 (Table 2). The mean CI of the two morphotypes were statistically different (Welch´s t test, t=-9.12, p<0.01).
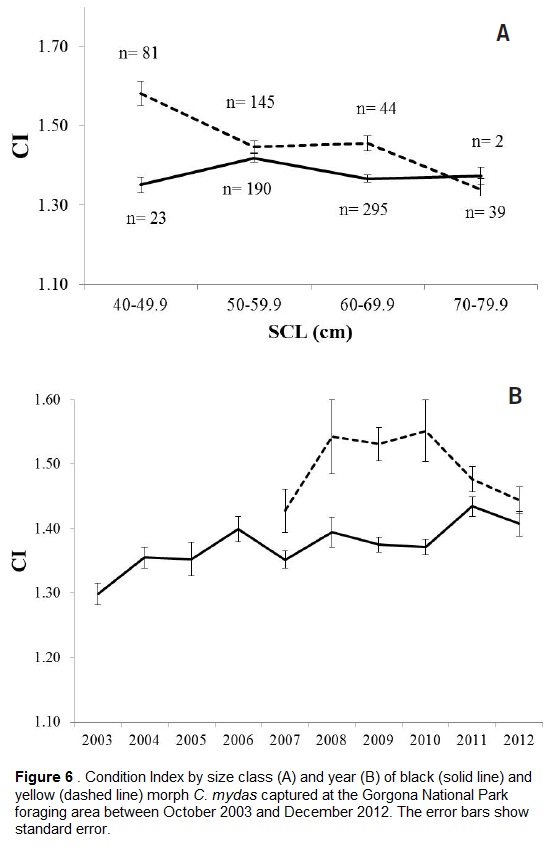
A comparison of CI values among size classes for each morphotype was carried out, taking all sampled years for each morphotype separately (2003-2012 for black turtles, 2007-2012 for yellow turtles). This analysis showed that for both morphotypes the different size classes were statistically different (Kruskal-Wallis, H=17.46, p=0.001 for black turtles and H=23.86, p<0.01 for yellow turtles). For black turtles, the 50-59.9 cm size class had a higher CI than the 60-69.9 cm class, while for yellow turtles, the smaller turtles 40-49.9 cm had a higher CI than larger turtles (Fig. 6A; Multiple comparisons of mean ranks, p<0.01).
There were significant differences in the CI of black turtles among years (2003 to 2012) (Kruskal-Wallis, H=37.34, p<0.01), with 2003 being significantly lower than 2006, 2008, 2011, and 2012, and 2011 being significantly higher than 2007 (Fig. 6B; Multiple comparisons of mean ranks, p<0.05). Mean CI of yellow turtles was not statistically different among years (2007 to 2012) (Fig. 6B; Kruskal-Wallis, p>0.01; Multiple comparisons of mean ranks, p>0.05).
DISCUSSION
All C. mydas individuals captured at the GNP foraging area were below the mean size at maturity (SM). It is therefore safe to say that GNP is a juvenile developmental habitat used by black and yellow C. mydas. There were reports in the 90's of eight black morphotype C. mydas nesting at a beach on the western side of Gorgona Island (McCormick-Anzola, 1996), but there were no reports of nesting activity during the years of the present study.
There were more black turtles than yellow turtles captured from 2003 to 2012, which is in accordance with what has been reported for the Galapagos Islands (Pritchard, 1971; Green, 1993). Of 6,743 C. mydas adults tagged between 1976 and 1980 at the Galapagos Islands, only 23 were of the yellow morphotype (Green, 1981). However, there was an increase in the number of yellow turtles captured at GNP since 2007, which could be indicative of changes in the migratory routes taken by this morphotype. It should be noted that, since capture effort was not quantified, the change in numbers of captured turtles could be an artifact of sample effort. However, we do not think there was bias in the captures, and we believe that our sample is representative of the proportion of black and yellow turtles present at GNP during the years of this study.
Most black and yellow turtles arrived at GNP at >40 cm SCL (except two black morphotype turtles 36-38 cm SCL). Green turtles arrive to Hawaiian foraging grounds at 35 cm SCL (Arthur and Balazs, 2008), at 35-40 SCL to foraging grounds in Baja California (Seminoff et al., 2003), and at 40 cm CCL in the southwestern Pacific Ocean (Chaloupka et al., 2004). Parker et al., (2011) proposed that the black eastern Pacific turtles remain longer in the oceanic region than the yellow morphotype, which would explain a larger recruitment size at GNP than at Hawaii.
There were more yellow turtles at the smaller size class. This difference in the proportion of small turtles (40-49.9 cm SCL) could be due to black turtles recruiting to the neritic foraging ground of GNP at a larger size (>50 cm SCL) than yellow turtles (Table 1). It might also be indicative of a difference in the migratory behavior of black and yellow turtles, because the fact that two black turtles <40 cm SCL were captured indicates that this morphotype can arrive at this size to coastal areas. Black turtle juveniles could be recruiting to coastal areas other than GNP and moving along the coast to this foraging area, while yellow turtles could be arriving directly to GNP from the open ocean.
Yellow turtles captured at GNP are travelling mainly from rookeries in the western Pacific (French Polynesia and Hawaii), probably being transported along west-east currents, while black turtles arrive from the Galapagos and Michoacan rookeries (Amorocho et al., 2012; Correa-Cárdenas, 2013). Satellite telemetry studies would be necessary to confirm the movements of both morphotypes in the region, and future stable isotope analyses comparing the carbon values of these two morphotypes could also help elucidate their migratory behavior.
Black turtles reached larger sizes than yellow turtles at this study site, which indicates that black turtles stay in the neritic zone of GNP until almost reaching their SM (~79.4 cm SCL), while yellow turtles would only be using the area for part of their migratory developmental phase, since their SM is much larger (~96.0 cm SCL). Tagging data indicate that C. mydas individuals stay at GNP for several years (unpublished data). GNP is probably a stopover point in the migratory route of both C. mydas morphotypes.
The data showed that black turtles were significantly larger during the last years of this study (2011 and 2012), and that yellow turtles were smaller in 2010. This could be indicative of a change in the contribution of the different rookeries (SM of Galapagos turtles is larger than that of Michoacan turtles, and that of Hawaii turtles is smaller than that of French Polynesia turtles), or to more time being spent by these turtles at different locations before arriving to GNP. According to genetic analyses, there were more turtles from Hawaii in 2010 than from French Frigate shoals, which would explain the smaller size of these turtles in that year (Correa-Cárdenas, 2013).
The CI values reported by several authors for eastern Pacific C. mydas are similar to those found in this study (Table 2). This indicates that the resources available for turtles at the GNP foraging area are equivalent to those of turtles further north (Baja California), since their CI is similar, and that resources at GNP are sufficient to maintain a healthy CI for C. mydas juveniles in the area. The lower availability and abundance of algae in GNP compared to Baja California might be compensated by the availability of other resources, such as tunicates that provide a high-protein food source (Amorocho and Reina, 2007; Fernández-García et al., 2011; Sampson and Giraldo, 2014).
Condition indices have been proposed as indicators of individual health; these indices can be used to compare the well-being of individuals from different populations and can be indicators of changes in the food supply (Bolger et al., 1989). The mean CI of yellow morphotype turtles was significantly higher than that of black turtles. This could be due to yellow turtles consuming more or better quality food, and coincides with the report by Pritchard (1971) of yellow turtles being larger and fattier than black turtles at the Galapagos Islands. It could also be due to smaller-sized turtles arriving to the foraging area with a higher CI: The mean CI of 40-49.9 cm SCL yellow turtles was 1.58, while the mean CI of 50-69.9 cm SCL turtles was 1.45, which would give support to the hypothesis that yellow turtles arrive at GNP in better condition (Fig. 6A). This suggests that, when yellow turtles recruit to the neritic area and switch from their protein-based oceanic diet, their condition decreases slightly, assuming that turtles stay in the neritic area and don´t go back and forth from the oceanic to the neritic area.
The CI of black morphotype turtles varied significantly among the years of this study, while that of yellow morphotype turtles did not. This could be explained by the larger sample size of black turtles (2003-2012, n =745), compared to that of yellow turtles (2007-2012, n =270), that would better reflect possible changes in CI among years. It could also be a consequence of yellow turtles recruiting to the GNP foraging area as their initial neritic foraging ground, and therefore reflecting conditions in the ocean, while black turtles had probably been feeding in other coastal areas and were dependent on coastal conditions that are more variable in terms of food availability.
Meylan et al. (2011) reported that at most Pacific sites the developmental habitats of juveniles overlap with the foraging range of adults. This is not the case at GNP, where no adults were observed. This site would function in a similar manner to those observed by Meylan et al. (2011) in the Caribbean and Florida, where only juveniles are present.
The variation in sizes at this foraging ground that point to variations in the migratory routes of the two morphotypes studied makes evident the importance of protecting oceanic migratory areas as well as coastal areas that provide resources for turtles that come from geographically separate rookeries.
CONCLUSIONS
GNP is a foraging ground used by both yellow and black morphotypes of C. mydas. These two morphotypes share the reef habitat of GNP while they grow to the size at sexual maturity. Most black turtles arrive at a larger size than yellow turtles, and remain until they reach larger sizes. Yellow turtles seem to arrive to GNP and to move out of the foraging area at smaller sizes. These turtles are possibly using GNP as a stopover point during their migration in the eastern Pacific before reaching their rookeries of origin. The black turtles seem to be using GNP until they reach their maturity size and then move either to the Galapagos or the Michoacan rookeries. The fact that the number of yellow turtles using the GNP habitat has increased in recent years, and the variation in the size of both morphotypes, imply changes in the migratory behavior of this species.
GNP is a foraging area where the stocks from different breeding areas converge to feed, making it an important area for conservation, since several populations are relying on this stopover point in their migratory route for growth.
ACKNOWLEDGMENTS
We thank all the agencies involved in collecting data for this study (National Parks of Colombia, CIMAD, WWF), as well as the National Parks personnel and volunteers involved. This paper is part of LS´s doctoral research, which was partly financed by the Natural Sciences and Engineering Research Council of Canada, and by the Postgraduate Biology Program of the Universidad del Valle through a Teaching Assistantship.
REFERENCES
Acevedo-Bueno CI, Beltrán-León BS, Caicedo-Tulante RA. Plan básico de manejo 2005-2009 Parque Nacional Natural Gorgona. Cali: Parques Nacionales Naturales de Colombia. Dirección Territorial Suroccidente; 2004. p. 54-134. [ Links ]
Amorocho DF, Reina RD. Feeding ecology of the East Pacific green sea turtle Chelonia mydas agassizii at Gorgona National Park, Colombia. Endanger. Species Res. 2007;3:43-51. [ Links ]
Amorocho DF, Abreu-Grobois FA, Dutton PH, Reina RD. Multiple distant origins for green sea turtles aggregating off Gorgona Island in the Colombian Eastern Pacific. PLoS ONE. 2012;7(2):e31486. DOI: 10.1371/journal.pone.0031486 [ Links ]
Arthur KE, Balazs GH. A comparison of immature green turtle (Chelonia mydas) diets among seven sites in the main Hawaiian Islands. Pac Sci. 2008;62(2):205-217. DOI: Links ]doi.org/10.2984/1534-6188(2008)62[205:ACOIGT]2.0.CO;2" target="_blank">http://dx.doi.org/10.2984/1534-6188(2008)62[205:ACOIGT]2.0.CO;2
Balazs GH, Chaloupka M. Spatial and temporal variability in somatic growth of green sea turtles (Chelonia mydas) resident in the Hawaiian Archipelago. Mar Biol. 2004;145: 1043-1059. DOI: 10.1007/s00227-004-1387-6 [ Links ]
Bass AL, Epperly SP, Braun-McNeill J. Multi-year analysis of stock composition of a loggerhead turtle (Caretta caretta) foraging habitat using maximum likelihood and Bayesian methods. Conserv Genet. 2004;5(6):783-796. DOI: 10.1007/s10592-004-1979-1 [ Links ]
Bjorndal KA, Bolten AB, Chaloupka MY. Green turtle somatic growth model: evidence for density dependence. Ecol Appl. 2000;10(1):269-282. [ Links ]
Bolger T, Connolly PL. The selection of suitable indices for the measurement and analysis of fish condition. J Fish Biol. 1989;34(2):171-182. DOI:10.1111/j.1095-8649.1989.tb03300.x [ Links ]
Chaloupka M, Limpus C, Miller J. Green turtle somatic growth dynamics in a spatially disjunct Great Barrier Reef metapopulation. Coral Reefs. 2004;23(3):325-335. DOI: 10.1007/s00338-004-0387-9 [ Links ]
Correa-Cárdenas CC. Spatial-temporal analyses of Eastern Pacific green turtles (Chelonia mydas/Chelonia agassizii) foraging as mixed stock in Gorgona NNP, Colombian Pacific Ocean. (Master´s thesis) Departamento de Ciencias Biológicas, Universidad de los Andes; 2013, 67p. [ Links ]
Cortés J. Biology and geology of eastern Pacific coral reefs. Coral Reefs. 1997;15(1):S39-S46. DOI: 10.1007/s003380050240 [ Links ]
Engstrom TN, Meylan PA, Meylan AB. Origin of juvenile loggerhead turtles (Caretta caretta) in a tropical developmental habitat in Caribbean Panamá. Anim Conserv. 2002;5(2):125-133. DOI: 10.1017/S1367943002002184 [ Links ]
Fernández-García C, Riosmena-Rodríguez R, Wysor B, Tejada OL, Cortés J. Checklist of the Pacific marine macroalgae of Central America. Bot Mar. 2001;54:53-73. DOI: 10.1515/bot.2011.001 [ Links ]
Glynn PW, von Prahl H, Guhl F. Coral reefs of Gorgona Island, Colombia with special references to corallivores and their influence on community structure and reef development. An Inst Inv Mar Punta Betín. 1982;12:185-214. [ Links ]
Green D. The green sea turtle project in Galapagos: past, present and future. Not. Galapagos 33.1981;17-20. [ Links ]
Green D. Growth rates of wild immature green turtles in the Galapagos Islands, Ecuador. J Herpetol. 1993;27(3):338-341. [ Links ]
Heppell SS, Snover ML, Crowder LB. Sea turtle population ecology. In: Lutz PL, Musick J, Wyneken J, editors. The biology of sea turtles, Vol. II. Boca Raton: CRC Press; 2003. p. 275-306. [ Links ]
Hirth H. Synopsis of the biological data of the green turtle, Chelonia mydas (Linnaeus, 1758). U.S. Fish and Wildlife Service Biological Report; 1997. 120 p. [ Links ]
Koch V, Brooks LB, Nichols WJ. Population ecology of the green/black turtle (Chelonia mydas) in Bahía Magdalena, Mexico. Mar Biol. 2007;153(1):35-46. DOI: 10.1007/s00227-007-0782-1 [ Links ]
Lahanas PN, Bjorndal KA, Bolten AB, Encalada SE, Miyamoto MM, Valverde RA et al. Genetic composition of a green turtle (Chelonia mydas) feeding ground population: evidence for multiple origins. Mar Biol. 1998;130(3):345-352. DOI: 10.1007/s002270050254 [ Links ]
López-Castro MC, Koch V, Mariscal-Loza A, Nichols WJ. Long-term monitoring of black turtles Chelonia mydas at coastal foraging areas off the Baja California Peninsula. Endanger. Species Res. 2010;11(1):35-45. DOI: 10.3354/esr00264 [ Links ]
McCormick-Anzola CC. Contribución al conocimiento de la ecología y biología reproductiva de las tortugas marinas en la Isla Gorgona. (Tesis de pregrado). Cali: Departamento de Biología, Facultad de Ciencias, Universidad del Valle; 1996. 91p. [ Links ]
Márquez MR. An annotated and illustrated catalogue of sea turtle species known to date. FAO Fish. 1990;125(11):1-81. [ Links ]
Meylan PA, Meylan AB, Gray JA. The ecology and migrations of sea turtles 8. Tests of the developmental habitat hypothesis. Bull Am Mus Nat Hist. 2011;357:1-70. DOI: http://dx.doi.org/10.1206/357.1 [ Links ]
Parker DM, Dutton PH, Balazs GH. Oceanic diet and distribution of genotypes for the green turtle, Chelonia mydas, in the central North Pacific. Pac Sci. 2011;65(4):419-431. [ Links ]
Pritchard PCH. Galapagos sea turtles: preliminary findings. J Herpetol. 1971;5(1/2):1-9. [ Links ]
Pritchard PCH. Evolution, Phylogeny, and Current Status. In: Lutz PL and Musick JA (editors). The Biology of Sea Turtles. Boca Raton: CRC Press;1998. pp. 1- 28. [ Links ]
Rodríguez-Barón JM, Riosmena-Rodríguez R, Seminoff JA, Hernández-Carmona G. Chelonia mydas agassizii diet. Herpetol Rev. 2011;42(2):264. [ Links ]
Sampson L, Giraldo A. Annual abundance of salps and doliolids (Tunicata) around Gorgona Island (Colombian Pacific), and their importance as potential food for green sea turtles. Rev Biol Trop. 2014;62(1):149-159. [ Links ]
Seminoff JA, Alfaro Shigueto J, Amorocho D, Arauz R, Baquero A, Chacón D, et al. Biology and conservation of sea turtles in the eastern Pacific Ocean: A general overview. In: Seminoff JA and Wallace BP, editors. Sea Turtles of the Eastern Pacific Ocean: research advances, conservation challenges and signs of success. Tucson: University of Arizona Press; 2012. p. 11-38. [ Links ]
Seminoff JA, Jones TT, Resendiz A, Nichols WJ, Chaloupka MY. Monitoring green turtles (Chelonia mydas) at a coastal foraging area in Baja California, Mexico: multiple indices to describe population status. J Mar Biol Assoc UK. 2003;83:1355-1362.DOI: 10.1017/S0025315403008816 [ Links ]
Wallace BP, DiMatteo AD, Hurley BJ, Finkbeiner EM, Bolten AB, Chaloupka MY, et al. Regional Management Units for marine turtles: A novel framework for prioritizing conservation and research across multiple scales. PLoS ONE 2010;5(12):e15465. DOI: 10.1371/journal.pone.0015465 [ Links ]
Zapata FA, Vargas-Ángel B, Garzón-Ferreira J. Salud y conservación de las comunidades coralinas. In: Barrios LM, López-Victoria M, editors. Gorgona marina: contribución al conocimiento de una isla única. Santa Marta: INVEMAR, Ser Pub Esp. 7;2001. p. 41-50. [ Links ]
Zárate P. Informe final proyecto anidación de la tortuga verde, Chelonia mydas, durante la temporada de anidación 2002-2003. Fundación Charles Darwin. Presentado al National Marine Fisheries Service y Parque Nacional Galapagos; 2004. 105 p. [ Links ]