Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Acta Biológica Colombiana
Print version ISSN 0120-548X
Acta biol.Colomb. vol.21 no.1 Bogotá Jan./Apr. 2016
https://doi.org/10.15446/abc.v21n1.42894
Doi: http://dx.doi.org/10.15446/abc.v21n1.42894.
SOIL-PLANT NUTRIENT INTERACTIONS IN TWO MANGROVE AREAS AT SOUTHERN BRAZIL
Interacciones de nutrientes entre suelo y planta en dos áreas de manglares en el sur de Brasil
Interação entre a composição química de solo e planta em dois manguezais no sul do Brasil
Ana Paula LANG MARTINS MADI1, Maria Regina TORRES BOEGER2, Carlos Bruno REISSMANN3, Kelly GERONAZZO MARTINS4.
1 Graduate Program in Ecology and Conservation, Setor de Ciências Biológicas, Universidade Federal do Paraná. Centro Politécnico, Jardim das Américas. Curitiba, PR, Brazil.
2 Botany Department, Setor de Ciências Biológicas, Universidade Federal do Paraná. Centro Politécnico, Jardim das Américas. Curitiba, PR, Brazil.
3 Soil Department, Setor de Ciências Agrárias, Universidade Federal do Paraná. Rua dos Funcionários, 1540, Juvevê. Curitiba, PR, Brazil.
4 Environmental Engineering Department, Setor de Ciências Agrárias e Ambientais, Universidade Estadual do Centro-Oeste. PR 153 km 7 - Riozinho, Campus Irati, Paraná, Brazil.
For correspondence. langmartins@hotmail.com
Received: 23rd May 2014, Returned for revision: 21st January 2015, Accepted: 2nd March 2015.
Associate Editor: Hernán Mauricio Romero.
Citation / Citar este artículo como: Lang Martins Madi AP, Torres Boeger MR, Reissmann CB, Geronazzo Martins K. Soil-plant nutrient interactions in two mangrove areas at Southern Brazil. Acta biol. Colomb. 2016;21(1):39-50. doi: http://dx.doi.org/10.15446/abc.v21n1.42894.
ABSTRACT
Mangrove forests have a simple architecture. They shelter a few number of arboreal species that grow in a saline environment subject to tidal activity. The research objective was to evaluate possible interactions between physical-chemical soil attributes and plant-leaf nutrient concentrations of different mangrove species. Different mangrove species growing in the same soil, and the same mangrove species growing in two different soil classes were evaluated as to their leaf nutrient concentration patterns. The study was carried out in mangrove areas of the State of Paraná, southern Brazil, in two distinct soil classes: HISTOSOL THIOMORPHIC Salic sodic and GLEYSOL THIOMORPHIC Salic sodic; and three different species: Avicennia schaueriana, Laguncularia racemosa and Rhizophora mangle. Two subareas were delimited within each area from which soil and leaf samples were collected. Samplings from five individuals of each dominant mangrove species were taken from the soil (0-10 cm deep) under each tree crown projection. The data was submitted to statistical analysis using a set of simple and multivariate analysis in order to determine possible differences among mangrove species leaf nutrient concentrations, and whether these differences might be correlated with the soil attributes or not.
The results exposed that the nutritional state of the mangrove species is different and independent form the soil attributes in which they grow. Few correlations were found among leaf nutrient concentrations and soil attributes, suggesting differential selective nutrient uptake among species.
Keywords: Avicennia schaueriana, Laguncularia racemosa, mangrove, soil, plant nutrients, Rhizophora mangle, soil chemical attributes.
RESUMEN
Los manglares son bosques de arquitectura simple que albergan pocas especies arbóreas, creciendo en un ambiente salino sometido a la influencia de las mareas. El objetivo de este trabajo fue evaluar las posibles interacciones entre las propiedades fisicoquímicas del suelo y la concentración de nutrientes en hojas de diferentes especies de mangle. Se investigó si las diferentes especies que se desarrollan en la misma clase de suelo tienen concentraciones de nutrientes foliares similares, y si las plantas de mangles de la misma especie que se desarrollan en diferentes tipos de suelos tienen concentraciones foliares similares. El estudio se desarrolló en manglares del Estado de Paraná, sur de Brasil, en dos tipos de suelos diferentes (HISTOSOL TIÓNICO Salino sódico y GLEYSOL TIÓNICO Salino sódico). Se analizaron tres especies vegetales diferentes (Avicennia schaueriana, Laguncularia racemosa y Rhizophora mangle). En cada área se delimitaron dos subáreas para recolectar el suelo y las hojas de cada una de las especies. Se tomaron cinco individuos de cada especie del dosel dominante para recoger hojas y muestras de suelo de 0-10 cm, en la proyección de la copa de los árboles seleccionados. Se realizaron análisis univariados y multivariados para probar si las especies de mangle tienen perfiles nutricionales diferentes, y si existe alguna correlación entre las propiedades del suelo con la composición química de las hojas. Los resultados mostraron que el estado nutricional de las especies de mangle es distinto e independiente de los atributos de los suelos en los que se encuentran. Las concentraciones de elementos en las hojas presentan poca correlación con los nutrientes del suelo, lo que sugiere que la absorción de nutrientes por las plantas es selectiva.
Palabras clave: Atributos químicos del suelo, Avicennia schaueriana, Laguncularia racemosa, mangle, nutrientes, Rhizophora mangle.
RESUMO
Manguezais são florestas de arquitetura simples, abrigando poucas espécies arbóreas, vegetando em ambiente salino e sujeito ao regime de marés. O objetivo desta pesquisa foi avaliar as possíveis interações entre os atributos físico-químicos do solo e a concentração de nutrientes nas folhas de diferentes espécies de mangue. Assim, foi investigado se as diferentes espécies que se desenvolvem sobre a mesma classe de solo apresentam concentrações de nutrientes foliares similares, e se plantas de mangue da mesma espécie que ocorrem sobre diferentes classes de solo apresentam concentrações de nutrientes foliares similares. O estudo foi desenvolvido em manguezais no Estado do Paraná, Sul do Brasil, em duas classes distintas de solo (ORGANOSSOLO TIOMÓRFICO Sálico sódico e GLEISSOLO TIOMÓRFICO Sálico sódico), e três espécies diferentes de plantas (Avicennia schaueriana, Laguncularia racemosa and Rhizophora mangle). Em cada área foram delimitadas duas subáreas para coleta de solo e folhas das espécies. Cinco indivíduos do dossel dominante de cada espécie para coleta de folhas, na projeção da copa das árvores, e amostras de solo de 0-10 cm foram selecionados. Para testar se espécies de mangue apresentam perfis nutricionais distintos e se há correlação entre os atributos do solo com a composição química foliar um conjunto de análises univariadas multivariadas foram realizadas. Os resultados demonstraram que o estado nutricional das plantas é distinto e individualizado, independente dos atributos do solo em que se encontram as espécies. As concentrações dos elementos nas folhas pouco se correlacionaram com os nutrientes do solo, sugerindo absorção seletiva dos nutrientes pelas plantas.
Palavras-Chave: Atributos químicos do solo, Avicennia schaueriana, Laguncularia racemosa, mangue, nutrientes, Rhizophora mangle.
INTRODUCTION
Mangroves are ecosystems located between the highlands and ocean, characteristics of saline coastal habitats in the tropics and subtropics, occurring as an interface between land and sea (Schwarz, 2003). Such environments are dominated by many typical tree and shrub species adapted to a fine sediment substrate originated from periodical tide flood depositions, rich in organic matter, with varied salinity and low oxygen (Griffiths et al., 2008).
The mangrove forest areas of the world occupy around 14.65 million hectares (Wilkie and Fortuna, 2003). Brazil ranks third with one of the world's largest mangrove areas, representing 7 % of the world (Giri et al., 2011). The Brazilian mangroves are found along 6800 km of coast from the extreme north of Oiapoque (State of Amapá) until the southern region of Santa Catarina State (Schaeffer-Novelli et al., 1990). Although it is considered a Permanent Preservation Area (PPA) according to the Brazilian directive CONAMA 303/02, this ecosystem is under continuous anthropic activity threat (Krug et al., 2007). About 35 % of the Brazilian mangrove forests were lost between the 1980's and 1990's (Valiela et al., 2001).
Mangrove forests show low tree species diversity when compared to other tropical forests (Kathiresan, 2008). At southern Brazil, only three mangrove species are usually found: Rhizophora mangle L.; Laguncularia racemosa (L.) Gaertn; and Avicennia schaueriana Stapf & Leachman.
Despite the low plant diversity, mangroves play an important ecological role in the coastal areas (Kauffman et al., 2011), because they are a source of organic matter for the ecosystem. They also provide goods and food for the human population, thereby demonstrating their socio-economic role (Soares et al., 2003).
Species abundance is influenced by several factors such as geomorphology, climate, tide amplitude, salinity and edaphic characteristics (Boto and Wellington, 1984). Such abiotic factors are generally interrelated, as for instance soil salinity affecting leaf nutrient concentrations (Araujo et al., 2010). Besides the complex interactions among abiotic factors, many mangrove species may present differences in the absolute plant nutrient concentrations and the relative ratios between nutrients due to different plant species abilities in nutrient selectivity and uptake (Bernini et al., 2006).
Studies at the southern and northern coastal regions of Brazil showed that mangrove species presented differential nutrient concentrations. The Avicennia species was observed to show higher leaf concentrations of nitrogen (N), potassium (K) and magnesium (Mg) and lower concentrations of calcium (Ca) than Rhizophora mangle and Laguncularia racemosa (Cuzzuol and Campos, 2001; Bernini et al., 2010). Such variation is expected considering that these species have differential selectivity for nutrients even growing in the same environment (Bernini et al., 2006).
The spatial and temporal nutrient variability in the soil might also collaborate in differential nutrient uptake among species (Bernini et al., 2010). Nevertheless, other studies have suggested that soil physical-chemical attributes might also affect plant leaf nutrient concentrations, mainly when plants share the same pool of soil nutrients (Vitousek and Sanford, 1986).
Soil salinity is considered a determinant factor affecting mangrove vegetation structure and development (Bernini et al., 2006). Excessive soil sodium (Na) might induce other cation deficiencies in the plants. There are studies evidencing that mangrove foliar Ca concentrations are controlled by or dependent on the soil Na concentrations (Waisel, 1972). On the other hand, Ca is indispensable to the plant survival under mangrove environment conditions enhancing K uptake and Na inhibition (Lacerda et al., 1986).
Mangrove soils are classified as allomorphic soils, developed by the deposition of sediments from the ocean, rivers and rain runoff. The main soil classes in these environments are: thiomorphic Gleysols and salic Gleysols, and Histosols (Vidal Torrado, 2005). These soils are mainly characterized by fine sediments (clay and silt), high organic matter and soluble salts (from sea water) (Citrón and Schaffer-Novelli, 1983).
In the State of Paraná, Brazil, Gleysols and Histosols are commonly found and both may present thiomorphism (Embrapa, 2009). The Gleysol class consists of hydromorphic soils characterized by a grey color as a consequence of the presence of reduced iron (Fe2+) due to constant (periodical or permanent) water saturation; the Gleysols are constituted by minerals of varied textures. The Histosol class consists of soils with high proportion of vegetal residues in different degrees of decomposition, poor drainage conditions, very low density and high porosity; they are extremely fragile soils, but with high organic-C and water storage capacity (Embrapa, 2009).
Based on the soil-plant interaction evaluations, mainly on the relationships among soil physical-chemical attributes and plant leaf nutrient concentrations, two mangrove areas of southern Brazil with two distinct floristic structural organization such as tree abundance and basal area, were studied. The areas were characterized by two distinct soil classes (salic-sodic thiomorphic Histosol and salic-sodic thiomorphic Gleysol), with different organic-C contents. This study aimed to investigate if the leaf nutrient concentrations are soil class dependent, and if soil chemical-physical attributes would interrelate with leaf nutrients.
MATERIAL AND METHODS
Research area
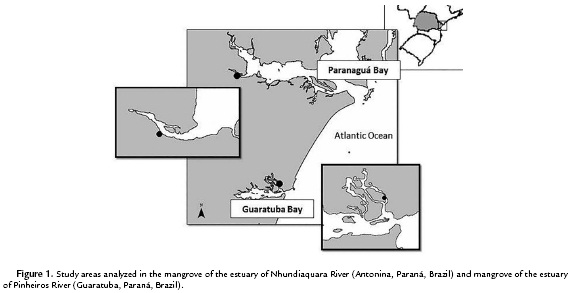
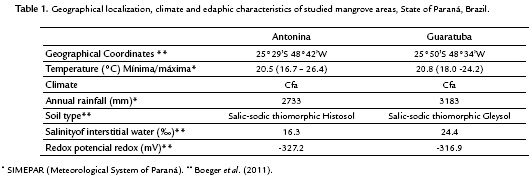
This study was carried out at Antonina and Guaratuba municipal districts, State of Paraná, Brazil. Antonina is located at the western region of Paranaguá Bay and covers an area of 460 km2. Guaratuba is located at the Guaratuba Bay and represents the second greatest estuarine complex of Paraná coast with 48.72 km2 (Fig. 1). The mangrove geographical position, edaphic and climatic characteristics are presented in Table 1. The average annual rainfall and daily temperature data is referred to the year of 2010, registered by the Paranaguá Station of the Meteorological System of Paraná (SIMEPAR) (Table 1).
Material sampling
For each mangrove site an area of about 1000 m2 (10 m x 100 m), was established parallel to the shore line for soil and plant leaf samplings from A. schaueriana, L. racemosa and R. mangle. Five dominant individuals were randomly marked per area along the entire mangrove strip. At Antonina, the individuals were marked in the superior middle part of the Nhundiaquara River estuary; and at Guaratuba, in the middle part of Pinheiros River.
Intact mature leaves were collected from the middle of the tree crown at north face (Borille et al., 2005), during July 2010, using pruning-scissors. Young and senescent leaves were not considered. Leaf samples were thoroughly rinsed and dried in a forced-air oven at 60 ºC until constant weight, grinded to powder and submitted to nitric-perchloric digestion, according to Jones and Case (1990). Phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), sodium (Na) and sulfur (S) were determined by ICP-OES (inductively coupled plasma optical emission spectrometry) with argon plasma. Nitrogen (N) was determined by Kjeldahl method (Jones and Case, 1990).
Soil samples were collected at 0-10 cm deep (Nielsen and Andersen, 2003) in four selected points under each tree crown projection, during June 2010. Soil samples were air-dried at room temperature and passed through 2 mm sieve to obtain the air-dried fine soil fraction (< 2 mm). Soil samples were then analyzed for pH CaCl2, P, K, Ca, Mg, Na and aluminum (Al), according to the "Manual of Methods for Soil Analysis" (Embrapa, 1997); Carbon (C) and N were determined by combustion using a VARIO-EL-III analyzer, Elementar® model. The mangrove thiomorphic character was confirmed according to classification of Embrapa (2009).
Statistical analysis
Plant and soil data were submitted to univariate and multivariate (data clustering and ordination) analyses in order to test whether mangrove species growing in different soil classes would show differential leaf nutrient concentrations. Univariate analysis was used to evaluate plant responses within and among mangrove areas. Statistical differences among areas were assured by means of two analyses of variance (one-way ANOVA), firstly including species as the variable factor, and secondly, the mangrove areas. Soil pedological attributes and leaf nutrient concentrations were the dependent variables. After ANOVA, variable means were compared by Fisher test LSD (p < 0.05). The variance homogeneity and Gaussian distribution were checked out by means of Bartlett test (p < 0.05) and Kolmogorov-Smirnov (p < 0.05) (Zar, 1999).
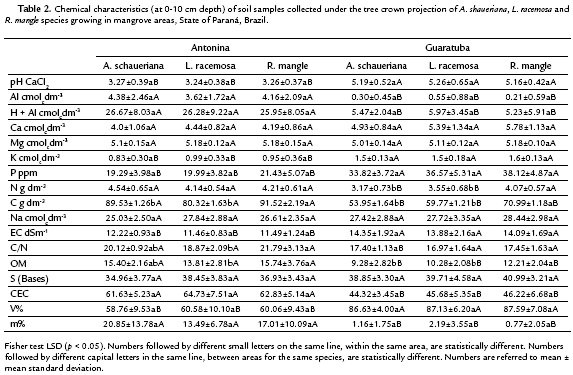
The K uptake efficiency in the presence of high Na concentrations was evaluated by means of the net selectivity efficiency index (net SK:Na), according to Flowers and Colmer (2008), calculated by the following formula:
Where leaf nutrient unities are in g.kg-1 and soil nutrient unities in mg.kg-1; the conversion factor for K is 0.391 and for Na is 0.230.
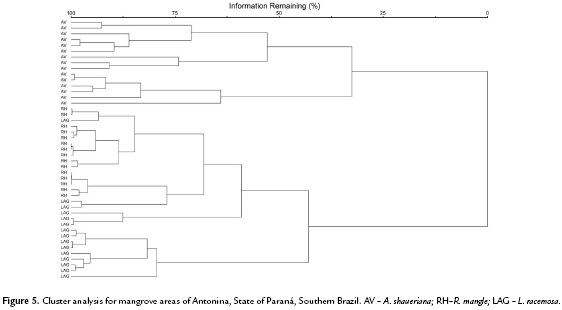
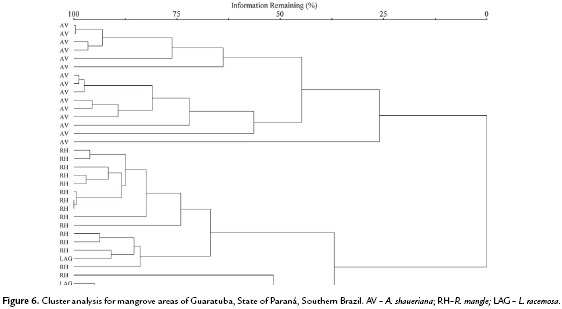
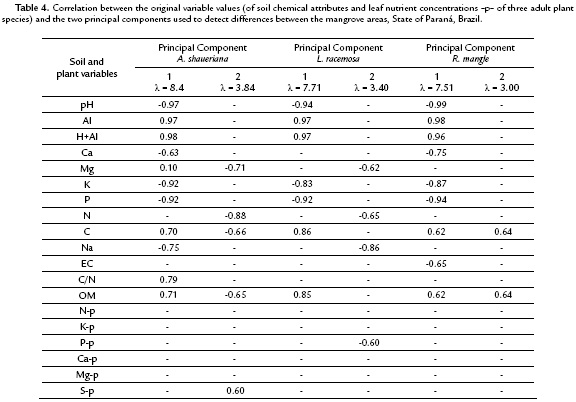
In order to detect differences among plant nutrient concentrations and soil chemical attributes in both mangrove areas, three Principal Components Analyses (PCA) were made with Euclidian distance. Pearson correlations among original variables and the PCA scores were made to identify which variables were the responsible for the variations. The number of axis was determined by the randomizing test with 999 permutations. Analyses were run by the PC-ORD 6.0 program (McCune and Mefford, 2011). In order to verify if the species present distinct strategies in nutrient absorption a hierarchical classification procedure was employed (cluster analysis, Euclidean distance and complete linkage-farthest neighbor), utilizing a matrix of leaf nutrient data, separately for each mangrove. The cophenetic correlation coefficient was calculated for each group with the objective to verify the deformation degree resulted from the dendrogram construction. In this study both coefficients were greater than 0.75. To test if the hierarquical classification could be significantly separated, a Permanova (Permutational Multivariate Analysis of Variance ) with 999 permutations of the residuals in the full model was performed with the same distances used in the hierarchical classification (McCune and Grace, 2002).
RESULTS
Soil analysis
Soil samples from Antonina showed lower pH values than those from Guaratuba, but no differences among soil for each species, within each area, were observed. However, differences between soils were observed when each species was individually compared (Table 2).
Higher electrical conductivity (EC) was found in soil samples from Guaratuba than from Antonina, but no significant differences among the species soil samples within the same area were found (Table 2).
Significant higher organic matter concentration values (OM) were found in soil samples from Antonina than from Guaratuba. In addition, significant differences within each area were observed, among the three species (Table 2). In the same way, higher soil carbon (C) values and C:N ratios were found in samples from Antonina, as expected, since these variables are related with organic matter.
No significant differences among the three species soil samples within each mangrove area were found for Ca, K, Mg, Na and P concentrations. But higher K and P concentrations were found in soil samples from Guaratuba than from Antonina. On the other hand, higher N concentrations were found in soil samples from Antonina, but only those collected under the A. shaueriana and L. racemosa species (Table 2).
Leaf analysis
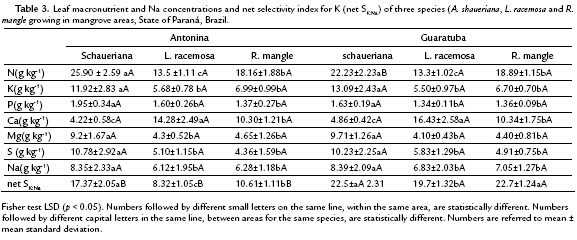
The leaf nutrient concentrations were ranked according to the following decreasing values for the three mangrove species: N > K > S > Mg > Na > Ca > P for A. shaueriana, in both mangrove areas; Ca > N > K > Na> S > Mg > P for L. racemosa in Antonina and Ca > N > Na> S > K > Mg > P in Guaratuba; N > Ca> K > Na> S > Mg > P for R. mangle, in Antonina, and the opposite Na > K in Guaratuba (Table 3).
In general, considering the same soil type or location, the three species differ significantly according to their nutrient leaf concentrations (Antonia F = 49.94; p < 0.01 e Guaratuba F = 51.52; p < 0.01) (Fig. 5 and Fig. 6).
No significant differences between areas for the same species leaf nutrient concentrations were found, except for A. schaueriana that showed higher leaf N concentration in Antonina (Table 3). Besides, leaf N concentrations were ranked in the same decreasing order for the species, within each mangrove area, as follows: A. schaueriana > R. mangle > L. racemosa. Potassium, Mg, S and Na showed higher and significant differences in A. schaueriana against L. racemosa and R. mangle (Table 3). Leaf P concentrations also showed the same tendency in both areas and within each area the following decreasing gradient was observed: A. shaueriana > R. mangle = L. racemosa for both Antonina and Guaratuba mangroves. As concerned to leaf Ca concentrations, A. shaueriana showed, in average, 3.5-fold lower Ca than L. racemosa in both mangrove areas (Table 3).
In Guaratuba, higher net selectivity efficiency index (net SK:Na) values for K (in presence of Na) were found for A. shaueriana than for L. racemosa and R. mangle. In Antonina, A. shaueriana and R. mangle showed similar index values, which were higher than L. racemosa index (Table 3).
Soil-plant interaction
The data matrix analysis revealed few correlations among soil and plant variables. Positive correlations between soil Na and R. mangle leaf N in Guaratuba (r = 0.75; p < 0.05) and L. racemosa leaf N in Antonina (r = 0.72; p < 0.05) were found, respectively.
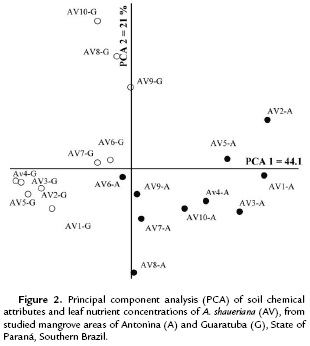
The principal coordinate analyses (PCA) of soil data collected under the three species crown projections and their respective leaf nutrient concentrations allowed clustering the species into two groups (Antonina and Guaratuba mangroves). The pedological attributes were the variables that mostly contributed, but correlation coefficients between principal coordinates and leaf nutrients were not above 0.70 (Table 4). This means that the data clustering and ordination found for A. shaueriana explained 65.1 % of the data (PCA 1= 44.1 % and PCA 2 = 21 %) (Fig. 2). The soil pH, Al, H+Al, K, P, C, Na and OM showed higher correlations with the first coordinate. The second coordinate also separated the plots into two groups, but it was mostly correlated with Mg and N variables.
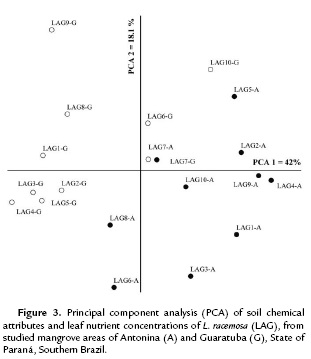
The ordination analysis for L. racemosa clustered the mangrove areas in two distinct groups (Fig. 3). The first and second coordinates together explained 60.1 % of data variation (PCA 1= 42 % and PCA 2 = 18.1 %). Soil pH, Al, H+Al, K, P, C, and OM were the attributes mostly correlated with the first coordinate, and Na with the second coordinate.
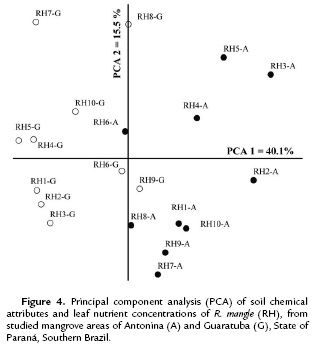
The ordination analysis for R. mangle also clustered the mangrove areas into two distinct groups (Fig. 4). Both coordinates together explained 55.6 % of the data variation (PCA 1= 40.1 % and PCA 2 = 15.5 %). Soil pH, Al, H+Al, K, P and Na were the attributes mostly correlated with the first coordinate, and C and OM with the second coordinate (r < 0.70) (Table 4).
DISCUSSION
Soil analysis
The soil chemical analysis showed intra and interspecific variations, and the results were similar to the average values of other Brazilian mangroves (Cuzzuol and Campos, 2001; Bernini et al., 2010; Bernini and Rezende, 2010). The main differential soil attributes between areas were Al, K, P, CEC, m % and V %, plus C-content that separated the soils in Histosol and Gleysol classes.
The expressive differential percentages between the soil classes represented by m = 92 %; Al = 91 %; P = 44 %; K = 39 %; pH = 37 %; V% = 31 %; C and OM = 29 %; and CEC = 28 %, are coherent with the clusters expressed in Fig. 2-4. Significant differences for N were only found in Guaratuba and for C/N ratio in Antonina. Therefore, the statistical analysis among and within mangrove areas showed that significantly different soil variables were related with the distinct soil classification.
Carbon concentration was the main attribute responsible for the mangrove soil classification, characterizing a Histosol in Antonina and a Gleysol in Guaratuba. The Guaratuba soil profile did not show a sufficiently expressive histic horizon to be classified as a Histosol. However, the gleysol samples showed higher OM concentrations nearby the R. mangle individuals than nearby other species. This higher OM concentration resulted from its accumulation in the depressions, where R. mangle occurs when growing in inland sites, despite its frequently occurrence on coastal locations. The present data corroborated other authors' results, when high OM contents were observed nearby R. mangle plants in mangrove areas of Mexico (Lópes-Portillo and Ezcurra, 1989) and southern Brazil (Carmo et al., 1998).
The lower pH values observed in Antonina's soil samples are probable due to the greater amount of sulfur radicals generated from OM and also to their greater potential acidity. However, two special considerations about Antonina's pH values must be done: (a) routine soil analysis showed very low pH values (3.5-4.5) indicating acidic medium; such low values are probably due to the soil sample procedure for chemical analysis, because oxidation of sulfur and derivates occur during soil drying (Bernini and Rezende, 2010); (b) under soil water saturation conditions (Embrapa, 2009), the same soil samples presented pH range of 6.8-7.4, which are more compatible with the high soil base saturation (V%) values found. Some of these mangrove soils may show pH ≤ 4.0, when incubated for until eight weeks, and then, they are characterized as thiomorphic soils (Embrapa, 2009).
The higher soil exchangeable base (Ca, Mg, K and Na) concentrations (SBCS, 2004) corroborated the in situ higher pH values found (Cuzzuol and Rocha, 2012). The same is evidenced by soil CEC values, because soil charges are originated from the high OM concentrations and mineral colloids of sediments. The OM higher CEC in Antonina than in Guaratuba soils is probably due to the organic constitution of Antonina's soils. Little variation of sum of bases (SB) was observed between areas and species. In this case Bases Saturation (V %), is inversely proportional to CEC, which in turn is dependent on the organic or mineral colloid characteristics.
The soil high electrical conductivity (EC) values (> 7dSm-1 at 25 ºC) conferred the Salic character to such mangrove soils (Embrapa, 2009). Furthermore, the high Na concentration values observed in these mangrove soils might be explained by the frequent flooding with saline waters in such areas, conferring the Sodic character to soil classification (Embrapa, 2009). Both studied areas presented high percentage of soil Na in the colloid exchange loci.
Leaf Analysis
Differential leaf nutrient concentrations were observed among mangrove species in both areas, although all three species were under the same synergistic and antagonistic relationships during root nutrient uptake from soil (Cuzzuol and Rocha, 2012).
In the present study, the leaf nutrient concentration means were within the range described in the literature for Brazilian mangroves (Cuzzuol and Campos, 2001; Bernini et al., 2010; Bernini and Rezende, 2010). However, different nutrient concentration patterns were observed for the three species, contrarily to the results reported by Bernini et al. (2010). They found similar nutrient patterns in L. racemosa and R. mangle species, when studying the Espírito Santo mangroves, at southern Brazil. On the other hand, in the present study highest leaf N and lowest P and S concentrations were found in A. shaueriana and R. mangle species, corroborating the results of Cuzzuol and Campos (2001), when studying the Mucuri mangrove in the State of Bahia, Brazil.
The differential leaf nutrient concentration ranking among mangrove species (K > Mg > Ca in A. shaueriana; Ca > K > Mg in L. racemosa and R. mangle) appears to be related to the reciprocal antagonism among cations (Mengel, 1984), as well as to the seasonal variation that directly influences the leaf nutrient concentrations.
There was a clear distinction among species as to their leaf N concentrations, significantly higher in A. shaueriana leaves, independently of the soil type (Table 2). This species' higher leaf N concentration might be the result of glycinebetain (N-rich quaternary ammonium compound) accumulation in the cytoplasm that is supposed to contribute to plant salt tolerance (Popp, 1984; Medina and Francisco, 1997).
Avicennia shaueriana's higher leaf Mg and Na concentrations evidenced a selective root uptake for these nutrients, fact that is a characteristic salt excretion mechanism present in the species of this genus (Lacerda et al., 1985; Medina and Francisco, 1997).
On the other hand, the differential leaf K concentrations observed among species suggested diverse mechanisms affecting soil K movement to the rhizosphere, and else, distinct root K selectivity and uptake (Meurer, 2006). The net K selectivity index (net SK:Na) values found (average = 19) were within the range indicated (9-60) by Flowers and Colmer (2008). The data evidenced that the medium physical characteristics might interfere on the K index values. High net SK:Na values for A. shaueriana in both studied areas expressed greater K uptake ability in the presence of high Na concentrations and higher K selectivity.
The high leaf Ca, superior than N concentrations observed in L. racemosa leaves do not represent the species usual nutrient pattern. Low leaf Ca was also found in A. shaueriana in mangroves of northern (Cuzzuol and Campos, 2001) and southern Brazil (Bernini et al., 2006) and seemed to be a characteristic of species of this genus, independently of the soil type where the individuals are grown. Else, it would depend on which plant category the species would belong to, as concerned to Ca acquisition and accumulation, such as the so-called oxalate containing plants (Hawkesford et al., 2012). The oxalate containing plants are characterized by the presence of free oxalate in the roots that would precipitate Ca and possibly restrict this nutrient transport to the xylem (Bernini et al., 2006).
Soil- plant interaction
The ordination analysis and Pearson correlations between soil and plant nutrient variables evidenced the soil was not a conditioning factor for the species leaf nutrient concentrations in the studied mangrove areas.
Higher soil N and C concentrations were found in Antonina, indicating greater soil OM enrichment compared to Guaratuba mangrove, and characterizing the Histosol class. The significant increases in such soil attributes determined the soil ordination under the respective species. The highest OM concentration values in Antonina might be consequence of harbor activities and urban conglomerations nearby the experiment sampling area, because the organic matter seemed to be a mixture of autochthonous and allochthonous OM depositions from continental origin (Bittencourt, 1998; Lana, 1998).
The soil K and pH data ordination might be explained by the strong relationship between the soil base saturation and soil pH (Raij, 1983). It is highlighted that in Guaratuba, where soil base saturation was close to 90 % and pH was higher than at Antonina, the base saturation and pH values might also be due to potassium feldspars of regional sediments originated from elements of the "Serra do Mar" mountain ridge (Angulo, 2004).
On the other hand, Antonina's mangrove soil showed relatively high Al saturation, lower base saturation, and lower K concentrations, although K was high enough in terms of plant nutrition. However, even with K-rich feldspar depositions, Antonina's mangrove lower soil K concentrations might be explained by the lower K-OM binding affinity, suggesting greater K leaching and lower extractable K concentrations.
Mangrove soil ordination was strongly defined by soil Al and H+Al concentration values. Antonina's mangrove higher potential acidity, as already discussed, is probably due to this environment higher OM content (Lin et al., 2009).
However, soil data ordination was not possible when using the variables responsible for mangrove soil classification (C, C/N and OM). This fact, in the case of R. mangle, might be attributed to its favorite localization at mangrove margins, which is a region of greater instability and more susceptible to tide action. Therefore, as this species follows along both mangrove margins, the spatial factor might have greatly influenced the R. mangle leaf chemical composition (Cuzzuol and Rocha, 2012). Contrarily to R. mangle, the other mangrove species seemed to more intensively respond to the soil matrix physical-chemical processes. Depending on the soil texture, soil factors such as redox potential, pH, CEC, C and EC might exert more or less influence on plant species nutrient uptake. The differential nutrient leaf composition of A. shaueriana and L. racemosa might also be attributed to the soil saturation degree and micro relief (Jimenez, 1988; Jimenez and Lugo, 1988).
From this study resulted that different mangrove species growing in the same soil class presented differential leaf nutrient concentrations. Avicennia shaueriana showed higher leaf nutrient concentrations, except for Ca, than the other two studied species (R. mangle and L. racemosa). Among the three species, A. shaueriana showed the highest net selectivity index for potassium in the presence of high saturation levels of sodium.
Differential and individual species nutritional patterns were observed, evaluated by the leaf nutrient concentrations, independently of the soil attributes in which they were growing. Differences observed in soil characteristics did not affect the species nutritional patterns. Few correlations were found between leaf and soil nutrient concentrations, suggesting differential selective nutrient uptake among mangrove plant species.
CONCLUSION
Different mangrove species growing in the same soil class presented differential leaf nutrient concentrations. Avicennia shaueriana showed higher leaf nutrient concentrations, except for Ca, than the other two studied species (R. mangle and L. racemosa). Among the three studied species, A. shaueriana showed highest values of selectivity index for potassium in the presence of high saturation levels of sodium.
Differential and individual species nutritional patterns were observed, evaluated by the leaf nutrient concentrations, independently of the soil attributes in which they were growing.
Differences observed in soil characteristics did not affect the species nutritional patterns. Few correlations were found between leaf and soil nutrient concentrations, suggesting differential selective nutrient uptake among mangrove plant species.
ACKNOWLEDGEMENTS
The authors are thankful to Petrobras and Araucária Foundation (Convention 412/09 protocol 12499) for the financial support. To the Coordination of Graduate Personnel Improvement (CAPES) for the research grant conferred to the first author. To the Council for Scientific and Technological Development (CNPq) for the scientific productivity (301561/2010-9) grant conferred to the second author. To Professor Dr. Celina Wisniewski for the soil classification of the mangrove areas under study.
REFERENCES
Alongi DM. The dynamics of benthic nutrient pools and fluxes in tropical mangrove forest. J Mar Res. 1996;54(1):123-148. Doi: http://dx.doi.org/10.1357/0022240963213475. [ Links ]
Angulo RJ. Cenozoic map of the state of Paraná coastal zone. Bol Par Geo. 2004;55(1):25-42. [ Links ]
Araujo QR, Krause RLO, Santana SO, Araujo TG, Mendonça JR, Trindade AV. Caracterização de Solo de Manguezal na Bacia Hidrográfica do Rio Graciosa, Bahia, Brasil [Internet]. Long Beach, Califórnia: Encontro Internacional Anual 2010 das Sociedades Americanas de Agronomia, Fitotecnica e Ciência do Solo; 2010. Available from: http://www.ateffaba.org.br/?p=6930. [ Links ]
Bernini E, Silva MA, Carmo TM, Cuzzuol GRF. Composição química do sedimento e de folhas das espécies do manguezal do estuário do rio São Mateus, Espírito Santo, Brasil. Rev Bras Bot. 2006;29(4):686-699. Doi: http://dx.doi.org/10.1590/S0100-84042006000400018. [ Links ]
Bernini E, Rezende CE. Concentração de nutrientes em folhas e sedimentos em um manguezal do norte do estado do Rio de Janeiro. Revista Gestão Costeira Integrada. 2010;2(2):1-10. [ Links ]
Bernini E, da Silva MAB, do Carmo TMS, Cuzzuol GRF. Spatial and temporal variation of the nutrients in the sediment and leaves of two Brazilian mangrove species and their role in the retention of environmental heavy metals. Braz Soc Plant Physiol. 2010;22(3):177-187. Doi: http://dx.doi.org/10.1590/S1677-04202010000300005. [ Links ]
Bittencourt AVL. Avaliação da disponibilidade hídrica. In: Lima RE, Negrelle RRB, editors. Meio Ambiente e Desenvolvimento no Litoral do Paraná: Diagnóstico. Curitiba: Editora UFPR; 2002. p. 41-48. [ Links ]
Borille AMW, Reissmann CB, Freitas RJS. Relação entre compostos fitoquímicos e o nitrogênio em morfotipos de erva-mate (Ilex paraguariensis St.Hil.). B Ceppa. 2005;23(1):183-198. [ Links ]
Boto KG, Wellington JT. Soil Characteristics and Nutrient Status in a Northern Australian Mangrove Forest. Estuaries.1994;7(1):61-69. Doi: http://dx.doi.org/10.2307/1351957. [ Links ]
Carmo TMS, Almeida R, Oliveira AR, Zanotti-Xavier S. Caracterização de um trecho do manguezal do rio da Passagem, baía de Vitória, Vitória-ES. In: Anais do IV Simpósio de Ecossistemas Brasileiros. Espírito Santo: ACIESP; 1998. p. 9-19. [ Links ]
Citrón G, Schaeffer-Novelli Y. Introdución a la ecologia del manglar. San Juan: Rostlac; 1983. p. 109. [ Links ]
Cuzzuol GRF, Rocha AC. Interação do regime hídrico com as relações nutricionais em ecossistema manguezal. Acta Bot Bras. 2012;26(1):11-19. Doi: http://dx.doi.org/10.1590/S0102-33062012000100003. [ Links ]
Cuzzuol GRF, Campos A. Aspectos nutricionais na vegetação de manguezal do estuário do Rio Mucuri, Bahia, Brasil. Rev Bras Bot. 2001;24(2):227-234. Doi: http://dx.doi.org/10.1590/S0100-84042001000200013. [ Links ]
Embrapa - Empresa Brasileira de Pesquisa Agropecuária. Centro Nacional de Pesquisas de Solo. Manual de Métodos de Análises de Solo. Rio de Janeiro: Embrapa-CNPS; 1997. p. 212. [ Links ]
Embrapa - Empresa Brasileira de Pesquisa Agropecuária. Sistema Brasileiro de Classificação de Solos. Rio de Janeiro: Embrapa; 2009. p. 306. [ Links ]
Flowers TJ, Colmer TD. Salinity tolerance in halophytes. New Phytol. 2008;179(4):945-963. Doi:10.1111/j.1469-8137.2008.02531.x. [ Links ]
Giri C, Ochieng E, Tieszen LL, Zhu Z, Singh A, Loveland T, et al. Status and distribution of mangrove forests of the world using earth observation satellite data. Global Ecol Biogeogr. 2011;20(1):154-159. Doi:10.1111/j.1466-8238.2010.00584.x. [ Links ]
Griffiths ME, Rotjan RD, Ellmore GS. Differential salt deposition and excretion on leaves of Avicennia germinans mangroves. Caribb J Sci. 2008;44(2):267-271. [ Links ]
Hawkesford M, Horst W, Kichey T, Lambers H, Schjoerring J, Mϕller I S, et al. In: Marschner P, editor. Marschner's Mineral Nutrition of Higher Plants. San Diego: Academic Press; 2012. p. 135-189. [ Links ]
Jimenez JA, Lugo AE. Avicennia germinans: black mangrove. Avicennae. Verbena Family. U.S. Department of Agriculture, Institute of Tropical Forestry; 1988. p. 6. [ Links ]
Jones Jr JB, Case VW. Sampling handling, and analyzing plant tissue samples. In: Westerman RL, editor. Soil testing and plant analysis. Madison: Soil Science Society of America; 1990. p. 389-427. [ Links ]
Kathiresan K, Bingham BL. Biology of mangroves and mangrove ecosystems. Adv Mar Biol. 2001;40(1):81-251. Doi: http://dx.doi.org/10.1016/S0065-2881(01)40003-4. [ Links ]
Kathiresan K. Methods of study mangroves. Handout of Training Course on Mangroves and Biodiversity [Internet]. Association?editor?University?; 2008. Available from: http://ocw.unu.edu/international-network-on-water-environment-and-health/unu-inweh-course-1-mangroves/unu-inweh-course-1-mangroves.zip/view. [ Links ]
Kauffman JB, Heider C, Cole TG, Dwire KA, Donato DC. Ecosystem carbon stocks of Micronesian mangrove forests. Wetlands. 2011;31(2):343-353. Doi:10.1007/s13157-011-0148-9. [ Links ]
Krug LA, Leão C, Amaral S. Dinâmica espaço-temporal de manguezais no Complexo Estuarino de Paranaguá e relação entre decréscimo de áreas de manguezal e dados sócio-econômicos da região urbana do município de Paranaguá - Paraná. Florianópolis, Brasil: Anais do XIII Simpósio Brasileiro de Sensoriamento Remoto, INPE; 2007. p. 2753-2760. [ Links ]
Lana PC. Manguezais: diagnóstico, conflitos e prognósticos. In: Lima RE, Negrelle RRB, editors. Meio Ambiente e Desenvolvimento no Litoral do Paraná: Diagnóstico. Curitiba: Editora UFPR; 1998. p. 105-115. [ Links ]
Lacerda LD, Rezende CE, José DV, Wasserman JC, Francisco MC. Mineral concentration in leaves of mangrove trees. Biotropica. 1985;17(1):260-262. [ Links ]
Lacerda LD, Rezende CE, José DV, Francisco MC. Metallic composition of mangrove leaves from the southeastern brazilian coast. Rev Brasil Biol. 1986;46(1):395-399. [ Links ]
Lin Yi-Ming, Liu X-W, Zhang H, Fan H-Q, Lin G-H. Nutrient conservation strategies of a mangrove species Rhizophora stylosa under nutrient limitation. Plant Soil. 2010; 326(1):469-479. Doi:10.1007/s11104-009-0026-x. [ Links ]
Lópes-Portillo J, Ezcurra E. Zonation in mangrove and salt marsh vegetation at Laguna de Mecoacán, México. Biotropica. 1989;21(2):107-144. [ Links ]
McCune B, Grace JB. Analysis of Ecological Communities. Oregon USA: MjM; 2002. p. 300. [ Links ]
McCune B, Mefford MJ. PC-ORD. Multivariate Analysis of Ecological Data. Version 6.0. Gleneden Beach, Oregon, U.S.A: MjM Software; 2011. [ Links ]
Medina E, Francisco AM. Osmolaty and d13C of leaves tissues of mangrove species from environments of contrasting rainfall and salinity. Est Coast Shelf Sci. 1977;45(2):337-344. [ Links ]
Medina E, Giarrizzo T, Menezes M, Carvalho-Lira M, Carvalho EA, Peres A, et al. Mangrove communities of the "Salgado Paraense": ecologycal heterogeneity alone the Bragança península assessed through soil and leaf análisis. Amazoniana. 2001;16(3):397-416. [ Links ]
Mengel K. Ernährung und Stoffwechsel der Pflanze. Stuttgart: Gustav Fischer Verlag; 1984. p. 431. [ Links ]
Meurer EJ. Potássio. In: Fernandes MS, editor. Nutrição Mineral de Plantas. Viçosa: Sociedade Brasileira de Ciência do Solo; 2006. p. 281-298. [ Links ]
Nielsen T, Andersen FΦ. Phosphorus dynamics during decomposition of mangrove (Rhizophora apiculata) leaves in sediments. J Exp Mar Biol. Ecol. 2003;93(1):73-88. Doi: 10.1016/S0022-0981(03)00200-4. [ Links ]
Popp M. Chemical composition of Australian mangroves II. Low molecular weight carbohydrates. Z Pflanzenphysiol. 1984;113(5):411-421. [ Links ]
Raij B van. Avaliação da fertilidade do solo. Piracicaba: Instituto da Potassa; 1983. p. 142. [ Links ]
Schaeffer-Novelli Y, Cintrón-Molero G, Adaime RR. Variability of mangrove ecosystems along the Brazilian coast. Estuaries. 1990;13(2):204-218. Doi:10.2307/1351590. [ Links ]
Schwarz AM. Spreading mangroves: a New Zealand phenomenon or a global trend?. Water & Atmosphere. 2003;11(1):8-10. [ Links ]
Soares MLG, Chaves FO, Corrêa FM, Silva Jr CMG. Diversidade estrutural de bosques de mangue e sua relação com distúrbios de origem antrópica: o caso da Baía de Guanabara (Rio de Janeiro). Anuário do Instituto de Geociências-UFRJ. 2003;(26):101-116. [ Links ]
Sociedade Brasileira de Ciência do Solo. Comissão de Química e Fertilidade do Solo. Manual de adubação e de calagem para os estados de RS e SC. Porto Alegre: SBCS; 2004. p. 394. [ Links ]
Valiela I, Bowen JL, York OK. Mangrove forests: one of the world's threatened major tropical environments. BioSci. 2001;51(10):807-815. Doi: http://dx.doi.org/10.1641/0006-3568(2001)051[0807:MFOOTW]2.0.CO;2 [ Links ]
Vidal-Torrado P, Otero XL, Ferreira T, Souza Jr V, Bícego M, García-González MT, Macías F. Solos de mangue: características, gênese e impactos Antrópicos. Edafología. 2005;12:199-244. [ Links ]
Vitousek PM, Sanford Jr RL. Nutrient cycling in moist tropical forest. Ann Rev Ecol Syst. 1986;17(1):137-67. Doi:10.1146/annurev.es.17.110186.001033. [ Links ]
Waisel Y. Biology of halophytes. New York: Academic Press. 1972. p. 605. [ Links ]
Wilkie ML, Fortuna S. Status and trends in mangrove area extent worldwide [Internet]. Forest Resources Assessment Working Paper 63. Rome: Forest Resources Division. FAO; 2003. Available from: http://www.fao.org/docrep/007/j1533e/J1533E00.htm. [ Links ]
Zar JH. Biostatistical analysis. New Jersey: Prentice-Hall; 1999. p. 929. [ Links ]
Zöttl HW. Diagnosis of nutritional disturbance in forest stands International. In: Symposium on Forest Fertilization. Paris: FAO - IUFRO; 1973. p. 75-96. [ Links ]