INTRODUCTION
Mangroves are ecosystems with high productivity in terms of providing food and refuge for resident or migratory species (Schaeffer-Novelli, 1995). About 50 % of all coastal mangroves in Brazil are located within the states of Piauí, Maranhão, Pará, and Amapá (Kjerfve et al., 2002); however, this particular benthic fauna is still poorly identified compared to other coastal areas in the country (Amaral and Jablonski, 2005).
Polychaetes stand-out in benthic macrofauna as one of the most abundant groups with great diversity in body shapes and habitat occupation (Amaral et al., 2005). In addition, they are part of the diet of seabirds as well as many species of fish and crustaceans with commercial importance (Burder et al., 1997; Paiva, 2006). They play an important role in the structure and functioning of benthic communities because of their abundance, diverse eating habits, the occupation of different niches, and relation with different types of sediments (Rohr and Almeida, 2006).
The faunal structure of benthic organisms is influenced by various environmental factors such as rainfall, substrate type and structure, dissolved oxygen, depth, the degree of dynamism, and standard currents (Echeverría and Paiva, 2007; Rodrigues et al., 2016). Moreover, it is likely that the distribution of these organisms is influenced by biotic factors such as vegetation, which can be used as food and refuge, providing ideal conditions for the reproduction of some species (Lewis and Stoner, 1983).
Thus, the macrofaunal composition and abundance in mangroves tend to be influenced by the vegetation density and biomass because the spatial complexity of these habitats leads to a different distribution and abundance patterns in various animal groups (Maia and Coutinho, 2013; Pagliosa et al., 2016). The genus Rhizophora L. provides great substrate aeration and habitats through its root system, which favors occupation by the macrofauna (Gill and Tomlinson, 1977; Guerrero and López-Portillo, 2017; Silva-Camacho et al., 2017).
Mangrove trees interfere in the physical environment, modifying predation pressure and food resources, depending on the species, and creating distinct microhabitats and faunal communities (Kon et al., 2010). Areas vegetated by Rhizophora mangle L. have high polychaete abundance and diversity. Previous studies on associations between macrofauna and vegetation have been carried out on the Amazon coast of Pará, Brazil (Aviz et al., 2009; Braga et al., 2009). Our study analyzed the polychaete community structure in the Amazon coast of the State of Maranhão in order to determine if the community structure was directly associated with the mangrove's vegetation. This is one of the first studies in Brazil relating the distribution of polychaetes in mangroves with vegetation composition.
MATERIAL AND METHODS
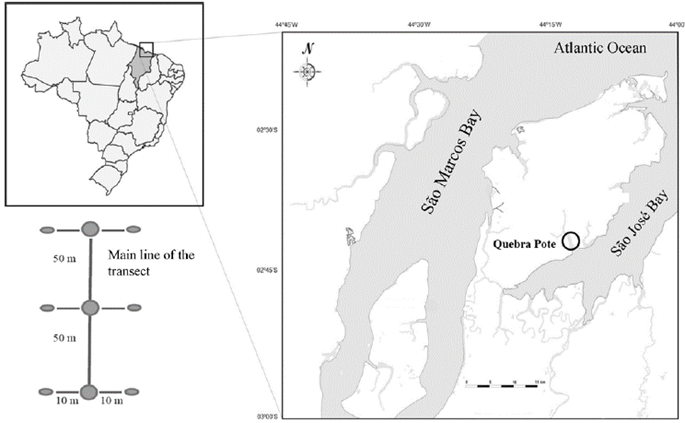
The coast of Maranhão is approximately 640 km long, located in the Northern Coast of Brazil. Mangroves in Maranhão occupy an area of 5.414.31 km2 and are displayed as fringes, behind beaches, around beach ridges and sand dunes, or bordering rivers and streams (Sousa-Filho, 2005). The Golfão Maranhense is located in the central region of the state's coast, divided into São Marcos Bay (where the Mearim, Pindaré, and Grajaú Rivers discharge) and São José Bay (where the Itapecuru and Munim Rivers discharge) (Teixeira and Sousa-Filho, 2009).
The Quebra Pote mangrove is located in the city of São Luís, in São José Bay, Maranhão (02°41.344' S; 44°12.604' W) (Fig. 1). It is bathed by the Tibiri River, which is approximately 13 km long and carries a nutrient-rich and terrigenous water load, mostly in the rainy season. The mangrove is strongly influenced by tides, which can reach 7.0 to 8.0 m in amplitude (Silva and Almeida, 2002), and affected by the direct interference of domestic sewage discharged in natura due to the absence of organic and inorganic waste collection or treatment.
Four sampling campaigns were performed during November 2013, January 2014, March 2014, and July 2014. One hundred meters long transects were set perpendicular to the waterline in a randomly selected area at each sampling event (Fig 1). Each transect was divided into three zones (50 m apart): Zone 1 (lower mesolittoral near the subtidal), Zone 2 (intermediate mesolittoral), and Zone 3 (upper mesolittoral). A total of 36 samples were collected, representing three samples per zone (10 m apart) at each sampling event.
The point-centered quarter method-PCQM (Schaeffer-Novelli and Citrón, 1986; Martins et al., 2011) was used to survey the vegetation following the same sampling transect, and the four nearest trees were identified at each sampling site. The type of mangrove forest and its ecological variables were considered in conjunction with the PCQM results to determine relationships between polychaetes distribution and type of mangrove.
Samples were collected using a PVC corer (10 cm in diameter and 20 cm in depth), fixed in 4 % formaldehyde, and transported to the Fisheries and Aquatic Ecology Laboratory at the Maranhão State University. Selected environmental parameters (salinity, temperature, and dissolved oxygen) were measured with multiparameter Hanna HI- 9828; sediment samples were collected with a PVC corer to determine granulometry and organic matter content at each sampling site (Walkley and Black, 1934; Suguio, 1973).
Samples were screened in a 0.5 mm pore sieve to prevent the loss of specimens and preserved in 70 % ethyl alcohol. Biological material was screened and identified to the possible lowest taxonomical category using stereoscopic and optical compound microscopes and specialized literature (Fauchald, 1977; Uebelacker and Johnson, 1984; Amaral and Nonato, 1996).
The frequency and abundance of polychaetes species were used as community descriptors. The Shannon-Wiener and Pielou ecological index were used to calculate diversity (H') and evenness (J'), respectively. The data from each studied zone were grouped by sampling campaigns and analyzed using multivariate ordination methods in the PRIMER software v6.0. The relative spatial-temporal variations were evaluated by Bray-Curtis similarities, calculated by zones at each sampling date, and based on the abundance data following square root transformation. Data were subsequently analyzed by non-metric multidimensional scaling (MDS) ordination.
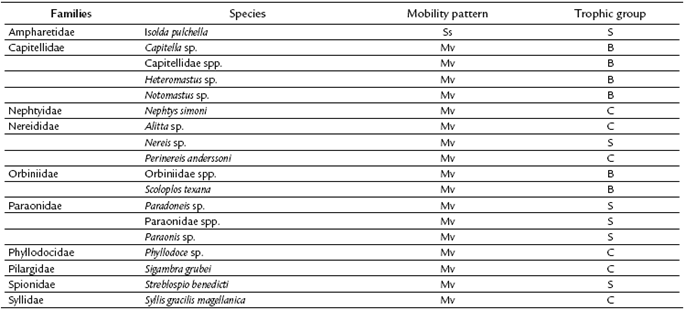
The classification of trophic guilds was based on Fauchald and Jumars (1979) and Jumars et al. (2015), which classified polychaetes into five trophic groups according to their diet (carnivores (C), herbivores (H), surface deposit-feeders (S) sub-surface deposit-feeders (B), and suspension-feeders (F)) and mobility patterns (mobile (Mv), discreetly mobile (Dm), and sessile (Ss)). The trophic group importance index (IT) was applied to each trophic group (Paiva, 1993).
RESULTS
Dissolved oxygen ranged from 3.2 mg/L (November) to 7.5 mg/L (July) during the sampling period. Salinity was higher in January (30) and lower in March (11) compared to the other sampling months. Dissolved oxygen and salinity were higher in samples collected in the dry season than in those in the rainy season. The water temperature varied from 25.7 °C (November) to 27.8 °C (March). The substrate was predominantly characterized as clay and silt with high organic matter content (Table 1).
Table 1 Granulometric composition and organic matter content in the sediment of Quebra Pote mangrove, Maranhão, Brazil.
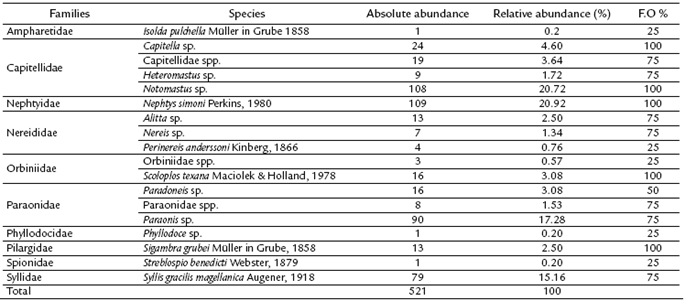
A total of 521 organisms, distributed among ten families and 15 species, were identified in the Quebra Pote mangrove. The most abundant species were Nephtys simoni, Notomastus sp., and Paraonis sp., which together accounted for 58.92 % of all samples. The least represented species were Isolda pulchella, Phyllodoce sp., and Streblospio benedicti. The species Capitella sp., Notomastus sp., N. simoni, Scoloplos texana, and Sigambra grubei were present in all samples (Table 2).
Table 2 Absolute abundance, relative abundance, and frequency of occurrence (F.O) of polychaete species as a function of the total number of sampled specimens during the sampling campaigns (n = 4) in the Quebra Pote mangrove.
The Quebra Pote mangrove showed diversity (H'), and evenness (J') values corresponding to 2.19 and 0.76, respectively. The group of deposit-feeders, consisting of surface deposit-feeders (IT = 5.187386) and sub-surface deposit-feeders (IT = 4.812184), showed the highest trophic importance in the trophic guilds. These groups were dominant in relation to mobility patterns (Table 3).
Table 3 Families identified and their respective mobility patterns and trophic groups (C = carnivores, B = sub-surface deposit-feeders, S = surface deposit-feeders, Mv = mobile, and Ss = sessile).
The following species were present in all mangrove areas: Alitta sp., Capitella sp., Capitellidae sp., Heteromastus sp., N. simoni, Notomastus sp., Paradoneis sp., Paraonidae spp., Paraonis sp., S. texana, S. grubei, and Syllis gracilis magellanica (Fig. 2).
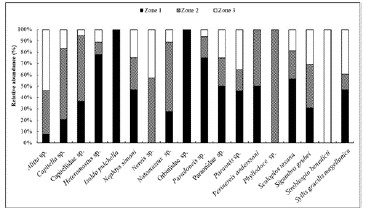
Figure 2 Spatial distribution of polychaete species in the study area, Quebra Pote mangrove, Maranhão.
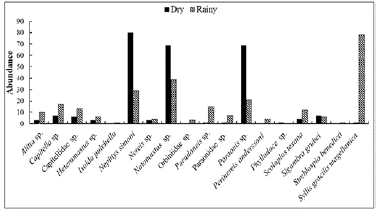
The highest abundance of polychaetes was observed in the rainy season. However, some species were more abundant in the dry season, such as N. simoni, Notomastus sp., and Paraonis sp.; the species Capitella sp., Paradoneis sp., and S. gracilis magellanica. We observed that Lumbrineris sp. and Lumbrineriopsis sp. were found only during the dry season, and P. anderssoni only in the rainy season (Fig. 3).
No significant differences were observed in the spatial-temporal distribution of polychaetes. The nMDS plots did not show clear correlations between zones or sampling dates (Fig. 4a, b). The total abundance of polychaetes decreased from the lower mesolittoral to upper mesolittoral areas (Fig. 5a). Among the most abundant species (N. simoni, Notomastus sp., and Paraonis sp.), only N. simoni followed this distribution pattern (Figure 5b). It is noteworthy that Notomastus sp. was associated with an area dominated by R. mangle, particularly in the intermediate mesolittoral (Zone 2), while N. simoni was associated with an area where Avicenniaschaueriana (Stapf and Leechm 1939) was present in the lower mesolittoral (Zone 1) (Fig. 5b).
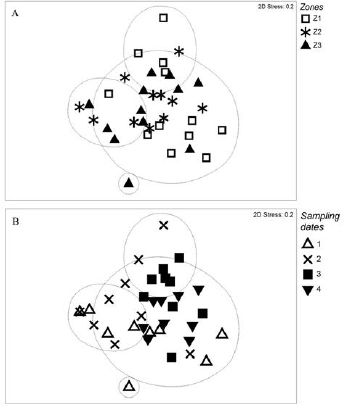
Figure 4 Ordination of mesolittoral zones in the Quebra Pote mangrove in Maranhão obtained from the MDS analysis and according to the similarity of Bray-Curtis.
DISCUSSION
The values of abiotic parameters were constant in the studied periods, except for salinity, which decreases in the rainy season. Similar results were observed by Oliveira and Mochel (1999) in a study about the macro-endofauna of the Parnauaçu mangrove located in the Maranhão coast where salinity showed large variations (between 12 and 30).
Salinity did not influence the abundance and diversity of benthic organisms in the present study unlike reports by Barros et al. (2008) of high abundance of Nereididae species in areas with low salinity in the Paraguaçu estuary. The contents of organic matter and predominance of silt and clay characterized the Quebra Pote mangrove. A study of the macrofauna living in unconsolidated substrates in the Santa Cruz channel (PE) showed that the type of sediment and salinity were important abiotic factors to the structure of macrofauna: wherein the area with the finest sediment showed greater polychaete abundance and density compared to the other studied macrofauna taxa (Paiva et al., 2005). The predominance of silt and clay in our study differed from other Amazonian mangroves. In the Caeté estuary in Pará, the sediment was characterized by fine sand and the species Namalycastis terrestris, Nephtys fluviatis, Mediomastus sp., and S. grubei were abundant (Silva et al., 2011).
In this study, the most abundant polychaetes were N. simoni, Notomastus sp., and Paraonis sp. According to Blake (2000), Capitellidae species such as Notomastus sp., are common in unconsolidated substrates and tolerant to wide variations in temperature and salinity. Such adaptations explain why Notomastus sp. was the most abundant species in Zones 2 and 3. These zones, which are located further away from the waterline and exposed to domestic sewage, represent the most stressful areas for organisms.
Most species of Capitellidae and Paraonidae are more opportunistic than organic deposit-feeders and non-selective feeders with short cycles of reproduction and recruitment. These characteristics probably favor greater abundances in organically enriched environments (Fauchald and Jumars, 1979; Saleh, 2012; Jumars et al., 2015).
The values of diversity and evenness were relatively high in the Quebra Pote mangrove, which would be expected in an estuary impacted by the discharge of domestic sewage that is inadequately treated. It is noteworthy that domestic sewage contained many toxic compounds (such as metals, oil and lubricants, pharmaceuticals, detergents, and others), which negatively affect the biota (Ugland et al., 2008; Dauvin and Ruellet, 2009). However, the organic enrichment from the discharge of untreated or inadequately treated domestic sewage leads to an increase in abundance and density of opportunistic polychaetes (Pearson and Rosenberg, 1978).
The observed ecological indexes (Shannon-Wiener and Pielou) were higher than those reported in areas with sewage discharge on the coast of Romania (Surugiu and Feunteun, 2008), and those found in other mangroves along the Brazilian coast (Aviz et al., 2009; Pires-Vanin et al., 2011; Garcia et al., 2014). In this study, the diversity was low in areas dominated by R. mangle. A similar situation was observed in the Rhizophora belt in an impacted area in Kenya, where the reduction in polychaete diversity was accompanied by an increase in the dominance of some species such as Perinereis vancaurica and Mediomastus sp., which are tolerant to organic enrichment (Penha-Lopes et al., 2013).
Mobile deposit-feeder polychaetes were abundant in this study, corroborating the hypothesis that unconsolidated sediments are more likely favorable environments to depositfeeder and suspension-feeder species (Snelgrove et al., 1997). The pattern of deposit-feeders found in the Quebra Pote mangrove was similar to that described by Barroso et al. (2002) in the Todos Santos Bay in Northeastern Brazil. The dominance of depositivorous species increases bioturbation activities, contributing to the exchange of material between the water column and sediment, as well as with deeper sediment layers (Sivadas et al., 2013).
Alitta sp., Capitella sp., Heteromastus sp., N. simoni, Notomastus sp., Paradoneis sp., Paraonis sp., S. grubei, and S. gracilis magellanica were observed in all mangrove zones. However, N. simoni was the most predominant species in Zone 1, and Notomastus sp. was the most predominant in Zones 2 and 3. This pattern of distribution can be related to disturbances because contaminants in polluted environments may also be responsible for conditioning the structure of benthic communities (Dauvin and Ruellet, 2009).
Sigambra grubei showed the highest density at the sites closest to the river mouth in the estuary of Caeté River in Pará due to increased salinity (Rosa Filho et al., 2006). The spatial distribution of macrofauna especially that of polychaetes, is determined by sediment grain diameter, salinity, sediment composition, and organic matter content (Omena and Amaral, 1997; Neves et al., 2013). However, abiotic factors, predation, and competition are also responsible for spatial and temporal variation (Day et al., 1989, Fanjul et al. 2011).
In this study, the greatest abundance of species occurred in the rainy season. Similar results were found in an estuary on the coast of Pará showing high macrofauna abundance and diversity during the rainy season, accompanied by low values of salinity and organic enrichment (Aviz et al., 2012). According to Rosa Filho and Aviz (2013), increased precipitation and low salinity in estuaries act positively on the benthic macrofauna, increasing the density, biomass, and diversity of tolerant species.
It is likely that Notomastus sp. is associated with the prevalence of R. mangle, mainly in the intermediate mesolittoral. Six species of polychaetes, including N. latericeus, were reported in association with R. mangle trunks in a mangrove forest in the State of Pará (Aviz et al., 2009).
It is noteworthy that vegetation can serve as a natural refuge for reproduction and development, as well as a food source, for a wide variety of animals, including polychaetes (Schaeffer-Novelli, 1995).
The diversity and abundance of polychaetes were greater in Zone 1 compared to the other zones in the study, with a dominance of A. schaueriana. Polychaete abundance was reduced in Zone 3 compared to Zone 2. Rhizophora mangle was the dominant species in these zones. According to Lana and Guiss (1991), the most vegetated areas generate high spatial heterogeneity and increased niches diversity, which affects the structure of the benthic community. Some polychaete species tend to dominate these areas using the rooting structures of trees as shelter and physical support (Lana and Guiss, 1992). Areas dominated by R. mangle usually have a great diversity of polychaete species (Metcalfe and Glasby, 2008). Nevertheless, in this study, regardless of the large population of R. mangle in Zone 3, the presence and influence of domestic sewage in this zone probably supplanted the effect of vegetation, resulting with low abundance and diversity of polychaetes. Local disturbances or increased stability in environmental conditions in the region, closest to the infralittoral and tidal variations, could explain this distribution pattern (Garcia et al., 2014).
CONCLUSIONS
Our results show that the abundance of polychaetes in the Quebra Pote mangrove does not follow a gradient in the intertidal zones. The polychaete fauna in this mangrove is very heterogeneous showing high values of diversity, richness, and evenness. In addition, this fauna is represented by species belonging to a variety of trophic groups, with deposit-feeders being the most abundant.
These results contribute to the understanding of the polychaete population structure in mangrove substrates in the Amazon coast (State of Maranhão) considering the complexity of mangrove formations; they indicate a possible association between polychaete communities and the formation of mangrove vegetation. Nevertheless, future detailed studies on polychaete-mangrove associations could improve the understanding of these distribution patterns considering the complexity of mangrove formations present in the Amazon coast.