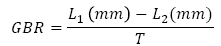Two main processes drive energy allocation in an individual: growth and reproduction (Lardner and Loman, 2003). Growth pattern influences life history traits such as age of sexual maturity, fecundity, and survival, all of which influence fitness (Andrews, 1982; Tomasevic-Kolarov etal., 2010). Slow growth results in late sexual maturity, greater life expectancy, and greater success in some aspects of reproductive biology like defending territory and hatchling survival, leading to larger offspring or clutches; in contrast, fast growth is associated with early sexual maturity, frequent reproduction and low survival rates of adults (Colbert et al., 1998).
Growth rates in ectotherms such as reptiles are strongly affected by abiotic factors like temperature (Gotthar, 2001; Angilletta and Dunham, 2003; Angilleta et al., 2004) and humidity (Sears and Angilleta, 2003). Growth rates and pattern have been studied extensively in lizards, particularly in those species distributed at lowland and/or template latitudes (Van Devender, 1978; Alfredo et al., 1998; Caley and Schwarzkopf, 2004; Sears, 2005; Ortega et al., 2007; Pandav et al., 2010; Robbins, 2010), and studies of highland species are restricted to those of temperate environments, which exhibit strong seasonal fluctuations (Lemos-Espinal and Ballinger, 1995; Tomasević-Kolarov et al., 2010; Guarino et al., 2010). Lizards inhabiting cold environments (such as those observed at high elevations) are expected to have low growth rates as a consequence of activity constraints due to thermoregulatory limitations, and also due to low food availability (Adolph and Porter, 1996; Shine, 2005). However, growth rates may be high if physiological adaptations such as low metabolic rates (which implies low energy consumption), occur in these environments, as has been observed in Sceloporus graciosus (Sears, 2005). Higher growth rates may also be a consequence of higher productivity associated with high precipitation levels (Iraeta et al., 2006).
The northern (tropical) Andes are characterized by extreme daily fluctuations in temperature, including very cold conditions, and this fluctuation is greater than that observed between dry and wet seasons (Sarmiento, 1986). Daily fluctuation in temperature has a direct effect on organism behavior and physiology (Navas, 1996), and surely also determines food resource availability, affecting individual growth rates directly. Nevertheless, these phenomena have not been studied in lizards inhabiting the northern Andes. In this study we describe the growth rates and pattern in a population of Anadia bogotensis collected from a subparamo locality and reared in captivity. A. bogotensis is an endemic species of the Andean forests and paramo ecosystems of the Cordillera Oriental mountain system in Colombia, distributed between 2000 and 4100 m (Jerez and Calderón-Espinosa, 2014). These lizards are small, diurnal and semifossorial, and exhibit sexual dimorphism, with males reaching a maximum body size (snout vent length) of 67.4 mm and females 61.2 mm; females may produce up to three clutches during the reproductive season, which seems to be continuous, and individuals of this species feed on coleopterans, orthopterans, dipterans, gastropods and arachnids (Clavijo and Fajardo, 1981, Jerez and Calderón-Espinosa, 2014). This is the first study that evaluates growth rates and pattern in a Gymnophthalmid lizard.
We collected 32 eggs from communal nests of Anadia bogotensis, in Las Moyas locality (N 4° 39' 45.612'' and W74° 0' 58.139''; datum WGS 84), in the municipality of La Calera, Cundinamarca, Colombia, at 2800 m, in August 2012 and July 2013. Eggs were transported to the city of Bogotá (2600 m a.s.l.), and incubated outdoors in glass terrariums (350 x 280 x 530 mm) that were exposed to environmental conditions. Bogotá is within the species' range, and is only 6 km from the Las Moyas locality. The mean temperature of Bogotá between August 2012-July 2013 was 14.09 °C (-3.57-27.24 °C), and mean humidity was 38.08% (3- 89.9 %) (IDEAM, 2018). The eggs were slightly covered with soil and leaf litter obtained from the natural nests at Las Moyas. Soil humidity was maintained by spreading water on the soil substrate every two days. Afterwards, hatching individuals were moved to other terrariums. We measured SVL and tail length of hatchlings by extending the lizard on graph paper with 1mm divisions, while weight was recorded with a digital balance (0.1 g precision). We repeated these measurements every seven days, during a year and a half. The lizards were fed every two days with spiders, opilions and crickets covered with powdered calcium, and water was provided with the same frequency by aspersion on the terrarium walls. Each hatchling was marked with enamel of different colors on the back and tail; we checked this mark continuously, marking each individual again if necessary. We did not separate individuals by sex, given that most of the lizards died young, before their sex could be identified with confidence.
We evaluated the best fitting model of growth pattern by evaluating the variables of body size (snout vent length and weight) and time, in non-linear logistic and Von Bertalanffy models as implemented in RWizard software (Guisande et al., 2014). To explore the relationship between growth rate and body size, we estimated growth rate per individual by using the following equation (Zamora et al., 2012):
Where GBR =growth body rate, L1 = initial SVL of lizards, L2 = final SVL, T= number of days from hatchling to death. Growth rates are then given in mm/day for each individual.
The lizards hatched at 24.9 ± 2.6 mm (SVL), and with an initial body weight of 0.31 ± 0.07 g (n=32). Individuals kept in captivity conditions during the study period had differential survivorship. Most individuals died during this study and survival time was between 1 and 19.75 months. Some individuals from the first group of hatchlings (eggs collected on August 2012) lived up to 19.75 months, while those from the second group (eggs collected on July 2013) lived up to 10 or 12 months; the lizards died at different times during their captivity, with some of the first cohort living longer. Most lizards died very young: 37.5 % of the individuals died after five months, and only three lived more than 10.5 months. Maximum body size (SVL) recorded for individuals were 89 and 97 mm, respectively, and corresponded to a female and a male respectively; individuals in this study reached larger body size than that recorded previously for individuals living in the wild (Clavijo and Fajardo, 1981). Given that in captivity lizards are released from predation pressure, this could explain the maximum body size observed. Some individuals survived for a very short period of time (the shortest survival time was four weeks). Exclusion of three individuals (the oldest) improved the model fitting of the growth data. We are aware that our data could be biased toward young adult individuals (those below 63 mm SVL), and that including older individuals would be necessary to characterize growth when lizards approach their maximum body size. Growth data of these young adult lizards followed a logistic pattern with both SVL (R2=0.91, all parameters with p < 0.001) and body weight (R2=0.81, all parameters with p < 0.001) as the dependent variable (Fig. 1). This means that initial growth of the lizards is moderate, followed by a period of fast growth, and according to Andrews (1982), this fast growth decreases when 50 % of the asymptotic body size is reached. Asymptotic size was estimated as 76.7±3.8 mm and 3.6± 0.71 g, which means that growth rates decelerate around 38 mm or 1.8 g, which is interesting since this SVL is close to the size suggested as the sexually mature body size for this species under wild conditions (Clavijo and Fajardo, 1981).
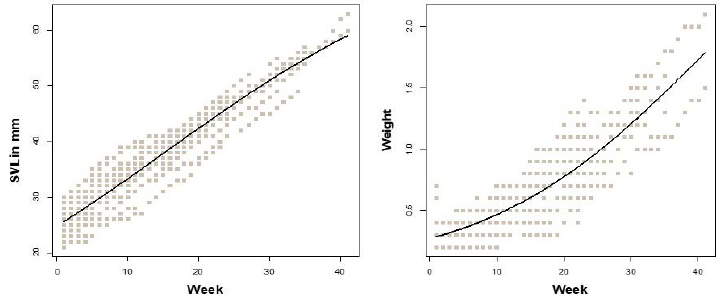
Figure 1 Logistic by length (A) and mass (B) growth pattern of Anadia bogotensis. Function parameters for logistic by length (b=0.78± 0.06, c=0.047±0.002) and logistic by mass (b=2.5± 0.19, c=0.06±0.004).
Growth pattern data in small species of squamate reptiles seem to be better fitted by logistic by mass models (Avery, 1994; Adolph and Porter, 1996); similarly, in A. bogotensis, a small lizard, it was the logistic by mass and logistic by length models that best fit the data. Individual growth rates fluctuated between 0.03 - 0.13 mm/day (per individual estimated over the whole survival period). Unfortunately, we do not have data on growth rates for other highland Andean lizard species to compare, but given the lifespan of these individuals (see below), we suggest these growth rates are higher than expected under fluctuating highland weather conditions.
However, captivity conditions affect growth rates by means of the higher and constant provision of food and water, it is a higher intake means higher growth rates (Sears and Angilletta, 2003). Meanwhile, a higher supplement of water results in higher growth rates but if individuals allow thermoregulating (Stamps and Tanaka, 1981; Lorenzon et al., 1999). Then, to know the effect of food and water supply during captivity on growth rates in A. bogotensis requires to describe the growth rates in the wild.
The largest (which were also the oldest) individuals died apparently by natural causes, so it seems that the lifespan of individuals of this species is slightly over a year and a half. Thus, this species can be considered as a fast growing lizard, which reaches sexual maturity at around the half of its maximum body size and with a lifespan below two years. Additionally, females of this species reproduce frequently (Clavijo and Fajardo, 1981, Jerez and Calderón-Espinosa, 2014). We consider that our data represent how lizards of A. bogotensis grow under captivity, and that potential differences in growth rates with individuals raised in the wild would be a consequence of differences other than the thermal regime. However, growth in the wild should be described to evaluate this hypothesis.