Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Acta Biológica Colombiana
Print version ISSN 0120-548X
Acta biol.Colomb. vol.21 no.1 Bogotá Jan./Apr. 2016
https://doi.org/10.15446/abc.v21n1.49240
Doi: http://dx.doi.org/10.15446/abc.v21n1.49240.
RICHNESS, CELLULOLYTIC ACTIVITY, AND FUNGICIDE SUSCEPTIBILITY OF FUNGI FROM A BIRD BIOLOGICAL COLLECTION
Riqueza, actividad celulolítica y susceptibilidad a fungicidas de hongos de una colección biológica de aves
Henry ARENAS-CASTRO1, Sergio A. MUNOZ-GOMEZ1, Lorena CASTAÑO-CASTAÑO1, Melissa URIBE-ACOSTA1, Pilar Ximena LIZARAZO-MEDINA1.
1 Grupo de Investigación Ecologia Microbiana y Bioprospección - EM&B, Instituto de Biologia, Universidad de Antioquia. Calle 67 n°. 53-108, laboratorio 7- 221. Medellín, Colombia.
For correspondence. pilarximenalizarazo.udea@gmail.com
Received: 17th February 2015, Returned for revision: 27th May 2015, Accepted: 19th June 2015.
Associate Editor: Enrique Arbeláez-Cortés.
Citation / Citar este artículo como: Arenas-Castro H, Munoz-Gomez SA, Uribe-Acosta M, Castano-Castano L, Lizarazo-Medina PX. Richness, cellulolytic activity,and fungicide susceptibility of fungi from a bird biological collection. Acta biol. Colomb. 2016;21(1):167-173. doi: http://dx.doi.org/10.15446/abc.v21n1.49240.
ABSTRACT
Biological collections in natural history museums serve important purposes to the scientific community and the general public, however, their value and utility might be diminished by biodeterioration. We studied a biological collection that represents more than sixty years of avifauna sampling of Colombia, the country with the highest bird diversity. An initial inspection of the collection showed that the general appearance of some specimens was compromised by mold-like growth on their surfaces. We aimed at (i) identifying the taxonomic affiliation of these fungi, (ii) evaluating their cellulolytic activity, and (iii) probing chemical agents that could be utilized to control their growth. The most common fungi genera were Aspergillus, Penicillium, Chaetomium, and Trichophyton, most of which can degrade cellulose. Zinc chloride and salicylic acid showed to be effective fungicides. Based on this, we propose some actions to control the fungi-pest in this biological collection of birds.
Keywords: Bird collection, cellulolytic activity, fungi, fungicides, museum.
RESUMEN
Las colecciones biológicas en los museos de historia natural juegan un papel importante tanto para la comunidad cientifica como para el público en general. Sin embargo, su valor y utilidad pueden verse afectados por la biodeterioración de sus ejemplares. Se estudio una colección biológica de aves que representa más de sesenta anos de esfuerzo de muestreo de la avifauna del pais más rico en aves. Una inspección inicial mostró que la apariencia general de algunos de los especimenes de la colección se encontraba afectada por hongos. Los objetivos de este estudio fueron (i) identificar la afiliación taxonómica de los hongos, (ii) determinar la actividad celulolítica y (iii) probar agentes químicos que puedan ser utilizados para controlar su desarrollo. Los géneros de hongos más comunes fueron Aspergillus, Penicillium, Chaetomium y Trichophyton, de los cuales la mayoria presentan la capacidad de degradar celulosa. Adicionalmente, el cloruro de zinc y el ácido salicilico actuaron como fungicidas efectivos. De acuerdo con en estos resultados proponemos algunas acciones para controlar la contaminación por hongos en la colección de aves.
Palabras claves: Actividad celulolitica, colección de aves, fungicidas, hongos, museo.
INTRODUCTION
Biological collections store the past and present ofworldwide biodiversity. They are unique examples of the evolutionary lineages extracted from the natural environment. Every specimen represents the long-term efforts of researchers as well as an investment of public funds. Their value as a whole increases when it is realized that contrary to art collections, where a unique piece is the focus, biological collections encompass many specimens of the same kind in order to reflect the natural variation present within populations and species (Simmons and Munoz, 2005; Lewis, 2008). These collections play important roles in educational purposes and biodiversity research (Lee and Balick, 2007; Delgadillo and Góngora, 2009). They can guide us in studying evolution, biological invasions, environmental and climate change, pathogens, vectors of disease, and conservation decision-making (Ponder etal., 2001). Many publications in ecology and evolutionary biology provide evidence for this, as they heavily rely on work previously done on biological collections. Therefore, conserving biological collections saves both institutions and taxpayers hundreds of millions of dollars per year (Páez, 2004; Suárez and Tsutsui, 2004).
Currently, budgetary shortfalls are not the sole threat to biological collections. Biodeterioration caused by bacteria, fungi, arthropods, and rodents threats cultural and natural heritage worldwide (Simmons and Munoz, 2005; Sterflinger, 2010). Fungi are especially problematic because they can degrade a wide array of biological compounds. Their preferences for keratin- and cellulose-based material, in addition to their great dispersal capability via spores and resistance to adverse environmental conditions, make them a pest hard to deal with (Saddler, 1985; Btyskal, 2009). Fungi cannot only corrode, color and digest organic materials, they can also cause allergies and respiratory problems in museum staff (Merritt, 2007). Their control demands active removal and reduction of growth rate and spore load in storerooms. Diverse chemical agents are commonly used to achieve this, but non-toxic alternatives are constantly pursued to avoid damage to biological specimens as much as possible (Knell, 1994).
Bird beaks, claws, scales, and feathers are characterized by the presence of keratin proteins (Frenkel and Gillespie, 1976; Sawyer et al., 2000). Wood supports, which bird specimens generally lay on, are a rich source of cellulose. Thus, museum material derived from animals is particularly vulnerable to biological attack due to its high carbohydrate and protein composition (Keopannha, 2008). Some surveys in biological collections and poultry birds have identified Aspergillus, Penicillium, Cladosporium, Trichophyton, Chrysosporium, and Rhizopus as the most common fungi genera present in their surfaces (Grunder et al., 2005; Shabbir et al., 2007). Among them, Penicillium, Aspergillus, and Rhizopus contain species with reported cellulolytic activity (Jahangeer et al., 2005; Devi and Kumar, 2012; Khokhar et al., 2012; Damaso, 2012).
We aimed at identifying the taxonomic affiliation of fungi isolated from bird specimens from the biological collection at Museo Universitario de la Universidad de Antioquia (MUUA). This collection represents 60 years of avifauna samples from Colombia, the country with the highest avian diversity in the world (Donegan et al., 2014). Having first diagnosed the fungi responsible for biodeterioration, we then evaluated their susceptibility to two previously reported low-toxicity chemical agents. We also evaluated the cellulolytic activity of these fungi. Interpretation of the results allowed us to propose actions to control the bird collection fungi-pests.
MATERIALS AND METHODS
Sampling
The storeroom where the MUUA bird collection is found also harbors other vertebrate collections. This space is interconnected with other major rooms and lacked an adequate ventilation system, its temperature ranged between 23.0-26.4 °C and humidity between 60.6-74.4 %. In total, 423 specimens were visually inspected for damages to their wood supports, as well as for any injury in their legs, feathers, and beaks. Samples were taken from specimens determined to have lesions that affected their general appearance and overall integrity; a specimen might have had more than one lesion. An injury was qualitatively defined as any visual sign of fungal or mold-like growth on the surface of the specimen that compromised its general appearance. These injuries stood out as major irregularities of the general pattern of the wood base, bird legs, feathers, or beak textures. Two methods were used for sampling depending on the type and location of the lesion: rubbing the affected region with sterile cotton swabs, or scraping harder surfaces with a sterile razor. Once samples were removed from the affected region of the specimen, they were then stored in sterile test tubes or Petri dishes for their subsequent transportation to the laboratory.
Identification
Taxonomic characterization of isolated fungi was carried out based on macroscopic and microscopic morphological features, using well established taxonomic keys (Ellis, 1971; Rebell and Taplin, 1974; Samson et al., 1984; Domsch and Gams, 1993; Barnett and Hunter, 1998; Gams et al., 1998; Ulloa and Hanlin, 2000; Klich, 2002). To observe the macroscopic traits, we inoculated fungi on Czapek solid media. For the microscopic traits, individual slides for each isolated fungus were prepared using lactophenol blue and subsequently observed through the optical microscope.
Cellulolytic activity assessment
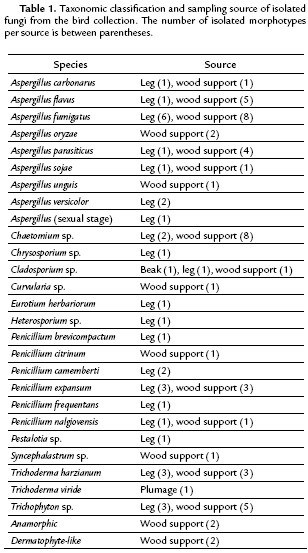
We screened a sample of 20 morphotypes looking for cellulolytic activity; they were selected based on the previously reported cellulolytic activity and prevalence of their genera among the total isolates (Peciulyté, 2007). A mycelial plug from five days old potato dextrose agar culture was inoculated on the center of a Petri dish containing carboxymethylcellulose (CMC) agar (2.0 g/L NaNO3, 1.0 g/L K2HPO4, 0.5 g/L MgSO4, 0.5 g/L KCl, 2.0 g/L carboxymethylcellulose sodium salt, 0.2 g/L peptone, and 17.0 g/L agar) (Kasana et al., 2008). Then, the CMC dishes were flooded with Gram's iodine (0.7 g/L KI and 0.3 g/L iodine) to reveal hydrolysis either after six days of inoculation or as soon as the colony reached 50 mm in diameter. Hydrolysis was evidenced by a zone of clearance around the colony. Fungi that were positive for hydrolytic activity were further selected to quantify their cellulolytic activity. As described above, three replicates of each selected morphotype were inoculated in different CMC agar dishes and revealed with Gram's iodine. The width of the hydrolysis area was measured five times per replicate per isolate. Fungal growth from the center of the plates was recorded daily to calculate the growth rate in Sabouraud and CMC agar. We used a strain of Aspergillus niger, whose cellulolytic activity was previously verified, as positive control.
Evaluation of fungicides
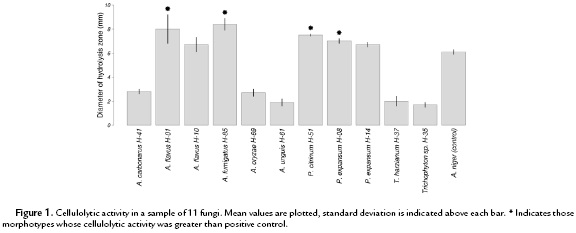
Two previously reported low-toxicity fungicides, zinc chloride (ZnCl) and salicylic acid, were assessed for their effect on the isolated fungi from the bird specimens (Babich and Stotzky, 1978; Hadi and Balali, 2010). Twenty fungal isolates, covering the total taxonomic diversity, were subjected to fungicide evaluation. In order to test the susceptibility of the samples to those chemical compounds, a small disc of 8 mm in diameter extracted from a Sabouraud agar culture of each selected isolate was inoculated on Petri dishes containing Sabouraud agar medium with either 10 mM ZnCl or 4 mM salicylic acid. The fungicides were added to the medium after autoclaving it and just before pouring it into Petri dishes. As negative and positive controls, the isolated fungi were also inoculated in plain and Benomyl-containing (3 g/L) Sabouraud agar medium, respectively. Each treatment consisted of five replicates per isolate. Fungal growth was recorded daily for nine days and growth rate was measured as mm/day. The percentage of growth rate reduction produced by fungicides was calculated as the difference between the growth rate in plain Sabouraud agar medium and fungicide-containing medium divided by the growth rate in plain Sabouraud agar medium and then multiplied by 100.
RESULTS
Fungal richness
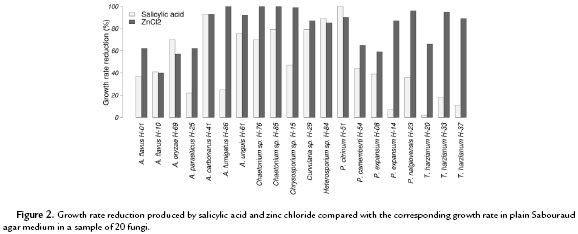
We identified 187 damages in 161 out of 423 bird specimens, of which 26 had more than one injury. Most of them occurred in wood supports (53.5 %) and legs (40.6 %), and to a lesser extent in plumage (4.8 %) and beaks (1.1 %).
Injuries were not diverse in aspect. They were primarily pale, gray shaded, flat, scabby, or downy. Their diameters ranged between 3-50 mm. At least 25 fungal species were represented by 86 isolated morphotypes. Of these, 64 % were identified at species level and 30.2 % at genus level only (Table 1). Isolated morphotypes came mainly from wood supports (58.1 %) and legs (39.5 %). Seven specimens had more than one fungal species. The most common genera were Aspergillus (45 %), Penicillium (15.3 %), Chaetomium (10.6 %), Trichophyton (9.4 %), and Trichoderma (7.1 %). The most common species were Aspergillus fumigatus (16.5 %), Aspergillus flavus (7.1 %), and Penicillium expansum (7.1 %). Two anamorphic fungi did not sporulate and two dermatophyte-like fungi could not be identified.
Cellulolytic activity
We detected cellulolytic activity in 11 out of the 20 tested morphotypes. Four of them exceeded A. niger (control) cellulolytic activity: A. flavus (H-01), A. fumigatus (H-85), Penicillium citrinum (H-51), and P. expansum (H-08) (all p<0.05, Student's t-test). Aspergillus flavus (H-10) and P. expansum (H-14) activity was nearly the same as that of A. niger (p = 0.41 and 0.12, respectively) (Fig. 1). All morphotypes exceeded A. niger growth rate in CMC agar. Although growth rate in CMC agar was on average 34.7 % lower than in Sabouraud agar medium, P. expansum (H-08) grew 9.8 % faster in CMC agar than in Sabouraud agar.
Fungicide effect
Zinc chloride was the most effective fungicide, it diminished fungal growth rate in all tested morphotypes (all p<0.05; Student's t-test). Salicilic acid diminished it in all morphotypes as well, except A. flavus (H-10; p = 0.18, Student's t-test), P. expansum (H-14; p = 0.53, Student's t-test), P. nalgiovensis (H-23; p = 0.09, Student's t-test), and T. harzianum (H-20; p = 0.13, Student's t-test). Zinc chloride and salicilic acid reduced the growth rate in 81.2 % and 49.2 % on average, respectively. The salicylic acid effect was greater than the ZnCl only in P. citrinum (H-51), Aspergillus oryzae (H-69), and Heterosporium sp. (H-84) (Fig. 2). Benomyl (positive control) reduced fungal growth rate by 95 % on average for all morphotypes except Curvularia sp. (H-29; 68 %).
DISCUSSION
Reports of fungi affecting birds are common, but according to our review, no survey has been conducted on bird collections.
We identified some genera, such as Aspergillus, Chaetomium, Chrysosporium, Cladosporium, Curvularia, Penicillium, Trichoderma, and Thichophyton, that have been previously isolated from free-living and poultry bird feathers (Pugh, 1972; Kaul and Sumbali, 1999; Deshmukh, 2004; Grunder et al., 2005). Those genera represent 91 % of isolated morphotypes. Most of them have also been associated with air contamination of museum's indoor environments (Arya et al., 2001; Shabbir et al., 2007; da Costa et al., 2011). This suggests that the fungi affecting the MUUA bird collection could come from both airborne spores and specimens themselves.
A sample of 11 fungi, representing 77 % of isolated genus diversity, exhibited cellulolytic activity. Nearly half of them exceeded or matched the cellulolytic activity of a control cellulolytic strain. These results suggest that the persistence ofthe MUUA bird collection's fungal contamination is mainly being driven by fungi that are able to degrade cellulose. Those environmental fungi found a rich cellulose substrate in the wood supports on which most of the avian specimens lay and from which more than a half of the injuries come from. The capability to degrade cellulose is not restricted to fungi, as some bacteria and insects produce cellulases too (Watanabe and Tokuda, 2001). However, fungi are especially ubiquitous due to the ability of their spores to facilitate the permanent colonization of new substrates. This scenario is favored by specimens being stored in close proximity to each other within interconnected cabinets. Although not tested here, keratin preference of some isolated fungi can also be responsible at some extent for fungal contamination and persistence. Indeed, some genera reported here, such as Aspergillus, Chaetomium, Cladosporium, Chrysosporium, Curvularia, Penicillium, Pestalotia, Syncephalastrum, Trichoderma, and Trichophyton, contain keratinolytic species (Btyskal, 2009). Keratin rich bird body parts like claws, beaks, feathers, and scales are ideal substrates for keratinolytic fungi (KorniWowicz-Kowalska and Bohacz, 2011).
Regardless of whether the cellulolytic or keratinolytic activity favor fungal contamination, an integrated pest management control program should focus on air quality monitoring in order to identify the optimal temperature, relative humidity, and other relevant environmental variables that might lead to a reduction in air-spore load (Abe and Murata, 2014). A temperature below 20 °C and humidity below 65 % is recommended to prevent the growth of fungi (Simmons and Munoz, 2005). This necessity is strengthened when it is considered that all fungal spores may be allergenic, especially those belonging to Aspergillus and Penicillium genera, which sum up 60.5 % of identified fungi (Kurup et al., 2002). If actions are not taken, an increase in biodeterioration of biological collections can be a hazard to the health of museum staff and visitors. This biological contamination in buildings can trigger temporal mucosal, skin, and general problems in workers, as is predicted by the sick building syndrome (Li and Yang, 2004).
A pest-control program should contemplate appropriate storage facilities for preventing contamination, as well as constant surveillance of specimens for accidental moldlike growth (Knell, 1994). Furthermore, lesions caused by occasional contaminations can be treated with fungicides that kill or retard fungal growth. In this study, we have shown the effectiveness of two low-toxicity chemical agents that significantly slow down the growth rate of fungal genera isolated from bird specimens. Incipient lesions can be controlled by spraying 10 mM ZnCl, which acts by interacting with sulfhydryl groups of proteins and altering their functions in the fungus (Xu and Imlay, 2012). Alternatively, salicylic acid disrupts ion transport within fungal cells (Amborabé et al., 2002). They have been effective controlling other fungi genera applied in solid and liquid media (Amborabé et al., 2002; Malachová et al., 2011). These two different fungicides emerge as promising candidates that can be implemented in bird biological collections, thus reducing risks to the health ofthe museum's staff. Although ZnCl was more effective that salicylic acid, the latter is nearly 40 % cheaper, being a cost-effective alternative.
CONCLUSIONS
Our study is the first survey done on a bird biological collection aimed to both identify fungi causing deterioration and characterize the properties that could be responsible for their colonization and persistence. We identified at least 25 fungi species, most of which were able to degrade cellulose. Target low-toxicity chemical fungicides reduced fungal growth rate to different extents. Further research should address the ecological aspects of the contamination, such as the provenance of fungi (environmentally acquired post-fixation or bird-indigenous pre-fixation), the relative contribution of fungi's ability to degrade cellulose, lignin, and keratin to the persistence of contamination, and the possible interactions among the surface microbiota on preserved bird specimens. The present study has opened up interesting new lines of research that will eventually contribute to the understanding of contamination dynamics in biological collections. It also offers a practical solution by proposing a set of strategies that can be implemented in a cost-effective manner in a small, local, and budget-limited university museum.
ACKNOWLEDGEMENTS
We thank to Ana Isabel Gutiérrez Gallo for her collaboration and technical support, to Isabel Carmona, Isabel Mesa, Marcelina Mendoza, and Diana L. Gómez for their laboratory assistance, and Alexis Acosta and Fernando Valencia for their assistance in sampling at the MUUA. We are grateful to Maicol Ospina-Bedoya for his crucial role in the initial conception of the research project and Tawni Voyles for her comments on the manuscript. We appreciate the efforts made by the MUUA to preserve its biological collections. We also thank to Corporación Académica para el Estudio de Patologias Tropicales (CAEPT). This project was funded by the program Incentivos a la Investigación of the Vicerrectoria de Extensión of the Universidad de Antioquia. Two anonymous reviewers made valuable comments that improved this work.
REFERENCES
Abe K, Murata T. A prevention strategy against fungal attack for the conservation of cultural assets using a fungal index. Int Biodeterior Biodegradation. 2014;88:91-96. Doi:10.1016/j.ibiod.2013.12.012. [ Links ]
Amborabé B, Fleurat-Lessard P, Chollet J, Roblin G. Antifungal effects of salicylic acid and other benzoic acid derivatives towards Eutypa lata: structure-activity relationship. Plant Physiol Bioch. 2002;40(12):1051-1060. Doi:10.1016/S0981-9428(02)01470-5. [ Links ]
Arya A, Shah AR, Sadasivan S. Indoor aeromycoflora of Baroda museum and deterioration of Egyptian mummy. Curr Sci India. 2001;81(7):793-799. [ Links ]
Babich H, Stotzky G. Toxicity of zinc to fungi, bacteria, and coliphages: influence of chloride ions. J Appl Environ Microbiol. 1978;36(6):906-914. [ Links ]
Barnett HL, Hunter BB. Illustrated genera of imperfect fungi. 4 ed. St. Paul: APS Press; 1998. p. 218. [ Links ]
Btyskal B. Fungi utilizing keratinous substrates. Int Biodeterior Biodegradation. 2009;63(6):631-653. Doi:10.1016/j.ibiod.2009.02.006. [ Links ]
da Costa ACA, da Silva LA, Hannesch O. Total microbial populations in air-conditioned spaces of a scientific museum: precautions related to biodeterioration of scientific collections. J Bioprocess Biotech. 2011;1(106):1-6. Doi:10.4172/2155-9821.1000106. [ Links ]
Damaso MCT, da Costa Terzi S, Farias AX, Pereira de Oliveira AC, Fraga ME, Couri S. Selection of cellulolytic fungi isolated from diverse substrates. Braz Arch Biol Techn. 2012;55(4):513-520. Doi:10.1590/S1516-89132012000400005. [ Links ]
Delgadillo I, Góngora F. Colecciones biológicas: estrategias didácticas en la ensenanza-aprendizaje de la biologia. Bio-grafia. 2009;2(3):148-157. [ Links ]
Deshmukh SK. Keratinophilic fungi on feathers of pigeon in Maharashtra, India. Mycoses. 2004;47(5):213-215. [ Links ]
Devi MC, Kumar MS. Isolation and screening of lignocellulose hydrolytic saprophytic fungi from dairy manure soil. Ann Biol Res. 2012;3(2):1145-1152. [ Links ]
Domsch KH, Gams W. Compendium of soil fungi. Eching: IHW-Verlag; 1993. p. 860. [ Links ]
Donegan T, Quevedo A, Verhelst JC, Cortés O, Pacheco JA, Salaman P. Revision of the status of bird species occurring or reported in Colombia 2014. Conservación Colombiana. 2014;21:3-11. [ Links ]
Ellis MB. Dematiaceous Hyphomycetes. Wallingford: CAB International; 1971. p. 595. [ Links ]
Frenkel MJ, Gillespie JM. The proteins of the keratin component of bird's beaks. Aust J Biol Sci. 1976;29(5):467-479. [ Links ]
Gams W, Hoekstra ES, Aptroot A. CBS course of mycology. Utrech: Centraalbureau voor Schimmelcultures; 1998. p. 165. [ Links ]
Grunder S, Mayser P, Redmann T, Kaleta EF. Mycological examinations on the fungal flora of the chicken comb. Mycoses. 2005;48(2):114-119. Doi:10.1111/j.1439-0507.2004.01074.x. [ Links ]
Hadi MR, Balali GR. The effect of salicylic acid on the reduction of Rhizoctonia solani damage in the tubers of marfona potato cultivar. Am Eurasian J Agric Environ Sci. 2010;7(4):492-496. [ Links ]
Jahangeer S, Khan N, Jahangeer S, Sohail M, Shahzad S, Ahmad A. Khan SA. Screening and characterization of fungal cellulases isolated from the native environmental source. Pak J Bot. 2005;37(3):739-748. [ Links ]
Kasana RC, Salwan R, Dhar H, Dutt S, Gulati A. A rapid and easy method for the detection of microbial cellulases on agar plates using Gram's iodine. Curr Microbiol. 2008;57(5):503-507. Doi: 10.1007/s00284-008-9276-8. [ Links ]
Kaul S, Sumbali G. Production of extracellular keratinases by keratinophilic fungal species inhabiting feathers of living poultry birds (Gallus domesticus): a comparison. Mycopathologia. 1999;146(1):19-24. Doi: 10.1023/A:1007086720237. [ Links ]
Keopannha V. Museum collections and biodeterioration in Laos (dissertation). Gothenburg: Gothenburg University; 2008. p. 73. [ Links ]
Khokhar I, Haider MS, Mushtaq S, Mukhtar I. Isolation and screening of highly cellulolytic filamentous fungi. J Appl Sci Environ Manag. 2012;16(3):223-226. [ Links ]
Klich M. Identification of common Aspergillus species. Utrech: Centraalbureau voor Schimmelcultures; 2002. p. 116. Doi:10.1007/s11046-005-5065-0. [ Links ]
Knell S, editor. Care of collections. London: Routledge; 1994. p. 315. [ Links ]
KorniWowicz-Kowalska T, Bohacz J. Biodegradation of keratin waste: Theory and practical aspects. Waste Manag. 2011;31(8):1689-1701. Doi:10.1016 j. wasman.2011.03.024. [ Links ]
Kurup VP, Shen HD, Vijay H. Immunobiology of fungal allergens. Int Arch Allergy Immunol. 2002;129(3):181-188. Doi:10.1159/000066780. [ Links ]
Lee RA, Balick MJ. The value of museum collections in the 21st century. Explore. 2007;3(2):145-148. Doi:10.1016/j.explore.2006.12.010. [ Links ]
Lewis R. A career at the museum. Nature. 2008;451:218-219. Doi:10.1038/nj7175-218a. [ Links ]
Li D, Yang CS. Fungal contamination as a major contributor to sick building syndrome. Adv Appl Microbiol. 2004;55:31-112. Doi:10.1016/S0065-2164(04)55002-5. [ Links ]
Malachová K, Praus P, Rybková Z, Kozák O. Antibacterial and antifungal activities of silver, copper and zinc montmorillonites. Appl Clay Sci. 2011;53(4):642-645. Doi:10.1016/j.clay.2011.05.016. [ Links ]
Merritt J. Mold: preservation of growth in museum collections. Conserve O Gram. 2007;3(4):1-5. [ Links ]
Páez VP. El valor de las colecciones biológicas. Actualidades Biológicas. 2004;26(81):97-98. [ Links ]
Peciulyté D. Isolation of cellulolytic fungi from waste paper gradual recycling materials. Ekologija. 2007;53(4):11-18. [ Links ]
Ponder WF, Carter GA, Flemons P, Chapman RR. Evaluation of museum collection data for use in biodiversity assessment. Conserv Biol. 2001;15(3):648-657. Doi:10.1046/j.1523-1739.2001.015003648.x. [ Links ]
Pugh GJ. The contamination of bird's feathers by fungi. Ibis;114(2):172-177. Doi:10.1111/j.1474-919X.1972.tb02602.x. [ Links ]
Rebell G, Taplin D. Dermatophytes: their recognition and identification. Coral Glabes: University of Miami Press; 1974. p. 49. [ Links ]
Saddler JN. Screening of highly cellulolytic fungi and the action of their cellulase enzyme systems. Enzyme Microb Tech. 1985;4(6):414-418. Doi:10.1016/0141-0229(82)90073-4. [ Links ]
Samson R, Hoekstra E, Van Oorschot C. Introduction to food-borne fungi. 2 ed. Utrech: Centraalbureau voor Schimmelcultures; 1984. p. 187. [ Links ]
Sawyer RH, Glenn T, French JO, Mays B, Shames RB, Barnes JL, et al. The expression of beta (P) keratins in the epidermal appendages of reptiles and birds. Am Zool. 2000;40(4):530-539. Doi:10.1093/icb/40.4.530. [ Links ]
Shabbir A, Khan MA, Khan AM, Iqbal M, Ahmad F. FUNGAL Biodeterioration: a case study in the Zoological Museum of the Punjab University. J Anim Plant Sci. 2007;17(3):90-92. [ Links ]
Simmons JE, Munoz-Saba Y, editors. Cuidado, manejo y conservación de las colecciones biológicas. Bogotá D.C.: Universidad Nacional de Colombia; 2005. p. 288. [ Links ]
Sterflinger K. Fungi: their role in deterioration of cultural heritage. Fungal Biol Rev. 2010;24(1):47-55. Doi:10.1016/j.fbr.2010.03.003. [ Links ]
Suárez AV, Tsutsui ND. The value of museum collections for research and society. BioScience. 2004;54(1):66-74. Doi:10.1641/00063568(2004)054[0066:TVOMCF]2.0.CO;2. [ Links ]
Ulloa M, Hanlin R. Illustrated dictionary of mycology. St. Paul: APS Press; 2000. p. 448. [ Links ]
Xu FF, Imlay JA. Silver(I), mercury(II), cadmium(II), and zinc(II) target exposed enzymic iron-sulfur clusters when they toxify Escherichia coli. Appl Environ Microbiol. 2012;78(10):3614-3621. Doi:10.1128/AEM.07368-11. [ Links ]
Watanabe H, Tokuda G. Animal cellulases. Cell Mol Life Sci, 2001;58(9):1167-1178. [ Links ]