Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Acta Biológica Colombiana
Print version ISSN 0120-548X
Acta biol.Colomb. vol.22 no.1 Bogotá Jan./Apr. 2017
https://doi.org/10.15446/abc.v22n1.57197
DOI: http://dx.doi.org/10.15446/abc.v22n1.57197
COMPLEMENTARY DESCRIPTION AND RECORD OF Neocyclops ferrarii (Cyclopidae: Halicyclopinae) FROM NORTHERN COLOMBIA
Descripción complementaria y registro de Neocyclops ferrarii (Cyclopidae: Halicyclopinae) en el norte de Colombia
Juan Manuel FUENTES-REINÉS1, Eduardo SUÁREZ-MORALES2.
1 Grupo de investigación en Biodiversidad y Ecología Aplicada, Facultad de Ciencias Básicas, Universidad del Magdalena. Carrera 32 n.º 22–08. Santa Marta, Colombia.
2 El Colegio de la Frontera Sur. A.P. 424, 77014 Chetumal, Quintana Roo, México.
For correspondence. juanmanuelfuentesreines@yahoo.com
Received: 28th April 2016, Returned for revision: 27th September 2016, Accepted: 9th October 2016.
Associate Editor: Santiago Gaviria Melo.
Citation/Citar este artículo como: Fuentes-Reinés JM, Suárez-Morales E. Complementary description and record of Neocyclops ferrarii (Cyclopidae: Halicyclopinae) from northern Colombia. Acta biol. Colomb. 2017;22(1):59-65. DOI: http://dx.doi.org/10.15446/abc.v22n1.57197
ABSTRACT
The interstitial cyclopoid copepod Neocyclops ferrarii Rocha, 1995 was found in samples obtained from littoral areas of Rodadero Bay, northern Colombia. The specimens from Colombia share the diagnostic features of N. ferrarii presented in the original description. However, the Colombian specimens show some degree of variation with respect to the type material in: 1) the number of teeth in the labrum; 2) the length of outer exopodal spine on female leg 5; 3) the relative length of the mandibular palp setae; 4) length/width ratio of caudal rami; 5) the length ratio of caudal setae VI/III; 6) the length ratio of caudal setae VII/VI; 7) the male body size; 8) the male caudal rami length/width ratio; 9) This is the second record of this species after its original description from Belizean waters. In the Caribbean region N. ferrarii most closely resembles N. vicinus Herbst, both of them bear an antennary exopod, a 12-segmented female antennule, P3ENP3 armature formula 3,III, but can be separated from the latter by difference in the length/width ratio of the female caudal ramus, the length ratio of caudal setae VI/III, the length ratio of caudal setae VII/VI, the male body size, the number of segment of P4ENP, the armature details of mandibular palp and the number of segments of male P5. This is the second species of Neocyclops recorded from Colombia and represents a distributional range expansion of N. ferrarii in the Caribbean Basin.
Keywords: coastal copepods, Caribbean Sea, distribution, zooplankton.
RESUMEN
El copépodo ciclopoide intersticial Neocyclops ferrarii Rocha, 1995 fue encontrado en muestras litorales obtenidas de la bahía Rodadero, al norte de Colombia. Los especímenes de Colombia comparten las características diagnósticas de N. ferrarii de la descripción original. Sin embargo, los especímenes colombianos muestran cierta variación con respecto al material tipo en: 1) el número de dientes en el labro; 2) la longitud de la espina exopodal externa de la pata 5 de la hembra; 3) la longitud relativa de las setas del palpo mandibular; 4) la relación largo/ancho de la rama caudal; 5) la longitud proporcional de las setas caudales VI / III; 6) la longitud proporcional de las setas caudales VII / VI; 7) la talla del macho; 8) la relación largo/ancho de la rama caudal del macho. Este es el segundo registro de esta especie después de su descripción original en aguas de Belice. En la región Caribe N. ferrarii se asemeja más estrechamente a N. vicinus Herbst, ambos poseen exópodo antenal, anténulas con 12 segmentos en la hembra, la fórmula P3ENP3 de 3, III, pero difieren en la proporción de la rama caudal en la hembra, la longitud de las setas caudales VI / III y VII / VI, el tamaño del macho, el número de segmento de P4ENP, en detalles del armamento del palpo mandibular y en el número de segmentos de la P5 del macho. Esta es la segunda especie de Neocyclops registrada en Colombia y representa una expansión de la distribución conocida de N. ferrarii en la Cuenca del Caribe.
Palabras clave: copépodos costeros, mar Caribe, distribución, zooplancton.
INTRODUCTION
The cyclopoid copepod genus Neocyclops Gurney, 1927 is one of the most speciose in the subfamily Halicyclopinae; currently, it is known to contain 25 species (Pesce, 2016; Walter and Boxshall 2016). Members of this genus are marine forms inhabiting epibenthic or interstitial environments (Lee and Chang, 2015). The genus Neocyclops is cosmopolitan; it has been recorded from coastal areas of the Indian, Atlantic, and Pacific Oceans; about 60% of all known species are recorded in the Indo-Pacific (Lee and Chang, 2015).
Petkovski (1986) split the genus into two subgenera according to the number of exopodal segments of the male P5: the subgenus Protoneocyclops, with four segments in the fifth leg as in N. ferrarii, and Neocyclops s. str. with three segments; this criterion has been questioned (Karanovic, 2008).
In the Americas, the countries with most records of species of Neocyclops are Brazil, Bahamas, and Cuba (Herbst, 1955; Pleşa, 1973; Pesce, 1985). In Colombia, only one species, N. stocki Pesce, 1985 from San Andres Island, has been reported so far (Petkovski, 1986).
The copepod fauna from Colombia has received little attention despite de diversity of fresh, marine and brackish systems in both the Atlantic and Pacific coasts of the country; more new species and records of Copepoda are expected to be found in future studies. The aim of this paper is to document the first record of N. ferrarii in Colombia, which expands the distributional range of this species in the Caribbean Basin. In addition, we present a complementary description of this species based on the Colombian specimens and a comparative analysis with the other known populations.
MATERIALS AND METHODS
Biological samples of littoral and limnetic habitats were obtained from Rodadero Bay, Magdalena, northern Colombia (11°14'10"N, 74°12'06"W) during fieldwork carried out from August 2015 to March 2016, mainly in the inshore areas covered by vegetation (mangrove) and a small bank of oysters but also from the shallow limnetic zones. Water salinity, pH, temperature was measured with a multiparameter WTW 350i. Water samples were collected manually using a 25-l bucket at both littoral and limnetic habitats. Samples were filtered with a zooplankton net (mesh size = 45 μm) and preserved in 70 % ethanol.
Copepods were sorted from all the samples and then processed for taxonomical identification including the examination of the whole specimen and dissection of selected appendages. Dissected appendages were mounted in slides with glycerine and sealed with Canada balsam. The specimens were measured in ventral position, from the anterior end of the rostral area to the posterior margin of the caudal ramus. Drawings were made with the aid of a camera lucida mounted on an Olympus BX51 compound microscope equipped with Nomarski DIC. The specimens examined were deposited at the Museo de Colecciones Biológicas de la Universidad del Atlántico, Barranquilla, Colombia (UARC273M-UARC281M), where they are available for consultation and/or further examination. Morphological terminology follows Huys and Boxshall (1991). The following abbreviations are used in the description: P1–P6= first to sixth swimming legs, EXP= exopod, ENP= endopod.
RESULTS
Taxonomy
Order Cyclopoida Burmeister, 1834
Family Cyclopidae Dana, 1846
Subfamily Halicyclopinae Kiefer, 1927
Genus Neocyclops Gurney, 1927
Neocyclops ferrarii Rocha, 1995
(Figures 1-3)
Description of female
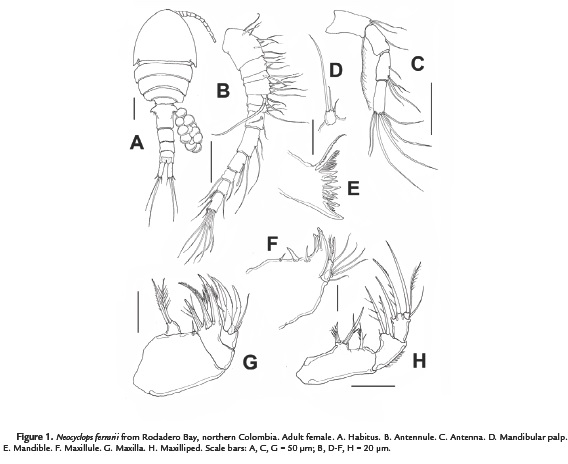
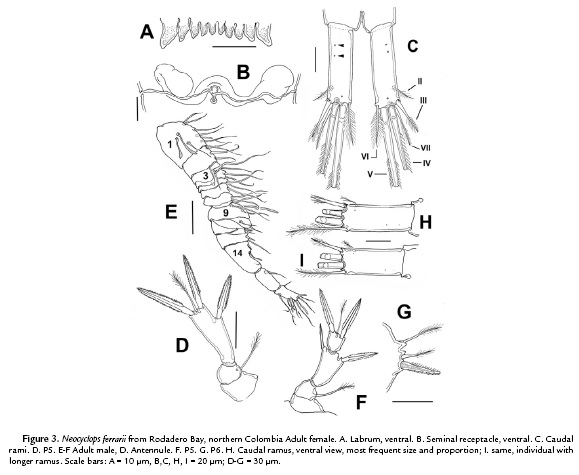
The morphology of the female specimens from Magdalena, Colombia agrees in general with previous descriptions and illustrations provided by Rocha (1995). Body widest at first pediger, slightly tapering posteriorly (Fig. 1A). Body length, excluding caudal setae, 756–826 μm (average = 780 μm; n = 7). Labrum trapezoidal, armed with ten blunt teeth of different sizes, outermost being largest and thickest (Fig. 3A). Urosome with four somites, genital double-somite 1.1 times as long as wide, with paired backwardly directed spinous proximal processes visible in dorsal and ventral views (Fig. 1A). Seminal receptacle as shown in Fig. 3B. Anal operculum at middle of anal somite, not strongly convex, with smooth posterior margins.
Caudal ramus (Fig. 3C) about 2.7–3.1 as long as wide, with six setae, lateral seta II located slightly dorsally, outer seta III short, spiniform and bipinnate, about 0.7 times as long as ramus, a little longer than 1.3 length of inner seta VI. Terminal seta IV about 3.5 as long as the ramus, seta V is the longest. Dorsal seta VII slender, plumose, about 0.8 times as long as inner seta VI, and slightly shorter (0.5 times) than caudal ramus. Integumental pores pattern of caudal rami as in figure 2C.
Antennules 12-segmented; armature as in type specimens (Rocha, 1995) (Fig. 1B). Antenna (Fig. 1C) slender, distinctly 4-segmented, comprising basipod and 3-segmented endopod. Surface of basipod smooth, about 3.2 times as long as wide, with one long outer uniserially setulose seta representing exopod, and two naked setae at inner distal corner. First endopodal segment about 1.76 times as long as wide, with one naked seta at halfway the inner margin. Second endopodal segment longer than first endopodal segment, about 2.1 times as long as wide, with minute spinules along outer margin (not illustrated); armed with one short medial, two short subapical and two long apical setae along inner margin. Third endopodal segment elongate, about 2.7 times as long as wide, ornamented with one row of spinules along outer margin (not illustrated), bearing seven apical setae including four geniculate and three slender setae.
Mandible (Figs. 1D-E), palp reduced to small segment armed with three slender naked apical setae; longest seta not reaching gnathobasal teeth, about five times as long as shortest one; the middle seta is twice as long as the shortest (Fig. 1D). Coxal gnathobase well-developed; cutting edge armed with inner group of three stout teeth and one spinous element, middle group of six teeth and outer group of one unipinnate spine and one outer unipinnate dorsal seta.
Maxillule (Fig. 1F), maxilla (Fig. 1G), and maxilliped (Fig. 1H) as described by Rocha (1995).
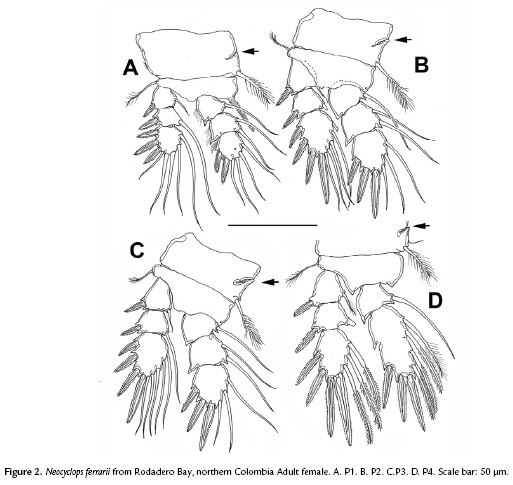
P1-P4 EXP and ENP three-segmented, except for P4ENP which is two segmented (Figs. 2A-D), armed as in Table 1. Spine inserted at inner corner of P1 basis reaching the half of second endopodal segment of P1 (Fig.2A). Intercoxal sclerites of P1–P4 with distal margin smooth. Praecoxal sclerites not expressed. Coxae unornamented, with transverse internal chitinous ridges originating from medial (arrowed in Figs 2A-D). P1-P4 EXP3 with setal formula 5,5,5,5 and spine formula 3,4,4,3; each leg bearing two inner setae on enp-2, and one inner seta on enp-1 and exp-1. P4ENP2-3 (Fig. 2D) fused; inner distal spine 1.36 times longer than outer distal spine.
P5 (Fig. 3D) three-segmented; coxa unarmed, about 0.65 times as long as wide, basis about 1.7 times wider than long, with outer plumose seta, exopod gradually broadening distally, about between 2.0 (Belizean specimens) and 2.3 times longer than wide, armed with outer pinnate spine slender apical plumose seta flanked by two pinnate spines. Inner distal spine 1.1 times longer than outer spine, about 1.34 times as long as lateral spine, 0.97 times as long as segment.
Male (allotype): Body 602–630 μm long (average 610±10 μm, n = 8). Urosome with six somites. Caudal rami shorter than in female, 2.2 times longer than wide (Fig. 3I); one specimen with relatively longer caudal ramus (2.6) (Fig. 3H); armature of rami as in female.
Antennule (Fig. 3E) 16-segmented; geniculate, armature and segmentation as in N. vicinus (Lotufo and Rocha, 1993).
P5 (Fig. 3F) 4-segmented, with small intercoxal sclerite; coxa unarmed, subquadrate; basis with outer plumose seta. First exopodal segment subrectangular, about 1.7 times as long as wide, armed with an outer spine and single inner seta, second exopodal segment as long as wide, bearing one plumose apical seta plus two spines.
P6. Consisting of low plate armed with one inner spine and two setae, outer seta about two times length of middle seta (Fig 3G). Male identical to female in all other respects.
DISCUSSION
Following Pesce and Galassi (1993) and Rocha (1995), up to eight valid species of Neocyclops have been recorded in the Caribbean region: N. medius Herbst, 1955; N. vicinus Herbst, 1955; N. affinis Pleşa, 1961; N. improvisus Pleşa, 1973; N. stocki Pesce, 1985, N. papuensis Fiers, 1986, N. geltrudeae Pesce & Galassi, 1993; and N. ferrarii Rocha, 1995. Only three, N. vicinus, N. medius, and N. ferrarii have been reported in South America (Herbst, 1955, Lotufo and Rocha, 1993, present data).
Neocyclops ferrarii is assigned to the subgenus Protoneocyclops for its four-segmented male P4, this group contains nine species (Lee and Chang, 2015); this characteristic is shared with other two American congeners: N. papuensis and N. geltrudeae.
Neocyclops ferrarii can be easily recognized from its congeners by a unique combination of characters including: 1) large scar-like integumental ridges originating from the medial margins of the coxa in all swimming legs; 2) P4ENP2-3 fused; 3) mandibular palp with three elements, 4) dorsal caudal seta from 0.8-1.0 as long as ramus, 5) inner seta on first exopodal segment of male fifth leg plumose. Rocha (1995) proposed the integumental pore pattern as a useful character to distinguish closely related species of Neocyclops. He compared the pore topography of N. ferrarii with that of N. vicinus and N. medius (Rocha, 1995, fig. 20, 21). We were able to observe pores on the caudal rami (arrowed in Fig. 3C); out of this group of species, and only N, ferrarii has pores in this position.
The transverse chitinized scars occurred in all specimens observed. Similar structures have been previously reported for four species from Australia: N. australiensis (Karanovic, 2008, figure 54A, D), N. sharkbayensis (Karanovic, 2008, figs. 58D, 59B), N. trajani (Karanovic, 2008, fig. 61C) and N. hoonsooi (Lee and Chang, 2015, figs 4A-D) but in the first three species the scars are less pronounced.
Specimens from Colombia are identical in most aspects to those reported from the type locality in Belize (Rocha, 1995), however, these two population show subtle differences: 1) labrum with ten teeth in specimens from Colombia (present data, fig, 3A) vs. nine in populations from Belize (Rocha, 1995), 2) the outer exopodal spine on female leg five is relatively shorter in the Colombian specimens (0.7 times as long as segment) than in the type population (0.9) (Rocha, 1995, fig. 17). 3) the relative length of the mandibular palp setae differs in the two populations; the Belizean specimens have two short setae that are subequally long, whereas the Colombian specimens one of these setae is at least twice as long as the other one; 4) length/width ratio of caudal rami is about 2.7–-3.1 in the specimens from Rodadero Bay (present data, Fig 3C ) whereas it is 2.4 in specimens from Belize (Rocha, 1995; fig 10; Karanovic, 2008; Lee and Chang, 2015); 5) the length ratio of caudal setae VI/III differs in these species, it is clearly longer in specimens from Belize (ratio 1.37) (Rocha 1995, fig. 10) whereas the figure for our specimens from Colombia is 1.25; 6) the length ratio of caudal setae VII/VI is smaller in specimens from Colombia (ratio = 0.8) vs. 1.0 in type specimens from Belize; 7) The male body size of this species has a new range: the specimens from Colombia are larger (602-630 μm) than those reported from Belize (520-580 μm) (Rocha, 1995), 8) the male caudal rami length/width ratio is another variable structure; it ranged between 2.2–2.6 in the Colombian population vs. 2.2 in the Belizean specimens (Rocha, 1995). Overall, we do not regard such differences as evidence enough to suggest the erection of a new species to accommodate our specimens from Colombia.
Among the species of Neocyclops reported from the Caribbean region and adjacent areas (see Pesce, 2016), N. ferrarii closely resembles N. vicinus Herbst, 1955 from Brazil. Both of them bear an antennary exopod, a 12-segmented female antennule, and a 3, III P3ENP3 armature formula. However, N. ferrarii can be separated from N. vicinus by several characters. In N. ferrarii the length/width ratio of the female caudal rami is 2.4 – 3.1(Rocha, 1995, fig 10, Present data, fig 3C), vs. 2.5–2.9 in N. vicinus (Herbst, 1955, taf. 33a-b; Lotufo and Rocha, 1993, fig 20). The length ratio of caudal setae VI/III differs in these species, it is quite longer in N. ferrarii (ratio: 1.25-1.37) (Rocha 1.995, fig 10, present data, fig 3C) while in N. vicinus ranges from 0.9-1 (Herbst, 1955, taf. 33a-b; Lotufo and Rocha, 1993, fig 20). The length ratio of caudal setae VII/VI is smaller in N. ferrarii (ratio 0.8-1) vs. 1.4 in N. vicinus.
The number of segments of P4ENP is an important difference between these two species; in N. ferrarii P4ENP it is two-segmented (Rocha, 1995, fig 16, present data, fig 2D) vs. three-segmented in N. vicinus (Herbst, 1955, fig 33E). In addition, the mandibular palp is armed with 3 setae in N. ferrarii (Rocha, 1993, fig. 11, present data, fig. 3D) vs. two setae in N. vicinus (Herbst, 1955; Lotufo and Rocha, 1993, fig. 21). The male P5 of N. ferrarii is four-segmented (Rocha, 1995, fig 18, present data, fig 3F) vs. a three-segmented condition in N. vicinus (Lotufo and Rocha, 1993, fig 16).
The information presented in this paper allows an increase of the total number of free-living cyclopoid copepods reported for Colombia (Fuentes-Reinés and Suárez-Morales, 2015) to 48 species. Neocyclops stocki was the first species of the genus recorded from Colombia (see Gaviria and Aranguren, 2007); the present record of N. ferrarii represents the second from this country the second from this country.
Distribution and ecology
Neocyclops ferrarii was originally described from a pond adjacent to mangroves in Belize (Rocha, 1995); it was recorded later in the northern sector of the Yucatan Peninsula from the stomach contents of a coastal fish (Suárez-Morales et al., 2002); the species was advanced by these authors as a possible endemic of the Yucatan Peninsula, but its finding in Colombia allows us to discard this idea; hence, N. ferrarii is now deemed to be restricted to the western Caribbean. In Colombia this species was found in the littoral zone of the Rodadero Bay in an area covered by mangrove and at a depth of 0.70 m where water temperature varies over the seasons in the range of 30 – 32 ºC, salinity is 36.1 psu, and pH 8.3. These environmental conditions are within the ranges of salinity (19–39 psu) and temperature (30–35 °C) reported by Rocha (1995) for the type locality.
CONCLUSIONS
The information presented in this paper documents in detail the first record of Neocyclops ferrarii in Colombia and reveals a number of subtle morphological variations between the Colombian specimens and the Belizean population. Its presence in Colombia increases its known distributional range in the western Caribbean. This species can be confused with its congener N. vicinus in the Caribbean region but they can be separated by some important characters including the length/width ratio of the female caudal ramus, the number of segments of P4ENP, the armature details of mandibular palp, and the number of segments of the male P5.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
REFERENCES
Burmeister H. Beiträge zur Naturgeschichte der Rankenfüsser (Cirripedia). Berlin: G. Reimer; 1834. 60p. [ Links ]
Dana JD. Notice of some genera of Cyclopacea. Ann Mag Nat Hist Lond. 1940;18:181-185. [ Links ]
Fuentes-Reinés J, Suárez-Morales E. Checklist of planktonic Copepoda from a Colombian coastal lagoon with record of Halicyclops exiguus Kiefer. Bol Invest Mar Cost. 2015;44(2):369-389. [ Links ]
Gaviria S, Aranguren N. Especies de vida libre de la subclase Copepoda (Arthropoda, Crustacea) en aguas continentales de Colombia. Biota Colomb. 2007;8(1):53-68. [ Links ]
Gurney R. Zoological results of the Cambridge Expedition to the Suez Canal, 1924. XXXIII. Report on the Crustacea-Copepoda (littoral and semi-parasitic). Trans Zool Soc London. 1927;22:451-577. [ Links ]
Herbst HV. Cyclopoida Gnathostoma (Crustacea, Copepoda) von der Brasilianische Atlantik Küste. Kiel Meeresforsch. 1955;11(2):214-229. [ Links ]
Huys R, Boxshall GA. Copepod Evolution. London: The Ray Society; 1991. 468 pp. [ Links ]
Karanovic T. Marine interstitial Poecilostomatoida and Cyclopoida (Copepoda) of Australia. Crustaceana. 2008;9:1-336. [ Links ]
Kiefer F. Versuch eines Systems der Cyclopiden. Zoologischer Anzeiger. 1927;73:302-308. [ Links ]
Lee J, Chang CY. A new marine cyclopoid copepod of the genus Neocyclops (Cyclopidae, Halicyclopinae) from Korea. ZooKeys. 2015;520:131-146. Doi: 10.3897/zookeys.520.6006. [ Links ]
Lotufo GR, Rocha CEF. Neocyclops Gurney from Brazilian sandy beaches (Copepoda: Cyclopoida). Bijdr dierkd. 1993;63(3):163-172. [ Links ]
Pesce GL. Amsterdam Expeditions to the West Indian Islands, Report 45. Cyclopids (Crustacea, Copepoda) from West Indian groundwater habitats. Bijdr dierkd. 1985;55(2):295-323. [ Links ]
Pesce GL. Cyclopoida: Halicyclopinae Kiefer, 1927: Neocyclops Gurney, 1927. Copepod 2016; Available in: http://www.luciopesce.net/copepods/neocy.htm. Accessed 26 March 2016. [ Links ]
Pesce GL, Galassi DP. The genus Neocyclops Gurney in the West Indies: an update including of Neocyclops (Protoneocyclops) geltrudeae n. sp. (Crustacea, Copepoda, Cyclopoidae). Bijdr dierkd. 1993;63(2):115-120. [ Links ]
Petkovski TK. Zur Taxonomie des Genus Neocyclops Gurney 1927 (Crustacea, Copepoda, Cyclopoida). Acta Mus Maced Sci Nat. 1986;18(2/148):27-46. [ Links ]
Pleşa C. Un nouveau cyclopide interstitiel de la Mer des Caraïbes: Neocyclops improvisus sp. n. (Crustacea, Copepoda). Rés Expéd Biosp Cub Roum Cuba. 1973;1:119-122. [ Links ]
Rocha CEF. Neocyclops (Protoneocyclops) ferrari, a new species of cyclopid (Copepoda, Cyclopoida) from Belize, with remarks on the morphology of the genus Neocyclops. Contr Zool. 1995;65:41-51. [ Links ]
Suárez-Morales E, Avilés-Torres S, Rocha CEF. Extensión del ámbito geográfico de dos copépodos haliciclópinos (Copepoda: Cyclopoida: Halicyclopinae) en el suroeste de México. An Inst Biol Univ Nac Autón Méx Ser Zool. 2002;73(1):113-115. [ Links ]
Walter TC, Boxshall G. Neocyclops Gurney, 1927. World of Copepods database. 2016; Available in: http://www.marinespecies.org/copepoda/aphia.php?p=taxdetails&id=106434 Accessed 26 March 2016. [ Links ]