Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Acta Biológica Colombiana
Print version ISSN 0120-548X
Acta biol.Colomb. vol.21 no.3 Bogotá Sept./Dec. 2016
https://doi.org/10.15446/abc.v21n3.53650
DOI: http://dx.doi.org/10.15446/abc.v21n3.53650
EVOLUTION OF ALLOMETRIC CHANGES IN FRUIT FLY LEGS: A DEVELOPMENTALLY ENTRENCHED STORY
La evolución de los cambios alométricos en las patas de las moscas de la fruta: una historia enraizada en la biología del desarrollo
Juan Nicolas MALAGON1; Waleed KHAN1.
1 University of Toronto, Department of Cell and Systems Biology, 25 Harbord street M5S 3G5, Toronto, Canada.
For correspondence. nicolasmalagon@gmail.com
Received: 28th October 2015, Returned for revision: 24th November 2015, Accepted: 21st January 2016.
Associate Editor: Rodolfo Jaffé Ribbi.
Citation/Citar este artículo como: Malagon JN, Khan W. Evolution of allometric changes in fruit fly legs: a developmentally entrenched story. Acta biol. Colomb. 2016;21(3):509-519. DOI: http://dx.doi.org/10.15446/abc.v21n3.53650
Este artículo fue presentado por invitación de la Red Colombiana de Biología Evolutiva (COLEVOL) y Acta Biológica Colombiana con el fin de incentivar la investigación en el área de biología evolutiva.
ABSTRACT
Allometric studies measure the scaling changes between different body parts and these often have implications on understanding ecology and evolution. Although most work on allometry has described its importance during phenotypic evolution, few studies have focused on studying how entrenched developmental processes can affect allometric changes. To explore this problem, here we used the sex comb, a male-specific group of bristles with a spectacular morphological diversity among Drosophila species. By combining morphometric analysis in wild type and genetically perturbed Drosophila melanogaster and Drosophila species, we studied the allometric changes that occur in leg length and other bristle rows in concert with sex comb radiation. We show that bristle-developmental processes are important for understanding the allometric changes of Drosophila first tarsal segments. Different lines of evidence suggest that a complicated interaction between bristle spacing and movement are crucial for understanding the evolution of allometry in this system. As a result, this work shows that although the emergence of a new trait, the sex comb, can modify the allometric relationships, there is a hierarchy of ancestral developmental processes with respect to how easily they can be modified. As a result, the interconnection of developmental processes can bias the direction of morphological changes.
Keywords: rotation, sex combs, transverse rows.
RESUMEN
La alometría estudia los cambios de tamaño entre las diferentes partes del cuerpo de los seres vivos y sus implicaciones ecológicas y evolutivas. Aunque la mayoría de los estudios en esta área se han centrado en investigar la importancia de los cambios alométricos en la evolución fenótipica, pocos estudios han analizado como la interconexión de los diferentes procesos del desarrollo afectan dichos cambios de tamaño. Para investigar la relación entre los mecanismos de desarrollo y los cambios alométricos, utilizamos los peines sexuales de diferentes especies del género Drosophila. Dichas estructuras, constituidas por un grupo de sedas ubicadas en las patas anteriores de los machos, presentan una variedad morfológica sobresaliente durante la evolución. Por medio de análisis morfométricos entre diferentes especies de Drosophila, incluidas líneas de Drosophila melanogaster modificadas genéticamente, investigamos los cambios alométricos que ocurren en el tamaño de las patas y diferentes tipos de sedas como resultado de la radiación de los peines sexuales. En este trabajo presentamos evidencia que sugiere una interacción compleja entre los mecanismos del desarrollo encargados de definir la distancia entre las sedas y su movimiento. Además, mostramos que dichos mecanismos son fundamentales para entender cómo evoluciona la alometría en los segmentos tarsales. Aunque la emergencia de una nueva característica puede modificar las relaciones alométricas, los procesos ancestrales de desarrollo varían en su susceptibilidad de ser modificados. De igual forma, este trabajo muestra que la interconexión entre los diferentes procesos de desarrollo puede sesgar la dirección de los cambios morfológicos.
Palabras clave: hileras transversales, peines sexuales, rotación.
INTRODUCTION
Allometry can be defined as the scaling relationship between different body parts and its ecological and evolutionary impact (Thompson, 1917; Huxley, 1972; Gould, 1976; Klingenberg, 1996; Klingenberg, 1998). A proper regulation of allometry is crucial for the survival of an organism and defects in different pathways regulating allometry have constantly been linked to different types of cancer including those caused by insulin-like growth factor (Pollak, 2008) and by TGF-β, transformation growth factor-Beta (Massagué, 2008). Although previous studies have shown the importance of allometric changes for adaptive evolution (Beldade and Brakefield, 2002; Frankino et al., 2005; Pélabon et al., 2014), few studies have concentrated on studying how the inextricable relationships between developmental processes can affect the evolution of allometry (Malagón et al., 2014). To explore this problem, we study the evolution of various leg bristle rows and their relationship to leg length among Drosophila species. In this study allometric changes mainly refer to changes in leg length.
The majority of the allometric studies are mainly based on morphometric changes between populations of the same species (Young et al., 2010; Gutierrez and Eberhard, 2015) or phylogenetic comparisons among various species or clades (Baker and Gerald, 2001; Langlade et al., 2005; Marroig and Cheverud, 2010). In addition to this approach, a few studies have combined these morphological measurements with various genetic manipulations to understand how allometry evolves in more detail (Brakefield, 2006; Carreira et al., 2009; Vasseur et al., 2012; Warren et al., 2014). For example, artificial selection experiments in the butterfly, Bicyclus anynana, show the potential for incredible flexibility in allometry during evolution (Beldade and Brakefield, 2002; Frankino et al., 2005). Here we use D. melanogaster legs as a system to study the evolution of allometry. This system provides the opportunity to integrate morphometric studies in D. melanogaster and several Drosophila species to multiple types of genetic perturbations including mutations, artificial selection, and producing spatial and temporal developmental perturbations by manipulating them genetically. (Ahuja and Singh, 2008; Tanaka et al., 2011; Malagón et al., 2014).
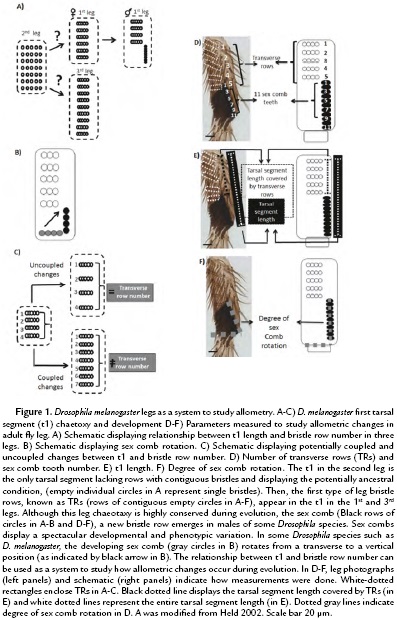
Although D. melanogaster legs have apparently similar structures, each one of the three legs displays a different length and bristle patterns (Hanna-Alava, 1958a; Hanna-Alava, 1958b). In particular, the first tarsal segment (t1) of each leg displays a spectacular diversity of bristle patterns during Drosophila evolution (Hanna-Alava, 1958a; Hanna-Alava, 1958b; Held, 2002) (Fig. 1A). While the 2nd leg seems to conserve the ancestral bristle pattern, mainly formed by separated bristles evenly spread in the t1, the 1st and 3rd legs display an evolutionary innovation, the emergence of contiguous bristles attached to each other forming rows (Held, 2002). D. melanogaster legs display two main types of bristles rows: 1) transverse rows (TRs) and 2) sex combs. TRs are formed by lightly pigmented bristles, which seem to be used as cleaning utensils for the eyes and wings (Kopp, 2011). Sex combs, on the contrary, are heavily pigmented and are a male-specific group of bristles, used in the courtship ritual of more than 150 Drosophila species (Atallah, 2008, Kopp, 2011). Sex combs display spectacular diversity (Kopp, 2011); some of those morphological changes have been suggested to play a role in modifying the t1 allometry (Atallah, 2008, Atallah et al., 2014). The variation in sex comb orientation is an example of their rapid morphological diversification (Kopp, 2011, Atallah et al., 2012).
Sex comb orientation can be grouped into three categories: transverse (0-30°), diagonal (30-60°), and vertical (60-90°). Sex combs with a transverse orientation resemble the ancestral Drosophila chaetotaxy, in which leg bristle rows are relatively parallel to the leg joints. Diagonal and vertical orientations, in contrast, represent an evolutionary novelty among Drosophila species. However, those changes in position are accompanied by developmental changes, which can promote or limit changes in leg length (Malagón et al, 2014). For, example, in some Drosophila species such as D. melanogaster, sex combs move from a transverse to a vertical orientation (Fig. 1B). This ~90° rotation is accompanied by male-specific lengthening of the tarsal segment, which increases t1 male leg (Atallah, 2009). Similarly, the increase in D. melanogaster sex comb length seems to be rapidly limited, since there is not enough space available for the rotation (Malagón et al, 2014). The present work evaluates the relationship between t1 allometry and bristle numbers during evolution.
Previous work showed that similar to that in the B. anynana's study, the evolution of sex comb length a the high flexibility to decrease or increase the bristle number in D. melanogaster (Ahuja and Singh, 2008). (Any mention in this paper regarding sex comb length strictly refers to bristle number per comb). However, despite the flexibility of sex comb length, the spacing between bristles is conserved in D. melanogaster and Drosophila species (Malagón et al., 2014). As a result, long D. melanogaster sex combs bend, showing atypical shapes, while multiple allometric changes take place during evolution (Malagón et al., 2014). Here, we extended our previous work and studied in more detail how the sex comb evolution affects the t1 allometry in short and long term evolution. We found a highly conserved coupling between two traits, the t1 length and TR number during Drosophila evolution. Although the appearance of the sex combs modifies the t1 allometry, those changes usually occur without disrupting this conserved coupling. These results are consistent with previous findings, suggesting a bias in variation of some phenotypes during evolution (de Bakker et al., 2013). Although many potential evolutionary paths are conceivable, evolutionary changes generally follow only a few routes (Schluter, 1996; Renaud et al., 2006; Weinreich et al., 2006; Marroig, 2007).
MATERIALS AND METHODS
Flies were grown in standard culture conditions on yeast-cornmeal-molasses media at a temperature of 25 °C. To change sex comb length and tarsal segment length three different types of genetic perturbations were used: mutations, artificial selection and UAS-GAL4 system. The following three mutations were studied in t1 or 2nd tarsal segment (t2): bric à bracPR72(babPR72) (Godt et al., 1993), Sex comb reduced13A/6 (Scr13A/6) (Sivanantharajah and Percival-smith, 2009) and sex comb distal (Scd) (Randsholt and Santamaria, 2008). Artificial selection was performed following the protocol described by Ahuja and Sing (2008). Hence, two lines were generated, a line with a low and a line with a high sex comb tooth number; Low and High lines respectively. Based on the bristle number, sex combs were divided into three groups: 1) short (≤7 bristles), 2) intermediate (9-11 bristles), and 3) long sex combs (12-15 bristles). Finally, the UAS-GAL4 system was used to perturb sex comb development. rnGAL4-5 (Ng and Kopp, 2008), babGAl4 (Godt et al., 1993) and dll-GAL4 (Held, 2010) were used to drive gene expression in the distal part of the t1. As a responder (UAS lines), four different pathways were perturbed: bristle morphogenesis (rab11 RNAi, flamingo RNAi. Dishevelled RNAi), leg morphogenesis (Scr RNAi, Dll RNAi, Dachousond RNAi), the sex determination pathway (Transformer RNAi, Double-sex RNAi) and epithelial morphogenesis (Shg RNAi and αCat RNAi).
Introgression of fluorescent protein into D. melanogaster lines with different sex comb length
The ubi-DEcad::GFP line used was generated by others for a previous study (Oda and Tsukita, 2000). Four genotypes were studied: male wild type (wt), artificially selected lines for high and low number of sex comb teeth and the mutant babPR72. High and Low sex comb bristle number lines were developed by artificial selection as previously described (Ahuja and Singh, 2008). Wild type males came from the outbred base population described in Ahuja and Singh (2008). The introgression of the fluorescent marker into the artificially selected lines required several generations of backcrossing between the ubi-DE::cadGFP and the artificially selected lines. In addition, the mutant babPR72 was generated by Godt et al. (1993). Different from artificially selected lines, standard genetic crosses were enough to introgress the fluorescent marker into this mutant strain.
Data collection
Adult legs were dissected for image acquisition. They were then mounted in Hoyer`s medium in 22X22 mm No 1 (VWR) coverslips, and imaged with a Cool Snap camera U-CMAD (Photometrics) using protocols that have been previously described (Atallah, 2008). Image J software (http://rsb.info.nih.gov/ij) was then used to measure the length of the t1.
Data collection of developing legs
For imaging developing legs, pupae were mounted in halocarbon oil (series 700; Halocarbon Products) on a coverslip (Sigma) and imaged with a laser 510 scanning confocal microscope (ZEISS) at 25 degrees with a 40× objective, using LSM Browser software (ZEISS). Z-stacks had a 3 μm step size. For post-acquisition image analysis and manipulation, maximum intensity projections were performed with LSM software (ZEISS). The removal of the pupal case background from images and the generation of 2D and 3D projections were manually performed as described by Atallah (2008).
Morphological measurements in D. melanogaster lines and Drosophila species with different sex comb length
In D. melanogaster, the 1st and 3rd legs were dissected in both males and females, to compare the allometric relationship between the sexes. The following measurements were done in adult legs: number of TRs, t1 length, sex comb tooth number and degree of sex comb rotation (Fig. 1D-F). The Drosophila species used were D. willistoni, D. virilis, D. mojavensis, D. guanche, D. rhopaloa, D. nikananu, D. ficusphila, and D. serrata.
Statistical Analyses
To determine whether t1 length is coupled to the number of TRs, the correlation coefficient was calculated using Excel (Microsoft). In addition, to compare how different degrees of rotation affect t1 length, initial comparisons were made using analysis of variance while post hoc comparisons were performed using Tukey's test. Similar statistical analysis were done for comparing various cellular parameters in lines with different sex comb length, including the number of cells in the distal region, the distance between TR and top sex comb tooth, and apical cell area. In both cases, the statistical package Sigma Plot11.0 was used. Error bars represent the standard deviation.
RESULTS
Coupling between t1 length and TR number: An ancestral leg relationship
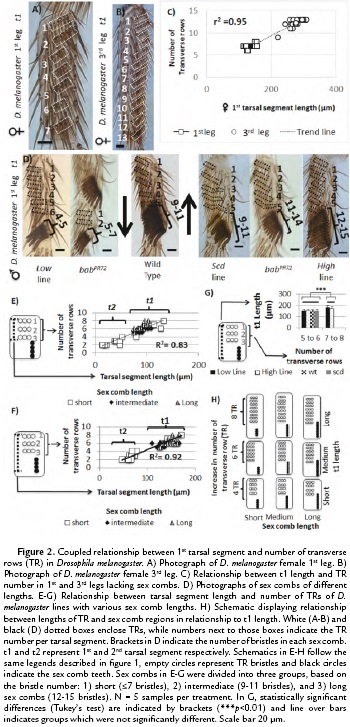
To study the relationship between chateotaxy and allometry during evolution, we asked whether variation in t1 length was coupled to bristle row number (Fig. 1B), we used the t1 of the female 1st and 3rd leg as a system (Fig. 1A). Those TRs have been suggested to represent the ancestral pattern in t1 bristle rows (Fig.1A) and vary in number depending on leg length (Hanna-Alava, 1958a; Hanna-Alava, 1958b). To do so, we measured the t1 length and the number of TRs in the 1st and 3rd leg of female D. melanogaster as indicated in Fig 1D-E. The measurements of 20 adult female legs (Fig. 2A-C) confirmed the results of previous studies, showing that the t1 is longer in the 3rd leg than in the 1st leg (Average of t1 length in 3rd leg = 277.49, +/- 22.60 µm; 1st leg = 139.76, +/-12.02 µm) (Hanna-Alava, 1958a; Hanna-Alava, 1958b). The TR number is also higher in the 3rd leg than the 1st leg (Average of TR number in 3rd leg = 12.3, +/-1.03 µm; 1st leg = 7, +/-0.46 µm). In addition, these measurements revealed a coupled relationship between these two traits, as indicated by the high correlation coefficient (r2 = 0.95, N = 40). An increase in t1 length was associated with an increase in the number of TRs along the t1.
Then we asked whether this coupled relationship between t1 length and TR number is still observed after appearance of the sex comb and whether changing sex comb length, a common evolutionary modification, could modify this tarsal segment allometry. For this purpose, adult male legs with different sex comb lengths were measured as shown in Fig. 1D-F. To modify sex comb length, we used mutations and artificial selection in t1 and t2 as indicated in the material and methods section. The analysis of light microscope pictures revealed that the coupled relationship is independent of sex comb length (Fig. 2D-H). The TR number increases when studying t1 and t2 as indicated by the high value of the correlation coefficient, whether measuring from top to bottom TR (r2 = 0.83, N = 25; Fig. 2E) or from top TR to the bottom sex comb teeth (r2 = 0.92, N = 25; Fig. 2F). As a result, these data suggest that modifying sex comb length occurs without disrupting the ancestral morphological relationship, a coupled relationship between t1 length and the TR number. In addition, we also found that t1 length was independent of sex comb length while displaying complete rotation. When comparing short or long sex combs, we found that the t1 length is statistically homogeneous if those legs have similar numbers of TRs (Figure 2G-H; 5 to 6 TRs Tukey's test, HSD, F3,16 = 0.03, p = 0.99; 7 to 8 TRs Tukey's test, HSD, F1,18 = 2.34, p = 0.13). However, when comparing between t1 with different numbers of TRs, the length is significantly different (5 -8 TRs Tukey's test, HSD, F5,34 = 7.85, p < 0.001). These results indicate an unexpected finding, changes in sex comb length can occur independently of the t1 allometry. In the next section, we show how sex comb morphogenesis can provide various mechanisms to explain how dramatic changes in sex comb length can have only a small effect in t1 length.
Developmental aspects of relationship between t1 and sex comb length
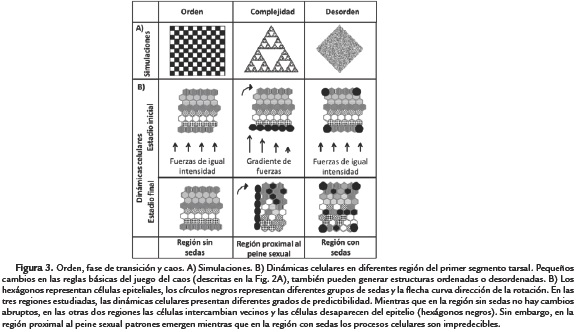
Malagón et al., (2014) show that the space available for the rotation of the D. melanogaster sex comb is independent of the sex comb's length. This finding can explain the reason why changing sex comb length occurs without affecting t1 allometry. To understand in more detail, the developmental basis of this phenomenon we measured four lines of D. melanogaster with variable numbers of bristles per comb. We used five developing legs per line with three groups of sex comb length: short (Low line ≤ 7 bristles), intermediate (wt = 9-11 bristles) and long (babPR72 and High line = 12-15 bristles) sex combs. We introgressed a fluorescent protein in these lines to visualize cell boundaries as described in the materials and methods section. We measured different cellular parameters that can indicate allometric changes in the sex comb field (group of cells around this bristle row), including cell density and the distance between the distal TR and the sex comb (Fig. 3A-F). Our results confirmed and extended our previous findings (Malagón et al., 2014), suggesting that the sex comb field is limited to increase in size, and as a result, changing sex comb length occurs without changing t1 allometry.
First, we asked whether the size of the sex comb field changes with the number of sex comb teeth. We found that increasing sex comb length also significantly increases the number of cells underneath this row of bristles (Tukey's test, HSD, F4,15 = 21.9, p < 0.001) (Fig. 3C). These preliminary results seem to indicate that increasing sex comb length could also increase the t1 length. However, when analyzing the apical cell area of cells distal to sex combs (Fig. 3B), we found that cells close to the distal TR are significantly more crowded in long sex combs (>9 bristles) than short combs (<5 bristles) (Tukey's test, HSD,F4,15 = 10.16, p < 0.001; Fig 3B). In contrast, cells far from the distal TR are evenly packed independent of the sex comb length (F4,15 = 1.71, p = 0.203; Fig 3B). As a result, increasing cell numbers will lead to tighter cell packing rather than producing longer t1s (Fig. 3F).
A potential explanation for the difference in cell density is that selection or mutations can remove or add bristles, but those changes occur without a proportional change in space in the sex comb field. To test this hypothesis, we quantified the distance between the sex comb and the most distal transverse row after rotation (Fig. 3D-F). We found that the distance between the distal TR and the top proximal sex comb tooth is significantly higher in short combs (<7 bristles) than in wt or long combs (>10 bristles) (Tukey's test, HSD, F4,15 = 160, p < 0.001). In other words, although the sex comb changes in length, the vertical space allocated for rotation remains without significant changes. These results together can begin to explain why changes in sex comb bristle number unexpectedly occur without affecting t1 length. In the next section, we discuss another unexpected developmental effect on allometry; reducing the angle of sex comb rotation, in this instance, does contribute to change in D. melanogaster t1 length (Atallah, 2008; Atallah et al., 2009).
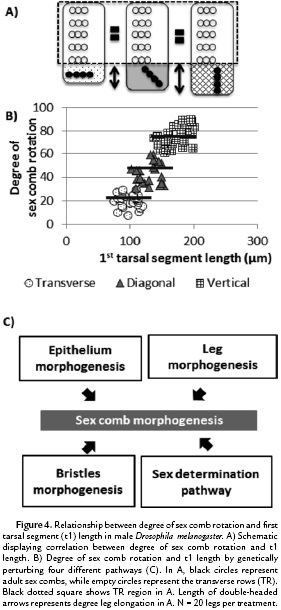
Changes in degree of rotation produce changes in 1st tarsal segment allometry in D. melanogaster
As previously discussed, in some Drosophila sex combs such as that of D. melanogaster, the sex combs rotate up to 90° during development (Fig. 1B). The change in position of this bristle row produces a male specific-tissue elongation, leading to a sexually dimorphic t1 length in D. melanogaster (Atallah, 2008). To test whether different degrees of sex comb rotation can modify the allometry of t1 length, we used highly specific perturbations of the expression of the gene Dll. To do so, we use the UAS-GAL4 system along with the temporal regulation of tubGAL80ts (Atallah et al., 2014). As a result, three different orientations were observed: 1) transverse (0°-30°), 2) diagonal (30°-60°), and 3) vertical (60°-90°). Analysis of the adult leg chaetotaxy showed that this genetic perturbation varies the degree of sex comb rotation without affecting virtually any other t1 traits including sex comb tooth number and TR formation (Atallah et al., 2014). Similar genetic perturbations of different pathways (Fig. 4A-C) again showed that increasing the degree of sex comb rotation produces significant lengthening of the t1 (genetic perturbations of Dll and four different pathways, Tukey's test, HSD, F3,76 = 238.3, p < 0.001 ). As a result, the developmental degree of rotation of the sex comb can produce a change in the leg allometry in the distal region, without altering the proximal region, where the TRs are located. As Drosophila sex combs display a great diversity of morphologies, the next step is to test whether patterns observed in D. melanogaster are also conserved among other Drosophila species, both in terms of coupling between orientation and t1 length as well as TR number and t1 length.
Changes in various bristles row parameters and t1 length in other Drosophila species
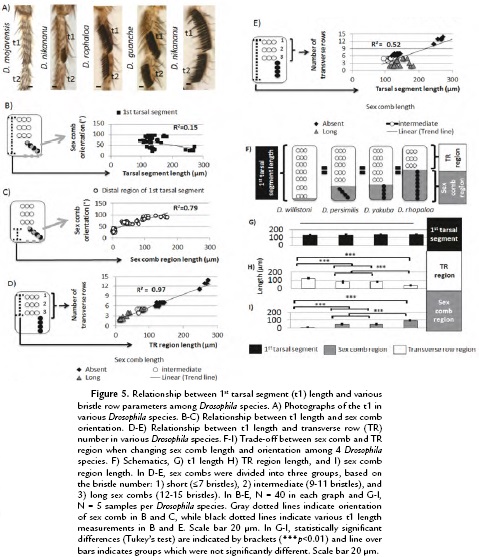
To test whether the Drosophila t1 length displays similar patterns observed in D. melanogaster, we studied males of eight Drosophila species (Fig. 5A). We integrated the different parameters studied in D. melanogaster adults including t1 length, TR number, sex comb orientation and length. To do so, the Drosophila species were carefully chosen to represent a good approximation of sex comb developmental and morphological diversity. We found that despite great developmental differences observed among Drosophila species, the same relationship between t1 length and bristles patterns observed in D. melanogaster also occur in other Drosophila species. However, those rules seem to be used in novel ways to produce a diversity of morphologies during evolution.
First, we examined whether there is a relationship between t1 length and the orientation of the sex comb as observed in D. melanogaster. To do so, we measured the t1 length as well as the distance between the joint to top part of the sex comb or homologous distal TRs in adult male legs (Fig. 5B-C). Different from that in D. melanogaster, we found that there was no relationship between sex comb orientation and t1 length as indicated by the low correlation coefficient (r2 = 0.15, N = 40; Fig. 5B). However, there is an elongation of the distal region of t1 based on the sex comb orientation. The lowest distance was observed in transverse bristle rows and highest in vertical rotating combs, thus displaying a high correlation coefficient (r2 = 0.79, N = 40; Fig. 5C). These data indicate that although there is a local modification in the sex comb field, the top part of the t1, the region covered by TRs is also fundamental to understand how the t1 length changes during evolution.
To study the changes observed in top part of t1, we measured how t1length changes among Drosophila species and test whether the coupling between TR number and t1 length is still conserved during evolution. Similar to D. melanogaster, t1 length was measured from the top TR to the bottom sex comb tooth or TR region as indicated in Fig 5D-E. We observed that in Drosophila species that lack a comb or have a short comb, the coupling is still conserved as indicated by the correlation coefficient (r2 = 0.97, N = 25) (Fig. 5D). However, those morphological changes are different from those observed in D. melanogaster, because when sex comb length increases, the TR region decreases in length (Fig. 5E). This trade-off between region lengths is indicated by reduction in the correlation coefficient (r2 = 0.52, N = 25) (Fig. 5E).
To examine in more detail, the trade-off in length between TR and sex comb regions, we focused on 4 Drosophila species: D. willistoni, D. persimilis, D. yakuba, and D. rhopaloa (Fig. 5F). Although these species show differences in sex comb length and orientation, their t1s have a statistically similar length (t1 length among the 4 different Drosophila species chosen, Tukey's test, HSD, F3,16 = 1.23, p = 0.33) (Fig. 5G). However, when studying the TR and sex comb regions, we observed statistically significant changes in length between these two regions (sex comb region length among the four different Drosophila species chosen, Tukey's test, HSD, F3,16 = 224.8, p < 0.001; TR region length among the four different Drosophila species chosen, Tukey's test, HSD, F3,16 = 188.2, p < 0.001 ) (Fig. 5H-I). An increase in length in length in the sex comb region produces a reduction in the TR region. Together, the conservation in TR spacing and variation in TR and sex comb regions show an association between t1 length and chaetotaxy during Drosophila macro and micro-evolution.
DISCUSION
D. melanogaster and Drosophila species couple the t1 length to the TR number
The coupling between t1 and TR number is a highly conserved trait observed in all the legs studied, in both D. melanogaster and other Drosophila species. The first evidence of the conservation of this coupling emerges when comparing the t1 of the 1st and 3rd leg. As the t1 in the 3rd leg is much longer than the 1st and 3rd leg have extra TRs (Fig. 2A-C). In addition, the appearance of the D. melanogaster sex combs occurs without disrupting this coupling, independently of the sex comb length or degree of rotation (Fig. 3-4). The final evidence of this evolutionary conservation emerges when studying different Drosophila species (Fig. 5D-E). Despite the spectacular morphological diversity of the sex comb, the changes occur mainly in the sex comb region without modifying the coupling between the TR and t1 length in top region of the t1. These types of developmental correlation have been previously described in evolutionary studies (Marroig, 2007; Tobler and Nijhout, 2010). For example, the number of finger digits of frogs is associated with cell numbers in the presumptive digit region during development, suggesting a link between developmental changes and evolutionary variation (Alberch and Gale, 1983; Smith et al., 1985; Brakefield, 2006; Monteiro et al., 2011; Malagón et al., 2014; Pélabon et al., 2014).
We hypothesize that a developmental mechanism, lateral inhibition, is responsible for the coupling between t1 length and TR number (Held, 1990; Orenic et al., 1993; Held, 2002). Regular spacing between bristle rows occurs because the future bristle cells, the sensory organ precursors, inhibit the surrounding epithelial cells from having a neural cell fate (Held, 1990; Orenic et al., 1993; Held, 2002). Interestingly, although several genetic perturbations can modify the distance between bristles (Held, 1990; Held, 2002), our data show that this type of modification is not usually the path taken during evolution (Fig. 5D-E). Future studies are necessary to understand the extent to which the evolutionary conservation of this coupling, is due to internal factors, such as genetic or developmental factors, or an external factor such as selection (Gould, 1976; Weber, 1992). For example, since TRs are used as cleaning utensils (Kopp, 2011), it is necessary to test whether changing the spacing between TRs can potentially reduce cleaning efficiency.
Influence of sex comb length and rotation on t1 length changes in D. melanogaster and Drosophila species
Our data show that in D. melanogaster, the evolutionary innovation of producing a rotating sex comb plays an important role in determining t1 length (Atallah, 2008; Malagón et al., 2014). Previous studies show that the change in position of the sex comb leads to a male-specific lengthening of the distal part of t1, which are never seen in females (Atallah, 2008; Malagón, 2013). Consistent with this information, when genetically reducing the degree of rotation in different ways (Fig. 4A-C), a reduction in the t1 length takes place, thus returning to the allometric relationship observed in the t1 D. melanogaster females (Fig. 4). In contrast to rotation, changes in the sex comb length have a small effect on modifying t1 length. In D. melanogaster, the distal TR seems to function as a barrier preventing the D. melanogaster sex comb field from increasing beyond a certain size. As a result, although there is an increase in cell number underneath the sex comb (Fig. 3C), this increment translates into a higher cell density rather than longer t1s (3B-F).
In other Drosophila species studied, the relationship between t1 length and sex combs can be divided into two main groups: 1) species without combs or with transverse combs and 2) species with diagonal or vertical combs. In the first group, t1 length correlates to the number of TRs. In second group, the distal region of the t1 can increase in length and such elongation can be associated, in some cases, with sex comb orientation (Atallah, 2008; Tanaka et al., 2009). In addition, the evolution of Drosophila sex comb length can provide a good example of the differential effects of short vs. long term selection in allometric relationships (Malagón, 2013). In D. melanogaster, changing sex comb length through artificial selection, did not produce dramatic changes in t1 allometry (Fig. 3), in lines with similar degrees of rotation (Malagón, 2013). On the contrary, in Drosophila species males with long sex comb have a trade-off between lengths of the sex comb and and TR regions (Fig. 5E-I). As the sex combs increase in the lengths, the number of TRs is reduced. Future studies are necessary to study the genetic and developmental restrictions that limit dramatic changes in the short-term selection and how those restrictions are removed over the long term.
This work is also consistent with previous studies showing a bias in the morphological variation between traits (de Bakker et al., 2013; Kavanagh et al., 2013; Malagón et al., 2014), while changes in t1 allometry do occur, such changes generally occur without disrupting the ancestral coupling between t1 length and TR number. In addition, these results also suggest that in the fruit fly male t1 there can be a hierarchy of selection "accessibility" with spacing between bristle rows being the most conserved feature, bristle row numbers being more easily changed, and t1 length being the most easily modified given.
Entrenched developmental processes and t1 allometric changes in Drosophila evolution
Consistent with previous studies, this work highlights the importance of developmental studies for understanding the evolution of allometry (Gould, 1966; Stern and Emlen, 1999; Shingleton et al., 2007; Malagón et al., 2014). In particular, our data suggest that understanding the entrenchment of developmental processes can provide a better understanding of how allometric changes occur during Drosophila evolution.
We show a highly conserved correlation between t1 length and TR number. Independent of the allometric changes observed, the relationship between these two traits remain the same among D. melanogaster legs and Drosophila species. Our study hypothesizes that this simple spacing rule is important for understanding allometric changes in the t1 and sex comb. For example, as the D. melanogaster sex comb is a modified TR, the spacing between the D. melanogaster sex comb and the spacing between the TR, are independent of sex comb length (Malagón et al., 2014). In addition, although increasing sex comb length will increase the number of cells in the field, it seems that the distal TR acts as a barrier preventing the sex comb field from increasing in size (Fig 3B-F). As a result, unexpectedly long and small D. melanogaster sex combs can display similar t1 lengths. In contrast, as rotating the sex comb is accompanied by an elongation of the tissue surrounding it, a higher degree of rotation will also lead to a longer t1(Atallah et al., 2009). Finally, in long-term evolution, the spacing between bristle rows is still fundamental to understanding allometric trade-offs between t1 regions among Drosophila species. Rather than changing the spacing between bristles rows, Drosophila species with long sex combs reduce the number of TRs.
Finally, future studies are necessary to study the effect of changes in sex comb length on female chaetotaxy and t1 length. Although, it is believed that t1 female chaetotaxy is highly conserved among Drosophila species (Kopp, 2011), preliminary results indicate that in Drosophila species with long sex combs, female TRs are reduced in number or length. This biological phenomenon could be another example, showing the importance of development to understand morphological diversity.
CONCLUSIONS
Adaptation constantly requires changes in proportions between different body parts (Frankino et al., 2005; Brakefield, 2006; Pélabon et al., 2014). In order to study how such allometry evolves, we used t1 Drosophila legs. We compared morphometric measurements in wild type and genetically perturbed D. melanogaster with analysis of various Drosophila species. We suggest that bristle-developmental processes that are apparently unrelated to leg elongation, are crucial for understanding the evolution of Drosophila t1 allometry. In particular, we suggest that the complicated connections between various developmental processes such as bristle row spacing and movement are vital for understanding the allometric changes observed. These complex interactions between developmental processes can explain some biases in phenotypic variation (Brakefield, 2006; de Bakker et al., 2013; Malagón et al., 2014). Finally, although this work concentrated on how body scale proportions change over time, we consider that the entrenchment of developmental processes described in this work, can be an important feature for understanding how some phenotypes originate and evolve (Malagon and Larsen, 2015).
REFERENCES
Ahuja A, Singh RS. Variation and evolution of male sex combs in Drosophila: nature of selection response and theories of genetic variation for sexual traits. Genetics. 2008;179(1):503-509. Doi:10.1534/genetics.107.086363. [ Links ]
Aberch P, Gale E. Size dependence during the development of the amphibian foot. Colchicine-induced digit loss and reduction. J Embryol Exp Morphol. 1983;197:177-197. [ Links ]
Atallah J. The Development and Evolution of Complex Patterns: The Drosophila Sex Comb as a Model System (PhD thesis). Toronto: Department of Cell and Systems Biology; University of Toronto. p. 45-90. [ Links ]
Atallah J, Liu NH, Dennis P, Hon A, Godt D, Larsen EW. Cell dynamics and developmental bias in the ontogeny of a complex sexually dimorphic trait in Drosophila melanogaster. Evol Dev. 2009;11(12):191-204. Doi:10.1111/j.1525-142X.2009.00319.x. [ Links ]
Atallah J, Vurens NS G, Mavong S, Mutti A, Hoang D, Kopp A. Sex-specific repression of dachshund is required for Drosophila sex comb development. Dev Biol. 2014;386(2):440-447. Doi:10.1016/j.ydbio.2013.12.01. [ Links ]
Baker R, Gerald SW. Phylogenetic analysis of sexual dimorphism and eye-span allometry in stalk-eyed flies (Diopsidae). Evolution. 2001;55(7):1373-1385. Doi:10.1111/j.0014-3820.2001.tb00659. [ Links ]
Beldade P, Brakefield PM. The genetics and evo-devo of butterfly wing patterns. Nat Rev Gen. 2002;3(3):442-452. Doi:10.1038/nrg818. [ Links ]
Brakefield PM. Evo-devo and constraints on selection. Trends Ecol Evol. 2006;21(7):362-369. Doi:10.1016/j.tree.2006.05.001. [ Links ]
Carreira V, Mensch J, Fanara J. Body size in Drosophila: genetic architecture, allometries and sexual dimorphism. Heredity. 2009;102(3):246-256. Doi:10.1038/hdy.2008.117. [ Links ]
De Bakker M, Fowler D, Den Oude K, Dondorp EM, Navas MCG, Horbaczuk JO. Digit loss in archosaur evolution and the interplay between selection and constraints. Nature. 2013;500(7463):445-448. Doi:10.1038/nature12336. [ Links ]
Frankino WA, Zwann BJ, Stern DL, Brakefield PM. Natural selection and developmental constraints in the evolution of allometries. Science. 2005;307(5710):718-720. Doi:10.1126/science.1105409. [ Links ]
Godt D, Couderc J, Cramton S, Laski F. Pattern formation in the limbs of Drosophila: bric à brac is expressed in both a gradient and a wave-like pattern and is required for specification and proper segmentation of the tarsus. Development. 1993;119(7463):799-812. Doi:10.1038/nature12336. [ Links ]
Gould SJ. Allometry and size in ontogeny and phylogeny . Biol Rev. 1966;41(4):587-640. [ Links ]
Gutierrez EE, Eberhard WG. Male Dimorphisms in beetles and earwigs and the question of developmental constraints. Evolution. 19915;45(1):18-28. Doi: 10.2307/2409478. [ Links ]
Hanna-Alava A. Developmental genetics of the posterior legs in Drosophila melanogaster. Genetics. 1958a;43(5):878-905. [ Links ]
Hanna-Alava A. Morphology and chaetotaxy of the legs of Drosophila melanogaster. J Morph. 1958b;103(2):281-310. Doi:10.1002/jmor.1051030205. [ Links ]
Held L. Arrangement of bristles as a function of bristle number on a leg segment in Drosophila melanogaster. Roux's Archiv Dev Bio. 1990:48-62. Doi:10.1007/BF01681532. [ Links ]
Held L. Imaginal Discs: The Genetic and Cellular Logic of Pattern Formation. Cambridge. [ Links ]
Cambridge University Press; 2002. p. 5-29. [ Links ]
Held LIJ. How does Scr cause first legs to deviate from second legs? Dros Inf Serv. 2010;93:132-146. [ Links ]
Huxley JS. Problems of Relative Growth. London: Johns Hopkins University Pres; 1972. p. XX-XL. [ Links ]
Kavanagh KD, Shoval O, Winslow BB, Alon U, Leary BP, Kan A. Developmental bias in the evolution of phalanges. Proc Natl Acad Sci U.S.A. 2013;110(45):18190-18195. Doi:10.1073/pnas.1315213110. [ Links ]
Klingenberg CP. Multivariate allometry. In: Advances in morphometrics. Marcus L. F., Corti M, Loy A., Naylor G. J. P., Slice D. E., editors. Multivariate Allometry. Advances in morphometrics. New York: Plenum Press; 1996. p. 23-49. [ Links ]
Klingenberg CP. Heterochrony and allometry: the analysis of evolutionary change in ontogeny. Biol Rev Camb Philos Soc. 1998;73(1):79-123. Doi:10.1111/j.1469-185X.1997.tb00026.x. [ Links ]
Kopp A. Drosophila sex combs as a model of evolutionary innovations. Evol Dev. 2011;13(6):504-522. Doi:10.1111/j.1525-142X.2011.00507.x. [ Links ]
Langlade NB, Feng X, Dransfield T, Copsey L, Hanna AI. Evolution through genetically controlled allometry space. Proc Nat Acad Sci USA 2005;10221-10226 Doi:10.1073/pnas.0504210102. [ Links ]
Malagón JN. Sex combs in motion: Cellular processes involved in sex comb rotation in Drosophila melanogaster (PhD thesis). Toronto: Department of Cell and Systems Biology, Arts and Science Faculty, University of Toronto. 2013. p. 120-150. [ Links ]
Malagón JN, Ahuja A, Sivapatham G, Hung J, Lee J, Muñoz-Goméz SA, Atallah J, Singh R, Larsen E. Evolution of Drosophila sex comb length illustrates the inextricable interplay between selection and variation. Proc Nat Acad Sci USA. 2014;111:E4103-E4109. Doi:10.1073/pnas.1322342111. [ Links ]
Malagon JN, Larsen E. Heredity and self-organization: partners in the generation and evolution of phenotypes. Int Rev Cell Mol Biol. 2015;315:153-181. Doi:10.1016/bs.ircmb.2014.12.003. [ Links ]
Marroing G. When size makes a difference: allometry, life-history and morphological evolution of capuchins (Cebus) and squirrels (Saimiri) monkeys (Cebinae, Platyrrhini). BMC Evol Biol. 2007;7:20. Doi: 10.1186/1471-2148-7-20. [ Links ]
Marroing G, Cheverud J. Size as a line of least resistance II: direct selection on size or correlated response due to constraints? Evolution. 2010;64:1470-1488. Doi:10.1111/j.1558-5646.2009.00920.x. [ Links ]
Massgue J. TGFbeta in Cancer. Cell. 2008;134:215-30. Doi:10.1101/cshperspect.a022053. [ Links ]
Monteiro A, Brakefield PM, French V, Url S. Butterfly Eyespots: The Genetics and Development of the Color Rings. Evolution. 2011;51:1207-1216. Doi:10.2307/2411050. [ Links ]
Ng CS, Kopp A. Sex combs are important for male mating success in Drosophila melanogaster. Behavioral genetics. 2008;38:195-201. Doi:10.1007/s10519-008-9190-7. [ Links ]
Oda H, Tsukita S. Real-time imaging of cell-cell adherens junctions reveals that Drosophila mesoderm invagination begins with two phases of apical constriction of cells. J Cell Sci. 2000;114:493-501. [ Links ]
Orenic TV, Held LI, Paddock SW, Carroll SB. The spatial organization of epidermal structures: hairy establishes the geometrical pattern of Drosophila leg bristles by delimiting the domains of achaete expression. Development. 1993;118:9-20. [ Links ]
Pélabon C, Firmat C, Bolstad GH, Voje KL, Houle D, Cassara J. Evolution of morphological allometry. Ann N Y Acad Sci. 2014;1320:58-75. Doi:10.1038/nrc2536. [ Links ]
Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nature Rev Cancer. 2008;8(12):915-928. Doi:10.1111/nyas.1247. [ Links ]
Randsholt NB, Santamaria P. How Drosophila change their combs: the Hox gene Sex combs reduced and sex comb variation among Sophophora species. Evol Develop. 2008;10(1):121-133. Doi:10.1111/j.1525-142X.2008.00219.x. [ Links ]
Renaud S, Auffray J, Michaux J. Conserved phenotypic variation patterns, evolution along lines of least resistance, and departure due to selection in fossil rodents. Evolution. 2006;60(8):1701-1717. Doi: 10.1554/05-330.1. [ Links ]
Schluter D. Adaptive radiation along genetic lines of least resistance. Evolution. 1996;50:1766-1774. Doi:10.2307/2410734. [ Links ]
Shingleton AW, Frankino WA, Flatt T, Nijhout HF, Emlen DJ. Size and shape: the developmental regulation of static allometry in insects. BioEssays. 2007;29(6):536-548. Doi: 10.1002/bies.20584. [ Links ]
Sivanatharajah L, Percival-Smith A. Analysis of the sequence and phenotype of Drosophila Sex combs reduced alleles reveals potential functions of conserved protein motifs of the sex combs reduced protein. Genetics. 2009;182(1):191-205. Doi:10.1534/genetics. [ Links ]
Smith M, Burian R, KauffmanN S, Alberch P, Campbell J, Goodwin B. Developmental constraints and evolution: A perspective from the Mountain Lake Conference on development and evolution. Quart Rev Biol. 1985;60(3):256-287. [ Links ]
Stern DL, Emlen DJ. The developmental basis for allometry in insects. Development. 1999;1101(6):1091-1101. [ Links ]
Tanaka K, Barmina O, Kopp, Sanders LE, Arbeitman MN, Kopp A. Evolution of sex-specific traits through changes in HOX-dependent doublesex expression. PLoS biology. 2011;9(8):e1001131. Doi:10.1371/journal.pbio.1001131. [ Links ]
Tanaka K, Barmina O, Kopp A. Distinct developmental mechanisms underlie the evolutionary diversification of Drosophila sex combs. Proc Natl Acad Sci USA. 2009;106(12):4764-4769. Doi:10.1073/pnas.0807875106. [ Links ]
Thompson DW. On Growth and Form. New York: Dover publications, INC; 1917. p. 78-85. [ Links ]
Tobler A, Nijhout HF. Developmental constraints on the evolution of wing-body allometry in Manduca sexta. Evol Dev. 2010;12(6):592-600. Doi:10.1111/j.1525-142X.2010.00444.x. [ Links ]
Vasseur F, Violle C, Enquist B, Granier C, Vile D. A common genetic basis to the origin of the leaf economics spectrum and metabolic scaling allometry. Ecol lett. 2012;15(10):1149-1157. Doi:10.1111/j.1461-0248.2012.01839.x. [ Links ]
Warren I, Vera J, Johns A, Zinna R, Marden NJ, Emle ND. Insights into the development and evolution of exaggerated traits using de novo transcriptomes of two species of horned scarab beetles. PloS one. 2014;9(2):88365. Doi:10.1371/journal.pone.0088364. [ Links ]
Weber KE. How small are the smallest selectable domains of form? Genetics. 1992;130(2):345-353. [ Links ]
Weinreich D, Delaney N, Depristo O M, Harlt D. Darwinian evolution can follow only very few mutational paths to fitter proteins. Science. 2006;7(5770):111-114. Doi:10.1126/science.1123539. [ Links ]
Young N, Wagner G, Hallgrimson B. Development and the evolvability of human limbs. Proc of the Nat Acad Sci. 2010;107(8):3400-3405. Doi:10.1073/pnas.0911856107. [ Links ]