Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Acta Biológica Colombiana
versão impressa ISSN 0120-548X
Acta biol.Colomb. vol.21 no.1 Bogotá jan./avr. 2016
https://doi.org/10.15446/abc.v21n1.47382
Doi: http://dx.doi.org/10.15446/abc.v21n1.47382.
EFFECT OF GENOTYPE ON THE in vitro REGENERATION OF Stevia rebaudiana VIA SOMATIC EMBRYOGENESIS
Efecto del genotipo sobre la regeneración in vitro de Stevia rebaudiana a través de embriogénesis somática
Esther Julia NARANJO1, Osman FERNANDEZ BETIN1, Aura Inés URREA TRUJILLO1, Ricardo CALLEJAS POSADA1, Lucía ATEHORTÚA GARCÉS1
1 Grupo de Biotecnología, Universidad de Antioquia. Medellín, Colombia.
For correspondence. esther.naranjo@udea.edu.co
Received: 20th November 2014, Returned for revision: 18th April 2015, Accepted: 19th May 2015.
Associate Editor: Leonardo Galindo.
Citation / Citar este artículo como: Naranjo EJ, Fernandez Betin O, Urrea Trujillo AI.Effect of genotype on the in vitro regeneration of Stevia rebaudiana via somatic embryogenesis. Acta biol. Colomb. 2016;21(1):87-98. doi: http://dx.doi.org/10.15446/abc.v21n1.47382.
ABSTRACT
Stevia rebaudiana (Asteraceae) is a plant of economic importance because of its medicinal properties and the presence of sweetener compounds on its leaves. These compounds can be a substitute for sucrose in a wide variety of products used by persons with diabetes and obesity problems. To standardize an efficient and effective propagation method for the different Stevia genotypes grown in Colombia, this study evaluated the effect of different combinations of the plant growth regulators 2,4-dichlorophenoxyacetic acid (2,4-D), indole-3-acetic acid (IAA), indole-3-butyric acid (IBA), 6-(gamma, gamma-dimethylallylamino) purine (2iP) and Zeatin on the induction and development of somatic embryos. Adenine and coconut water were also evaluated as supplements in the basal culture medium Murashige and Skoog Basal Salt Mixture (MS) with glutamine. The combination of 2,4-D (18.09 µM) and 2iP (7.38 µM) produced the highest number of somatic embryos per explant, which had well-defined characteristics. The genotype showed a significant effect on the embryogenic response. In the "SRQ-93" genotype, the formation and development of somatic embryos was achieved, whereas the genotypes "Bertoni" and "Morita II" only yielded embryogenic and non-embryogenic calli, respectively. The conversion to seedlings was achieved on the regeneration medium containing gibberellic acid (GA3) (0.29 µM) and activated charcoal.
Keywords: In vitro propagation, natural sweetener, somatic embryo, 2,4-dichlorophenoxyacetic acid (2,4-D), 2-isopentenyl adenine (2iP).
RESUMEN
Stevia rebaudiana (Asteraceae), es una planta de gran importancia económica debido a sus propiedades medicinales y a la presencia de compuestos endulzantes en sus hojas, los cuales pueden sustituir la sacarosa en gran variedad de productos utilizados por personas con problemas de diabetes y obesidad. Con el propósito de estandarizar un método de propagación eficiente y efectivo para diferentes genotipos de Stevia cultivados en Colombia, en la presente investigación se evaluó el efecto sobre la inducción y desarrollo de embriones somáticos de diferentes combinaciones de los reguladores de crecimiento vegetal 2,4-D, IAA, IBA, 2iP y Zeatina, además de los suplementos adenina y agua de coco en el medio de cultivo basal Murashige y Skoog (1962), adicionado con glutamina. Con la combinación 2,4-D (18.09 µM) y 2iP (7.38 µM) se obtuvo el mayor número embriones somáticos por explante con características bien definidas. El genotipo tuvo un efecto significativo en la repuesta embriogénica, en el genotipo "SRQ-93" se logró la formación y el desarrollo de embriones somáticos, mientras que en los genotipos "Bertoni" y "Morita II", solo se obtuvo callo embriogénico y no embriogénico respectivamente. La conversión a plántulas se alcanzó en el medio de regeneración conteniendo GA3 (0.29 µM) y carbón activado.
Palabras claves: Ácido 2,4-diclorofenoxiacético (2,4-D), embriones somáticos, endulzante natural, propagación in vitro, 2-isopenteniladenina (2iP).
INTRODUCTION
Stevia rebaudiana Bertoni is a plant that belongs to the Asteraceae family and is native to Paraguay. On its leaves, the plant produces low-calorie sweeteners composed of diterpene glycosides that are 30 to 320 times sweeter than sucrose (Ahmed et al., 2007). The compound that is found in the greatest proportion is stevioside, followed by rebaudioside and dulcoside (Mishra et al., 2010).
In addition to its sweetening properties, this plant is also a source of carbohydrates, proteins, raw fiber, minerals, amino acids, essential oils, etc. (Abou-Arab et al., 2010), and it possesses the following therapeutic properties: anti-hyperglycemic, anti-carcinogenic (Jayaraman et al., 2008; Gupta et al., 2013), antiviral (Kedik et al., 2009), anti-inflammatory (Ibrahim et al., 2007) and antioxidant (Zeng et al., 2013; Gupta et al., 2013). These health benefits highlight this plant's economic importance.
The conventional propagation of Stevia is difficult because of the low viability of its seeds and high cost of its propagation by cuttings results in a low number of seedlings obtained (Pande and Gupta, 2013). They also present difficulties in the rooting process on small-sized cuttings, which implies the selection of cuttings with a high number of nodes and a subsequent decrease in the amount of available material (Osman et al., 2013) and productivity, thus affecting its market. Therefore, in vitro propagation becomes an alternative technique to manage these limitations.
The globalization of agriculture requires improvements in the efficiency and competitiveness of existing production systems. Among plant tissue culture techniques, the regeneration of plants via somatic embryogenesis offers an efficient solution to propagation problems for different crops because this process yields a bipolar structure with the same characteristics and functions as a zygotic embryo originated from a somatic tissue. The process of developing the root and aerial parts of the plant is simultaneous, resulting in faster multiplication, development and acclimatization processes, and it also allows for an easier escalation process through bioreactors (Solís-Ramos et al., 2012; Saram et al., 2014).
Tissue regeneration in S. rebaudiana through tissue culture via organogenesis has been reported by Kumar et al. (2008), Anbazhagan et al. (2010), Das et al. (2011) and Suarez and Quintero (2014). Additionally, Bespalhok et al. (1993), Das and Mandal (2010), Banerjee and Sarkar (2010), and Pande and Khetmalas (2012) have described the process via somatic embryogenesis.
The induction and development process of somatic embryos can be influenced by several factors, such as the basal culture medium, growth regulators, nitrogen and carbon sources, various supplements and genotype. Genotype is important in all biological processes, and the differential response to in vitro culture of different genotypes of the same species has been well documented (Solís-Ramos et al., 2012). Based on reports of commercial-size cultures in different S. rebaudiana genotypes in Colombia (Martínez et al., 2007) and with the intention of contributing to the improvement of the multiplication process of elite material, this study aimed to evaluate the embryogenic response of three S. rebaudiana genotypes: "Morita II", "Bertoni" and "SRQ-93".
MATERIALS AND METHODS
Plant material
S. rebaudiana "Bertoni" plants served as the source of explants for the development of this study, and they were provided by the Botanical Garden Joaquín Antonio Uribe, which is located in the city of Medellín. The plants (hereafter referred to as genotype 1) were maintained under semi-controlled conditions at the biological station of the University of Antioquia. For the genotype known as mutant SRQ-93 (genotype 2), in vitro plants and plants adapted to semi-controlled conditions were used, and these plants were obtained from the Plant Biotechnology Laboratory of the University of Antioquia. In vitro plants of the Morita II variety (genotype 3) were also used, and they were donated by the bio-factory of the Technological Park of Antioquia (PTA) for this research.
In vitro establishment
Because in vitro material from genotype 1 was not available, a protocol of disinfection and in vitro establishment of apices from plants maintained under greenhouse conditions was established. The apices were cut, initially washed with Quirucidal® soap (Laboratorios Quirumedicas manufacturer's, Bogotá-Colombia) and then washed with 1 % Tween-20® for 5 minutes. Under aseptic conditions, the explants were then submerged in a Benomyl® (1 g/L) and streptomycin (1.5 g/L) mix for 4 hours. Subsequently, the material was treated serially with sodium hypochlorite (NaClO) at 0.8 and 0.5 % for five minutes each and then washed with sterile distilled water.
Culture media and growth regulators
Considering the positive effect of coconut water and adenine on the embryogenic response of different species (Apurva and Thakur, 2009; Bhattacharya et al., 2010; Milojevic et al., 2012; Nuño-Ayala et al., 2012; Rathore et al., 2012), the following three culture media were used in this experiment: MB1, composed of Murashige and Skoog Basal Salt Mixture (MS) salts (Murashige and Skoog, 1962) supplemented with glutamine (342.14 µM), thiamine-HCl (2 mg/L), nicotinic acid (2 mg/L), glycine (2 mg/L), pyridoxine (0.5 mg/L) and sucrose (30 g/L); MB2, composed of MB1 medium supplemented with adenine (5 mg/L); and medium MB3, containing MB1 medium supplemented with coconut water (10 %). The culture media were solidified with Gelrite® (3.0 g/L).
The initial assays, which were based on the protocols described for Stevia by Bespalhok et al. (1993), Bespalhok and Hattori (1997), Das and Mandal (2010), Banerjee and Sarkar (2010), and Pande and Khetmalas (2012), and combinations of the growth regulators 2,4-dichlorophenoxyacetic acid (2,4-D), indole-3-acetic acid (IAA), indole-3-butyric acid (IBA), 6-(gamma, gamma-dimethylallylamino)purine (2iP), Zeatin and Thidiazuron (TDZ) showed that for genotype SRQ-93, the formation of somatic embryos was favored by the 2,4-D - 2iP combination.
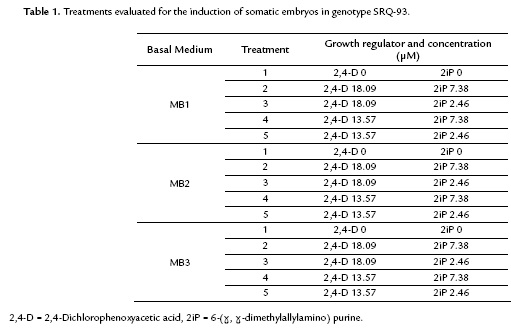
Based on these results, this study evaluated the effect of a broader range of concentrations of the growth regulators 2,4-D and 2iP on the induction of somatic embryos in the same genotype (Table 1).
Leaf segments (approximately 7 mm2) of in vitro plants in multiplication stages and after 20 days of subculture were planted in the different culture media. In all cases, adaxial side of the leaf was in contact with the culture medium.
Each of the treatments was composed of ten explants placed in separate Petri dishes (experimental unit). Every 20 days, the explants were transferred to fresh medium with the same composition. The response variable for all experiments was the number of somatic embryos formed per explant (SE). During the induction process, the cultures were maintained at 23 °C ± 2 and in continuous darkness.
Response of in vitro and ex vitro plant leaf segments to the formation of somatic embryos
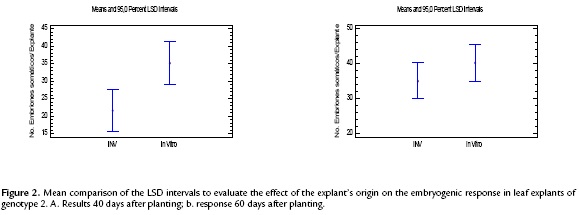
This experiment evaluated the effect of the origin of explants (in vitro or ex vitro) on the formation of somatic embryos. The explants were segments of in vitro plants and leaf segments from plants of genotypes 1 and 2 maintained under semi-controlled conditions. The culture medium used for this experiment was MB1 supplemented with the growth regulators 2,4-D (18.09 µM) and 2iP (7.38 µM).
The leaf segments of the ex o in vitro plants were treated with the same disinfection protocol described for the apices, and the cultures were maintained under complete darkness. Twenty five replicates per origin/genotype were performed. Every 20 days, the explants were transferred to fresh culture medium with the same composition, and the formation or absence of the formation of embryos and their number per explant were registered.
Effect of genotype on the induction of somatic embryos
Based on the results of previous experiment, the effect of the genotype on the formation of somatic embryos on Stevia was evaluated. Leaf segments of in vitro plants in the multiplication phase of the genotypes Bertoni, Morita II and SRQ-93 were planted in MB1 culture medium supplemented with growth regulators 2,4-D (18.09 µM) and 2iP (7.38 µM). The explants were transferred every 20 days to fresh culture medium with the same composition.
Ten replicates were performed for each genotype, and each explant was placed in a Petri dish (experimental unit). The response variable was the formation of an embryogenic callus and/or the number of somatic embryos per explant.
Histological analysis
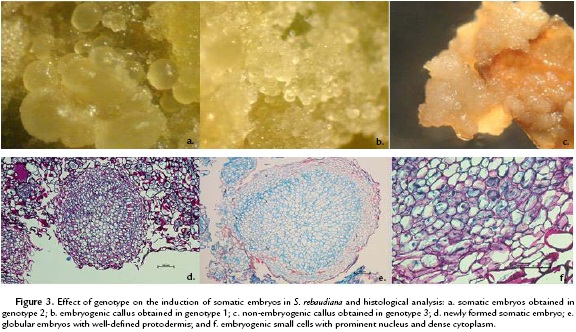
To corroborate the embryogenic nature of the obtained structures, a histological analysis was performed using portions of leaves with structures similar to somatic embryos. Such structures were submerged in formaldehyde - acetic acid - ethanol (FAA) fixing solution (10 mL of 37 % formaldehyde, 5 mL of glacial acetic acid, 50 mL of 96 % ethanol and 35 mL of distilled water) in Falcon® tubes for two days. The dehydration process was performed in an alcohol series (70 %, 80 %, 90 %, 96 %, 100 %) for two hours, and each process was followed by paraffin embedding (PARAPLAST). The embedded material was fractionated in a rotary microtome (LEICA Model RM 2125) in 5 µm-thick sections. The sections were stained with Safranin O and Alcian Blue to determine the presence of lignin, cutin, suberin and acid polysaccharides. The material was also stained using PAS (periodic acid-Schiff stain and naphthol blue black), which is commonly used to reveal the total insoluble polysaccharide content and proteins on cells. The stained sections were mounted with Entellan®, and the photographic registry was performed in a fluorescence microscope.
Regeneration of somatic embryos
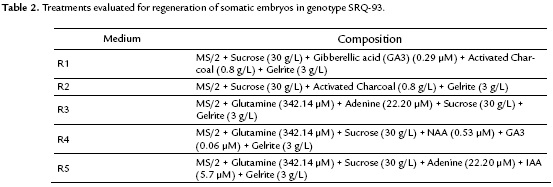
To assess the conversion of somatic embryos to complete plants, different treatments were proposed (Table 2).
The regeneration assays were performed in Petri dishes (100 x 15 mm) containing approximately 30 mL of medium. For each treatment, three clusters of embryos individually places on a Petri dish were evaluated. The cultures were maintained under continuous light conditions at 20 µmol m2 s-1 for their development. Subcultures were performed every 20 days, and the number of developed embryos per cluster and the formation or absence of root formation were registered.
Statistical analysis
The statistical analysis was performed using a one-way analysis of variance (ANOVA). The software Statgraphics Centurion was used for the randomization of the treatments, data analysis and verification of the ANOVA basic assumptions. To determine the differences between treatments (induction of somatic embryos), Tukey's test was performed. P values less than 0.05 (p < 0.05) were considered statistically significant.
RESULTS
Effect of culture medium and growth regulators on the induction of somatic embryos
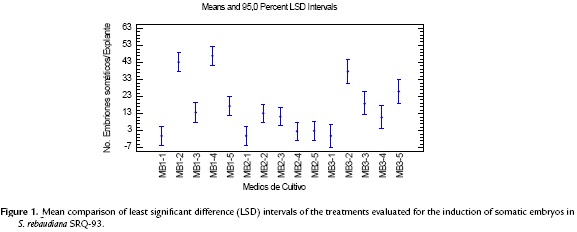
From the different supplements evaluated, the basal culture medium added with adenine (MB2) showed the lowest values of induced somatic embryos and produced an effect that was significantly different from the MB1 and MB3 culture media (Fig. 1). In the culture medium supplemented with coconut water (MB3), the mean number of somatic embryos did not show a statistically significant difference from the MB1 medium for the different growth regulator concentrations. However, the mean value was always lower than that of the non-supplemented treatments.
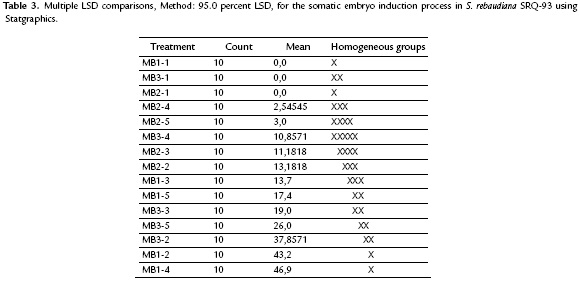
The induction of somatic embryos was achieved in all of the treatments containing growth regulators, and embryo formation started 23 days after planting. The statistical analysis showed significant differences between treatments (p < 0.00001) (Fig. 1). The treatments with a better response in the induction stage were MB1-2 containing 2,4-D (18.09 µM) plus 2iP (7.38 µM) and MB1-4 containing 2,4-D (13.57 µM) plus 2iP (7.38 µM). These treatments (Table 3) had a mean of 43.2 and 46.9 somatic embryos per explant, respectively, and morphological characteristics typical of the formation of somatic embryos. The highest mean number of embryos was achieved in the treatments containing the highest concentration of 2iP (7.38 µM) combined with 2,4-D (13.57 y 18.09 µM) (Fig. 1).
The induction of somatic embryos was not achieved in the preliminary tests that evaluated the growth regulators TDZ, IBA and IAA alone and in combination. Only callus formation was observed in media containing TDZ, whereas the formation of roots was observed in media containing IBA and/or IAA. The media containing Zeatin showed the formation of somatic embryos, but they were deformed and had irregular morphological characteristics.
Response of leaf segments from in vitro and ex vitro plants to the formation of somatic embryos
This assay revealed that the origin of the explant had an effect on the response time but not on the total number of somatic embryos. The leaf segments from ex vitro plants began forming somatic embryos between 25 and 27 days after planting, whereas the segments from in vitro plants responded after 20 days. The statistical analysis performed with the data from 40 days after planting showed significant differences in the number of embryos obtained in the leaf explants from these two origins (Fig. 2a). However, when this same parameter was evaluated at the 60th day, no statistically significant differences were observed (Fig. 2b). No statistical analysis was performed for genotype 1 because the only response was the formation of embryogenic calli. However, similar to genotype 2, a lower response time was observed in the material from in vitro conditions compared with the ex vitro plants under semi-control conditions at 30 and 45 days, respectively.
Effect of the genotype on the induction of somatic embryos
The response to embryogenesis in all three S. rebaudiana genotypes tested showed clear differences. Genotype 2 (SRQ-93) achieved a mean of 30 embryos per explant. In genotype 1 (Bertoni), the response was the formation of an embryonic callus, and only one explant achieved formation of a somatic embryo. For genotype 3 (Morita II), the response was the formation of a compact dark yellow-colored callus with no embryogenic characteristics (Fig. 3a-3c).
Histological analysis
The histological sections were used to identify the globular structures independent from the mother tissue with defined protodermis and small cells with a prominent nucleus and dense cytoplasm. All of these characteristics are typical of meristematic cells with regeneration potential; in this case, the potential is through somatic embryogenesis (Fig. 3a-3c). Similarly, the tissues surrounding the embryos were observed to correspond to the mother tissue and were characterized by parenchymal cells (Fig. 3a).
Regeneration of somatic embryos
The conversion of the somatic embryo to a plant was only obtained in the R1 media and achieved 60-80 days after transfer to the regeneration media (Fig. 4), with a mean of 5.6 seedlings per cluster. In the R2 media, the embryos remained intact after 100 days with no regeneration. However, when the embryos were transferred to the R1 medium, seedlings were obtained after 20 days. In R3, R4 and R5 media, the embryos were covered by calli after 20 to 25 days after transfer without achieving any conversion.
DISCUSSION
Effect of the culture medium and concentration of growth regulators 2,4-D and 2ip on the induction of somatic embryos
The organic supplements that were tested to induce and/or improve the embryogenic response in S. rebaudiana did not show the expected results.
Coconut water, a supplement for in vitro culture, has been reported as beneficial for induction processes such as morphogenesis, somatic embryogenesis, callus formation, and for cell cultures in suspension (Pervin et al., 2013). In embryogenic processes, the production of somatic embryos at a large scale was improved in Phoenix dactylifera (Hussam and Hussein, 2013). In addition, the promotion of the induction, growth and development of somatic embryos in other species was achieved (Bhattacharya et al., 2010); in others, the formation of somatic embryos was reduced (Apurva and Thakur, 2009).
Additionally, the adenine negatively affected the embryogenesis process in S. rebaudiana. These results are consistent with reports by Nuño-Ayala et al. (2012), who also found that the induction of somatic embryos was inhibited in Jarilla heterophylla. In other species, reports have noted the favorable effects of adenine on the efficiency of the induction and further development of somatic embryos (Jha et al., 2007; Wongtiem et al., 2011). This vitamin has been used as a supplement in culture media for the induction of somatic embryos (Milojevic et al., 2012; Rathore et al., 2012).
Bespalhok et al. (1993), Banerjee and Sarkar (2010) and Pande and Khetmalas (2012) reported achieved the formation of somatic embryos, although with low regeneration rates. In this study, certain treatments on genotype SRQ-93 showed the formation of somatic embryos, although in low amounts, and in other, embryos were not induced. Hence, new tests with a combination of growth regulators were performed.
In these experiments, a favorable response was achieved in the embryogenic process, the culture media that showed the highest number of somatic embryos per explant were those that contained 2,4-D (13.57 µM and 18.09 µM) combined with 2iP at the highest concentration (7.38 µM) (MB1-2 and MB1-4). The results of this study are the first reports of the favorable effects of this combination of regulators on the induction of somatic embryos in Stevia.
The formation of somatic embryos with the combination 2,4-D and 2iP in culture medium has been reported in several species (Zhu et al., 1996; Husain et al., 2010; Konieczny et al., 2010).
In the present study, the lowest 2iP concentration (2.46 µM) tested did not favor the formation of somatic embryos. Similar results were obtained by Zhu et al. (1996), who evaluated different concentrations of the 2,4-D/2iP combination on rice. The response to low 2iP concentrations has been described in other species regardless of the type of auxin used (Husain et al., 2010). However, Muñoz-Concha et al. (2012) found a favorable effect on the formation and development of somatic embryos with a low 2iP concentration combined with 2,4-D. This confirms once again, the variability in the response of different species to growth regulators and their concentrations.
Many of the obtained embryos showed a callus on their surface after 70 days of culture induction medium. This callus can be caused by extended exposure to the high 2,4-D concentration. This growth regulator has been widely reported as effective for inducing the embryogenic process (Joshi and Kumar, 2013) and has also been known to have deleterious effects on the development of embryos when they are treated for long periods of time and/or with high concentrations (Habibi et al., 2009). However, in other species, the prolonged exposure to 2,4-D is necessary to induce the formation of somatic embryos (Zouine and Hadrami, 2007).
The direct or indirect formation of embryos is another important aspect related to the possible epigenetic changes induced by tissue dedifferentiation and re-differentiation, which are ultimately related to the concentration of growth regulators. The capacity of an organism to form somatic embryos is determined by the types of cells present in the explant. If the explant possesses cells with embryonic capacity, only a stimulus is necessary to make the cells divide and form an embryo; this process is known as direct somatic embryogenesis. When the explant is a differentiated tissue with cells that have lost their embryogenic nature, the cells can mitotically divide, and under specific conditions, they can induce an embryogenic state and generate a callus that acquires embryogenesis capacity; this process is known as indirect embryogenesis (Kryvenki et al., 2008).
In S. rebaudiana, had reported direct somatic embryogenesis and indirect somatic embryogenesis (Bespalhok et al., 1993; Banerjee and Sarkar, 2010; Das and Mandal, 2010; Pande and Khetmalas, 2012). In this study, embryos were obtained by both ways. Therefore, it is necessary to study the explants, along with their parts and developmental stages that show the potential for a direct response. Consequently these parts and developmental stages could be utilized as an ideal explant that avoids the negative effects related to the genetic instability of the material being reproduced via indirect somatic embryogenesis.
Effect of the explant origin
In the formation of somatic embryos from genotype SRQ-93 and embryogenic calli of genotype Bertoni, the shorter response time on the leaf segments from in vitro plants compared with the longer response time for the explants from the greenhouse was the differentiating variable between both explant sources. The number of embryos per explant at 60 days of culture did not show any significant differences.
The in vitro plants shows differentiating characteristics compared with the plants maintained in the greenhouse, and these characteristics, such as thinner leaves and cuticles, underdeveloped palisade parenchyma, poorly formed epicuticular and cuticle waxes, different chemical compositions (less hydrophobic) and poorly defined stomata with reduced control (Kumar and Rao, 2012), can favor quicker responses.
The quickened response can influence the plant's responses to different processes in tissue culture, including the induction of somatic embryogenesis. However, as in most biological processes and because of the network of metabolic pathways, the responses cannot be predicted, especially when there are different genotypes.
Banerjee and Sarkar (2010) obtained a high number of Stevia embryos when they used leaves from plants recently established in vitro. However, Bespalhok et al. (1993) used leaves from greenhouse plants as explants and obtained a lower number of somatic embryos. In this study, both sources of explants were simultaneously tested and showed differences in response time but not in the final number of embryos per explant. Similarly, Beck et al., (1998) observed a differential response in the nodal explants of Acacia mearnsii from in vitro and greenhouse conditions. Contrary, Punyarani and Sharma (2012) reported not obtain differences in the response of nodal segments from greenhouse and in vitro conditions in the species Costus pictus D. Don.
Effect of the genotype on the induction of somatic embryos
Marked differences in the embryogenic response to the induction medium were found among the three S. rebaudiana genotypes, which were consistent with the genetic differences established by Martínez et al. (2007). This result suggests once again that genetic factors are important in the response to an in vitro culture. On the other hand, Jarma et al. (2005), Jarma et al. (2006), Jarma (2010) and Jarma et al. (2010) have reported variations in field at physiological response and productivity of different genotypes of Stevia rebaudiana grown in the Colombian Caribbean.
The effect of genetic constitution on the different development processes and culture of tissues is widely known. Similar to our results, the differential response to somatic embryogenesis caused by genotype has been described by several researchers in different species, including Phoenix dactylifera (Zouine and Hadrami, 2007), Theobroma cacao (Urrea et al., 2011), Cajanus cajan (Aboshama, 2011) and Elaeis guineensis (Carvalho et al., 2012).
The absence of embryos in the Bertoni and Morita II varieties under the evaluated parameters allows us to affirm that for these two genotypes, it is necessary to optimize the culture medium, growth regulators and/or culture conditions to perform propagation via somatic embryogenesis. This state of the art propagation method is recommended because of its escalation and automation potential, especially on species such as Stevia that have worldwide commercial value.
The results obtained in the present research allow us to conclude that the genotype has a significant effect on the response to somatic embryogenesis in Stevia. This result has not been previously studied, and it is of the upmost importance because the culture of this species on a commercial scale is performed in countries such as Paraguay, where it was reported to be greatest, followed by Brazil, Uruguay, Central America, Israel, Thailand, Australia, Japan, Korea and China. In addition, some cultures are performed in European countries, such as Italy, the United Kingdom, Ukraine, Spain, Germany and Switzerland (Ahmed et al., 2011; Sic Zlabur et al., 2013).
In Colombia, the genotypes commercially cultivated correspond to Bertoni and Morita II; therefore, it is necessary to continue this work to optimize the process of massive propagation of these genotypes.
Histological analysis
The histological technique allowed verifying that the formed structures correspond to somatic embryos. This study identified globular structures independent from the mother tissue and abundant meristematic cells with high starch content. These characteristics are typical of a somatic embryo as described by Avilés-Viñas et al. (2013).
According to the forming origin, the anatomic and morphological observations performed in different studies suggest that somatic embryos can be formed from a single cell or from a group of cells. When the somatic embryos have a unicellular origin, coordinated cellular divisions are observed, and the embryos are connected to the maternal tissue by the suspensor. In contrast, a multicellular origin is characterized by non-coordinated cellular divisions and somatic embryos that are observed as a protuberance fused to the maternal tissue (Avilés-Viñas et al. 2013). More in-depth research is required to precisely understand the unicellular or multicellular origin of the embryos formed in Stevia from leaf segments.
Conversion to complete plants
One of the critical points in somatic embryogenesis is the capacity of the embryos to convert to complete plants. In this study, the embryos from the genotype SRQ-93 that generated seedlings belonged to the induction media containing combinations of regulators 2,4-D (18.09 and 13.57 µM) and 2iP (7.38 µM). The regeneration was achieved only in the R1 medium supplemented with GA3 (0.29 µM) as the only regulator.
In general, to achieve the conversion to seedlings, the formed embryos are transferred to medium without regulators to eliminate the exogenous regulators and allow for their development. However, the stage of maturity of the embryos (previous stage to regeneration) is not always achieved in the same medium from which they are induced. To achieve such a state, the addition of adenine (Wongtiem et al., 2011), glutamine (Thiruvengadam et al., 2013) and coconut water (Pervin et al., 2013) has been described as a requirement. In addition, different concentrations and ratios of growth regulators are required from those used in the induction media (López-Pérez et al., 2006; Urrea et al., 2011).
This study did not find an effect of the supplements adenine and glutamine on the regeneration potential of the embryos. However, Das and Mandal (2010) and Thrivengadam et al. (2013) reported that the addition of exogenous glutamine improve the physiological development of the somatic embryos.
In the present research work, the effect of the regulators 2,4-D (18.09 and 13.57 µM) and 2iP (7.38 µM), which induced the formation of embryos, in the conversion stage, was evident. This result suggests that the auxin/cytokinin balance during the formation of embryos allowed them to reach the maturity stage required for subsequent development.
The positive effect of GA3 as the only regulator on the regeneration of seedlings observed in this study was evident in the R2 media, where in the absence of GA3, non-regenerating embryos were observed. The same results were observed when GA3 was combined with IAA.
For this same species, Bespalhok et al. (1993) found that transferring the embryos to a regulator-free medium did not result in a regenerating process, which is consistent with our results. However, Banerjee and Sarkar (2010) described a conversion to seedlings through the transference of the embryogenic callus to medium supplemented with IBA (7.38 µM).
The addition of GA3 to the regeneration media, significantly improved the number of seedlings regenerated, the regeneration process and elongation and was necessary for the improved development and subsequent regeneration of seedlings from somatic embryos in other species (López-Pérez et al., 2006; Afroz et al., 2009; Scherer et al., 2013).
CONCLUSIONS
Stevia rebaudiana is a commercially important species because of its sweetener characteristics and medicinal uses discovered in recent years. In this study, the somatic embryogenesis for this species was evaluated, and the genotype was found to affect the response to this massive propagation strategy. This result is the first world report for S. rebaudiana that may contribute to the development of protocols for other commercial varieties. However, the in vitro material had a lower response time, and the ex vitro material showed embryogenic potential as well. This result can be the starting point for establishing a propagation strategy using ex vivo vitro material selected by the grower on the field, which requires no previous steps. The development of somatic embryogenesis protocols in commercial varieties not only helps obtain a massive propagation of elite previously selected material but also aids in processes such as the production of interesting metabolites, improvement studies, material selection and conservation.
ACKNOWLEDGEMENTS
We would like to thank the CODI-Universidad de Antioquia and the "Convocatoria de Regionalización" (Regionalization Grant) for the funding of this project, and we would also like to thank The Convocatoria Estrategia de Sostenibilidad (Sustainability Strategy Grant)-CODI-Universidad de Antioquia.
REFERENCES
Aboshama H. Somatic embryogenesis proliferation, maturation and germination in Cajanus cajan. World J Agric Sci. 2011;7(1):86-95. [ Links ]
Abou-Arab A, Abou-Arab A, Abu-Salem M. Physico-chemical assessment of natural sweeteners steviosides produced from Stevia rebaudiana Bertoni plant. Afr J Food Sci. 2010;4(5):269-281. [ Links ]
Afroz A, Chaudhry Z, Khan R, Rashid H, Khan S. Effect of GA3 on regeneration response of three tomato cultivars (Lycopersicon esculentum). Pak J Bot. 2009;41(1):143-151. [ Links ]
Ahmed B, Hossain M, Islam R, Kumar A, Mandal A. A review on natural sweetener plant - Stevia having medicinal and commercial importance. Agron J. 2011;73(1):75-92. [ Links ]
Ahmed M, Salahin M, Karim R, Razvy M, Hannan M, Sultana R, et al. An efficient method for in vitro clonal propagation of a newly introduced sweetener plant (Stevia rebaudiana Bertoni.) in Bangladesh. Am-Euras J Sci Res. 2007;2(2):121-125. [ Links ]
Anbazhagan M, Kalpana M, Rajendran R, Natarajan V, Dhanavel D. In vitro production of Stevia rebaudianaBertoni. Emir J Food Agric. 2010;22(3):216-222. [ Links ]
Apurva P, Thakur P. Somatic embryogenesis and root proliferation from internode of Anthocephalus cadamba in vitro. Asian J Exp Sci. 2009;23(1):99-102. [ Links ]
Avilés-Viñas S, Lecona-Guzmán C, Canto-Flick A, López-Erosa S, Santana-Buzzy N. Morpho-histological and ultrastructural study on direct somatic embryogenesis of Capsicum chinense Jacq. In liquid médium. Plant Biotechnol Rep. 2013;7(3):277-286. Doi:10.1007/s11816-012-0261-0. [ Links ]
Banerjee M, Sarkar P. Somatic embryogenesis in Stevia rebaudiana Bertoni using different concentration of growth hormones. Int J Plant Sci. 2010;5(1):284-289. [ Links ]
Beck S, Dunlop R, Staden J. Micropropagation of Acacia mearnsii from ex vitro material. Plant Growth Regul. 1998;26(3):143-148. Doi:10.1023/A:1006109603715. [ Links ]
Bespalhok J, Hashimoto J, Esteves L. Induction of somatic embryogenesis from leaf explants of Stevia rebaudiana. Rev Bras Fisiol Veg. 1993;5(1):51-53. [ Links ]
Bespalhok J, Hattori K. Embryogenic callus formation and histological studies from Stevia rebudiana (bert.) Bertoni floret explants. Rev Bras Fisiol Veg. 1997;9(3):185-188. [ Links ]
Bhattacharya S, Bandopadhyay T, Ghosh P. Somatic embryogenesis in Cymbopogon pendulus and evaluation of clonal fidelity of regenerants using ISSR marker. Sci Hortic. 2010;123(4):505-513. Doi: 10.1016/j.scienta.2009.10.011. [ Links ]
Carvalho R, Gomes Z, Scherwinski J. Differential responses to somatic embryogenesis of different genotypes of brazilian oil palm (Elaeis guineensis Jacq.). Plant Cell Tissue Organ Cult. 2012;111(1):59-67. Doi:10.1007/s11240-012-0170-5. [ Links ]
Das A, Gantait S, Mandal N. Micropropagation of an elite medicinal plant: Stevia rebaudiana Bert. Int J Agric Res. 2011;6(1):40-48. Doi: 10.3923/ijar.2011.40.48. [ Links ]
Das A, Mandal N. Enhanced development of embryogenic callus in Stevia rebaudiana Bert by additive and amino acids. Biotechnol. 2010;9(3):368-372. Doi: 10.3923/biotech.2010.368.372. [ Links ]
Saram F, Mujib A. Morphological anomalies in somatic embryo structure in Catharanthus roseus: Improving embryo germination by amending plant growth regulators, activated charcoal and sucrose level. Br Biotechnol J. 2014;4(1):10-20. [ Links ]
Gupta E, Purwar S, Sundaram S, Rai G. Nutritional and therapeutic values of Stevia rebaudiana: A review. J Med Plant Res. 2013;7(46):3343-3353. Doi:10.5897/JMPR2013.5276 [ Links ]
Habibi N, Suthar R, Purohit S. Role of PGRs and inhibitors in induction and control of somatic embryogenesis in Themeda quadrivalvis. Indian J Exp Biol. 2009;47(3):198-203. [ Links ]
Husain M, Anis M, Shahzad A. Somatic embryogenesis and plant regeneration in Pterocarpus marsupium Roxb. Trees. 2010;24(4):781-787. Doi:10.1007/s00468-010-0448-3. [ Links ]
Hussam S, Hussein H. The role of coconut water and casein hydrolysate in somatic embryogenesis of date palm and genetic stability detection using RAPD markers. Res Biotechnol. 2013;4(3):20-28. [ Links ]
Ibrahim N, El-Gengaihi S, Motawe H, Raid S. Phytochemical and biological investigation of Stevia rebaudianaBertoni; 1-labdane-type diterpene. Eur Food Res Technol. 2007;224(4):483-488. Doi: 10.1007/s00217-006-0400-3. [ Links ]
Jarma A. Adaptación de dos clones de Estevia (Stevia rebaudiana Bert.) a tres ambientes del Caribe Colombiano (Tesis de doctorado). Bogotá: Escuela de Posgrados, Facultad de agronomía, Universidad Nacional de Colombia; 2010. p. 75-78. [ Links ]
Jarma A. Carranza C, Clavijo J. Captación y uso de la radiación en plantas de estevia (Stevia rebaudiana Bert.) en el Caribe colombiano. Agron Colomb. 2010;28(1):37-46. [ Links ]
Jarma A, Rengifo T, Araméndiz-Tatis H. Aspectos fisiológicos de estevia (Stevia rebaudiana Bert.) en el Caribe colombiano: I. Efecto de la rediación incidente sobre el área foliar y la distribución de biomasa. Agron Colomb. 2005;23(2):207-216. [ Links ]
Jarma A, Rengifo T, Araméndiz-Tatis H. Fisiología de estevia (Stevia rebaudiana Bert.) en función de la radiación en el Caribe colombiano. II. Análisis de crecimiento. Agron Colomb. 2006;24(1):38-47. [ Links ]
Jayaraman S, Saravanan M, Illanchezian. In vitro antimicrobial and antitumor activies of Stevia rebaudiana(Asteraceae) leaf extracts. Trop J Pharm Res. 2008;7(4):1143-1149. [ Links ]
Jha T, Mukherjee P, Datta M. Somatic embryogenesis in Jatropha curcas Linn., an important biofuel plant. Plant Biotechnol Rep. 2007;1(3):135-140. Doi:10.1007/s11816-007-0027-2. [ Links ]
Joshi R, Kumar P. Regulation of somatic embryogenesis in crops: a review. Agri Reviews. 2013;34(1):1-20. [ Links ]
Kedik S, Yartsev E, Stanishevskaya I. Antiviral activity of dried extract of Stevia. Pharm Chem J. 2009;43(4):198-199. Doi:10.1007/s11094-009-0270-7. [ Links ]
Konieczny R, Pilarska M, Tuleja M, Salaj T, Ilnicki T. Somatic embryogenesis and plant regeneration i zygotic embryos of Trifolium nigrescens (Viv.). Plant Cell Tissue Organ Cult. 2010;100(2):123-130. Doi:10.1007/s11240-009-9625-8. [ Links ]
Kryvenki M, Kosky R, Guerrero D, Dominguez M, Reyes M. Obtención de callos con estructuras embriogénicas de Stevia rebaudiana Bert en medios de cultivo semisólidos. Biotecnología Vegetal. 2008;8(2):91-98. [ Links ]
Kumar J, Kumari B, Castaño E. Cyclic somatic embryogenesis and efficient plant regeneration from callus of safflower. Biol Plant. 2008;52(3):429-436. Doi:10.1007/s10535-008-0087-3. [ Links ]
Kumar K, Rao U. Morphophysiologicals problems in acclimatization of micropropagated plants in-Ex Vitro conditions- A Review. J Ornam Hortic Plants. 2012;2(4):271-283. [ Links ]
López-Pérez A, Carreño J, Dabauza M. Somatic embryo germination and plant regeneration of three grapevine cvs: Effect of IAA, GA3 and embryo morphology. Vitis. 2006;45(3):141-143. [ Links ]
Martínez D, Rojas L, Urrea A, Jiménez E, Monsalve Z. Caracterización genómica mediante AFLP de Stevia rebaudiana Bertoni cultivada en el departamento de Antioquia (Colombia). Biotecnología Vegetal. 2007;7(2):107-113. [ Links ]
Milojevic J, Tubic L, Nolic V, Mitic N, Calic-Dragosavac D, Vinterhalter B, et al. Hygromycin promotes somatic embryogenesis in spinach. Plant Cell Tissue Organ Cult. 2012;109(3):573-579. Doi:10.1007/s11240-012-0117-x. [ Links ]
Mishra P, Singh R, Kumar U, Prakash V. Stevia rebaudiana - A magical sweetener. Global J Biotechnol Biochem. 2010;5(1):62-74. [ Links ]
Muñoz-Concha D, Mayes S, Ribas G, Davey M. Somatic embryogenesis from zygotic embryos and shoot-tips of the Chilean tree Gomortega keule. Plant Cell Tissue Organ Cult. 2012;109(1):123-130. Doi:10.1007/s11240-011-0080-y. [ Links ]
Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco cultures. Physiol Plant. 1962;15(3):473-497. Doi:10.1111/j.1399-3054.1962.tb08052.x. [ Links ]
Nuño-Ayala A, Rodríguez-Garay B, Gutiérrez-Mora A. Somatic embryogenesis in Jarilla heterophylla (Caricaceae). Plant Cell Tissue Organ Cult. 2012;109(1):33-39. Doi:10.1007/s11240-011-0070-0. [ Links ]
Osman M, Syamimi N, Faruq G, Nezhadahmadi A. Factors affecting microcuttings of Stevia using a mist-chamber propagation box. Sci World J. 2013;1:1-10. Doi:10.1155/2013/940201. [ Links ]
Pande S, Gupta P. Plant tissue culture of Stevia rebaudiana (Bertoni): A review. J Pharmacognosy Phytother. 2013;5(2):26-33. Doi:10.5897/JPP13.0258. [ Links ]
Pande S, Khetmalas M. Effect of concentration of sucrose on callus induction and somatic embryogenesis of anti-diabetic plant: Stevia rebaudiana. Asian J Biochem Pharm Res. 2012;1(2):27-31. [ Links ]
Pervin R, Azam S, Tanvir M, Morshed T, Rahman S, Anam K, et al. Natural growth substances has effective role in callus culture of Banana (Musa spp.) cultivar 'Anupam' (AAB Genome, Sapientum Subgroup). Am-Eurasian J Sustain Agric. 2013;7(3):149-154. [ Links ]
Punyarani K, Sharma G. Micropropagation and microrhizome induction in costus pictus Don using in vitro and ex vitro nodal segments as explant. Not Sci Biol. 2012;4(2):72-78. [ Links ]
Rathore J, Rai M, Shekhawat N. Induction of somatic embryogenesis in gum Arabic tree (Acacia Senegal (L) Willd.). Physiol Mol Biol Plants. 2012;18(4):387-392. Doi:10.1007/s12298-012-0130-x. [ Links ]
Scherer R, Correa A, de Freitas H, Dal L, Steinmacher D, Guerra M. Nodule cluster cultures and temporary immersion bioreactors as a high performance micropropagation strategy in pineapple (Ananas comosus var. comosus). Sci Hortic. 2013;151(28):38-45. Doi:10.1016/j.scienta.2012.11.027. [ Links ]
Sic Zlabur J, Voca S, Dobricevic N, Jezek D, Bosiljkov T, Brincic M. Stevia rebaudiana Bertoni- A review of nutritional and biochemical propierties of natural sweetener. Agric Conspec Sci. 2013;78(1):25-30. [ Links ]
Solís-Ramos L, Andrade-Torres A, Sáenz Carbonell L, Oropeza Salín C, Castaño de la Serna E [Internet]. In: Sato, editor. Somatic embryogenesis in recalcitrant plants, embryogenesis. InTech. 2012. Available from: http://www.intechopen.com/books/embryogenesis/somatic-embryogenesis-in-recalcitrant-plants. [ Links ]
Suarez I, Quintero I. Micropropagación de Stevia rebaudiana Bertoni, un endulzante natural a través de explantes con meristemos pre-existentes. Rev Colomb Biotecnol. 2014;16(1):29-33. [ Links ]
Thiruvengadam M, Jeyakumar J, Kamaraj M, Lee Y, Chung M. Plant regeneration through somatic embryogenesis from suspension cultures of gherkin (Cucumis anguria L.). Aust J Crop Sci. 2013;7(7):969-977. [ Links ]
Urrea A, Atehortúa L, Gallego A. Regeneration through somatic embryogenesis of an elite colombian Theobroma cacao L. variety. Rev Colomb Biotecnol. 2011;13(2):39-50. [ Links ]
Wongtiem P, Courtois D, Florin B, Juchaux M, Peltier D, Broun P, et al. Effects of cytokinins on secondary somatic embryogenesis of selected clone Rayong 9 of Manihot esculenta Crantz for ethanol production. Afr J Biotechnol. 2011;10(9):1600-1608. [ Links ]
Zeng J, Cai W, Yang W, Wu W. Antioxidant abilities, phenolics and flavonoids contents in the ethanolic extracts of the stems and leaves of different Stevia rebaudiana Bert lines. Sugar Tech. 2013;15(2):209-213. Doi:10.1007/s12355-013-0210-4. [ Links ]
Zhu Y, Ouyang W, Li Y, Chen Z. The effects of 2iP and 2,4-D on rice calli differentiation. Plant Growth Regul. 1996;19(1):19-24. Doi:10.1007/BF00024398. [ Links ]
Zouine J, Hadrami I. Effects of 2,4-D, glutamine and BAP on embryogenic suspension culture of date palm (Phoenix dactylifera L.). Sci Hortic. 2007;112(2):221-226. Doi:10.1016/j.scienta.2006.12.041. [ Links ]