INTRODUCTION
The degradation of coral reefs is a global phenomenon, both in populated and unpopulated human areas, largely due to diseases, algal blooms and anthropogenic impacts caused, mainly, by tourism (Eastwood et al., 2017). Because of this, many Caribbean ecosystems have lost important characteristics and functions as a result of the reduction in biodiversity (Mora et al., 2016). However, this loss, almost always reported for taxonomic species, has caused the role of the structural complexity of coral reefs to be underestimated (Alvarez-Filip etal,. 2015). For example, some aquatic activities, which sometimes involve the resuspension of the seabed sediment, have been reported as an important factor in the deterioration of key groups sensitive to sedimentation such as massive scleractinian corals, which play a fundamental role in the morphological and functional stability of reef complexity (Castro-Triana and Pereira-Chaves, 2016).
The archipelago of San Andrés, Providencia, and Santa Catalina accounts for more than 75 % of the coral reefs of Colombia (Díaz et al., 2000). However, the impact of anthropogenic activities, mainly, has induced the replacement of living coral cover with communities of algae and inert substrates (Geister, 1973; Garzón-Ferreira and Rodríguez-Ramírez, 2010).
San Andrés Island is an important tourist destination in the Caribbean and in the last few decades the number of people engaging in diving activities there has increased (James-Cruz and Márquez-Calle, 2011). After the island was declared a free port in 1953, there was an increase of visitors and immigrants, leading to changes in the socio-economic dynamic, going from subsistence fishing to commerce and tourism (Santos-Martínez et al., 2009). This brought with it excessive use of the resources from the coastal ecosystems of the island. These anthropogenic causes produced notable unfavorable changes in the complexity of the coral structure (e.g., low reef architecture), in addition to those arising due to natural causes (Alvarez-Filip et al., 2011). For that reason, efforts have been made to protect coral reefs through Marine Protected Areas (MPAs). These MPAs not only conserve ecosystems but also ensure the production and maintenance of commercial fish stocks, such as snappers, which are an island fishing resource (Santos-Martínez et al., 2009; Sala et al., 2017). In Colombia, the archipelago is a Biosphere Reserve called Seaflower recognized by UNESCO in 2000 and contains MPAs. It was implemented as a conservation and protection system to counteract the impact of intensive tourism, pollution, coastal infrastructure and the uncontrolled extraction of resources (Guerra-Vargas and Mancera-Pineda, 2015; CORALINA, 2016).
Currently, the coral ecosystems of the windward zone of the island receive impacts due to bathers, hotel infrastructure, docks, and ports. While, the reefs of the leeward zone are mostly affected by activities such as diving and wastewater discharge (James-Cruz and Márquez-Calle, 2011; Abdul azis et al., 2018).
Therefore, this study sought to establish the morpho-functional structure of the leeward reefs of the island of San Andrés and if there is a relationship with snappers. Additionally, (1) it was evaluated if there was a change in the structure of the reefs in the last decades and (2) categories of functional sensitivity of the corals was established. We established three main predictions of the current structure of the leeward reefs of the island: 1) Continuous and growing anthropogenic intervention in the reefs of the leeward zone of the island of San Andrés, would produce a change in the coral coverage, where the corals resistant to sedimentation and those of rapid growth would dominate. 2) The density of macroalgae cover would increase, compared with other studies due, among other reasons, to the loss of coral cover. 3) Density of snappers would be expected to be low due to the lack of coral complexity.
MATERIALS AND METHODS
Study area
San Andrés Island is located to the northwest of Colombia (12°28'-12°36' N; 81°40'- 81°44' W) approximately 240 km off Nicaragua and 800 km off Cartagena, Colombia (IGAC, 1986). Its climate is characterized by a unimodal pattern with an annual average rainfall of 1900 mm, dry season between February and April with an average rainfall lower than 50 mm and a rainy season between June and December with average rainfall is 150 mm. The average annual temperature is 27.4 °C (González and Hurtado, 2012). Marine currents are mainly directed towards the northeast (Geister, 1973).
Sample collection
We surveyed sampling sites both during dry and rainy seasons of 2013 and 2014, in the western leeward area of San Andrés Island, within the Seaflower Biosphere Reserve. We made five transects in three sites: Luna Verde (LV) in the extreme southern zone (12°29'7" N, 81°44'2" W), Wild Life (WL) in the south-center (12°30'30" N, 81°43'47" W) and Bajo Bonito (BB) in the northern zone (12°35'11" N, 81°42'59" W). The environmental variables of temperature and depth were measured. Previous studies have shown that these variables influence the structure of the reefs of San Andres Island (Sierra-Rozo et al., 2012; Abril, in press). Data used for this study were obtained from the Santos-Martinez, (2012) research project.
Fishes and coral reefs
Field research was endorsed using taxonomic guides and participation of experts to reduce uncertainty regarding size and abundance estimation. To estimate the abundance of Snappers species, visual censuses were carried out on sixty transects each of 50x2 m, using diving equipment (Sierra-Rozo et al., 2012; Abril, in press). Taxonomic keys of Greenberg (1986, Chaplin and Scott (1972), and Humann, (1996) were used. Seven size classes of 10 cm were used for the estimation of body length. Subsequently, size classes were discriminated in juveniles and adults, depending on maturity size described for each species in Hawkins, (2007), Claro and Lindeman, (2008) and Froese and Pauly, (2017).
Corals were sampled using sixty 50 x 0.6 m video transects. The form was estimated by the type of growth and the distribution of the hard coral, soft coral, and other biota was determined. Transects were located randomly in each area, at 10 m each (CARICOMP, 2001). Videos of the transects were also made, which were later analyzed, specialized researchers.
The cover was determined at coral species level when possible and was also classified into morpho-functional categories (CARICOMP, 2001; Abril, in press). The morpho-functional categories were: finger corals (FinC), fleshy corals (FleC), massive brain corals (BraC), leaf corals (LeaC), encrusting corals (EncC), pillar corals (PilC), branching corals (BranC), great star corals (StaC), submassive corals (SubC), flower and cup corals (F&C), hydrocorals (Hyd), octocorals (Oct), algae (Alg), sponges (Spo), organisms (Org) (ascidians, forams), seagrasses (SeaG), bare rubble (BARr), bare sediment (BARS) calcareous substrate (SCa) and other (Othe).
Based on the works of Nugues and Roberts, (2003) Mora et al., (2016), among others; we define a classification of functional sensitivity of corals to bleaching, fragmentation, and sedimentation. The following groups were established: corals susceptible to fragmentation (CSF) included LeaC, PilC, BranC and FinC; corals resistant to fragmentation (CRF) comprised of StaC and SubC; corals susceptible to sedimentation (CSS) composed of StaC, BraC, and SubC; and corals susceptible to bleaching (CSB) constituted by BranC, FinC and Hyd
Also, we performed the comparison of our data with coral coverage studies of Garzón-Ferreira and Rodríguez-Ramírez, (2000) and Rodríguez-Ramírez et al., (2005).
Numerical analysis
Reef cover data was transformed to relative abundance by transect and density ind/500 m2. Some relationships between the variables were calculated to identify deterioration indexes: total coral cover/encrusting coral cover (CTC/EncC), algae/ coral (Alg/COR), coral susceptible to sedimentation/ coral susceptible to bleaching (CSS/CSB). Shapiro-Wilk normality test was performed to select parametric or non-parametric methods. Spatial and temporal ce-diversity was calculated as the effective number of species: N1= exp (H'), where H' is the Shannon entropy index (Jost, 2006). W hittaker's beta diversity was calculated: 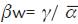 , where
, where 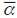 is the average number of species per unit obtained from a sample of n units within an area and ү is the total number of species for this area (Whittaker, 1960). This latter index indicates the replacement rate as a percentage. A non-metric multidimensional scaling (nMDS) was performed with the similarity index of Bray-Curtis (Shepard, 1962a; Shepard, 1962b; Kruskal, 1964), using the percentage of difference as a measure of similarity (Bray and Curtis, 1957), with the purpose of establishing possible spatial variations in the structure of reefs. The possible relationship between the structure of the reef cover and environmental variables (including snappers as an indirect predictor variable) were standardized and evaluated with a canonical correspondence analysis (CCA) (Terbraak, 1986). Analyzes were performed using Excel 2013, Past 3 and PRIMER V6.
is the average number of species per unit obtained from a sample of n units within an area and ү is the total number of species for this area (Whittaker, 1960). This latter index indicates the replacement rate as a percentage. A non-metric multidimensional scaling (nMDS) was performed with the similarity index of Bray-Curtis (Shepard, 1962a; Shepard, 1962b; Kruskal, 1964), using the percentage of difference as a measure of similarity (Bray and Curtis, 1957), with the purpose of establishing possible spatial variations in the structure of reefs. The possible relationship between the structure of the reef cover and environmental variables (including snappers as an indirect predictor variable) were standardized and evaluated with a canonical correspondence analysis (CCA) (Terbraak, 1986). Analyzes were performed using Excel 2013, Past 3 and PRIMER V6.
RESULTS
Cover of benthic organisms and non-living substrate
A total of 60 cover morphotypes were found, between benthic organisms and non-living substrate, which were grouped into 20 morpho-functional categories. Alg was the most abundant category, followed by SCa and Oct (Fig.1). By morphotypes, macroalgae (MaAlg), calcareous pavement (CALP) and sand sediment were the most abundant; others registered areas of less than 10 %. All sampling sites were dominated by algae cover (226 ind/500 m2), while all corals recorded very low cover (117 ind/500 m2), only SubC and EncC had cover greater to 12 ind/500 m2 in all sites. Total percentage of coral was mainly correlated with EncC (r= 0.76; p<0.001), SubC (r= 0.72; p<0.001) and pinnate branching octocoral (r= 0.81; p<0.001). Significant correlations were found among relative abundances using only corals; direct between CSF and FinC (r= 0.85; p<0.001) and inverse between Oct and CRF (r= -0.92; p<0.001) and; Oct and Alg/COR (r= -0.6; p<0.001).
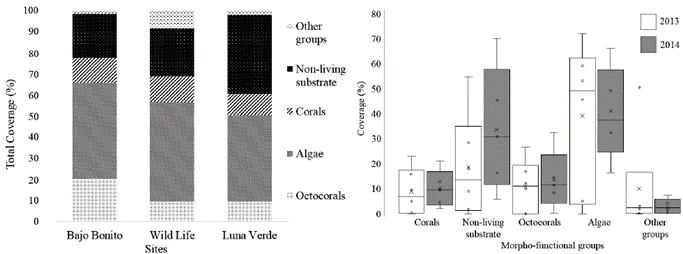
Figure 1 Cover percentages ofthe most abundant morpho-functional categories in the leeward reefs ofthe island of San Andrés by years (right) and sampling sites (left). White box= 2013, Grey box= 2014; Other groups = invertebrates, sea grasses and others.
CSS were correlated with Orbicella franski (r= 0.5; p<0.001), articulated calcareous algae (ArtCAlg; r= 0.5; p<0.001), ball-shaped sponges (BALS; r= 0.6; p<0.001) and dead coral (DEAC; r= 0.60; p<0.001). While the CSBs were correlated with the FinC (r= 0.6; p<0.001).
In general, the structure of coral diversity (N1) in the leeward zone of San Andrés is similar throughout the island, with low species turnover (BB= 9.37; LV= 8.77; WL= 10.3) and there is a low turnover between sites (βwLV= 2.34; (βwWL= 2.19; βwBB= 2.03). However, a greater difference (distance) was observed between the density of organisms of WL and LV, while BB had similar density with the other sites (Fig.2).
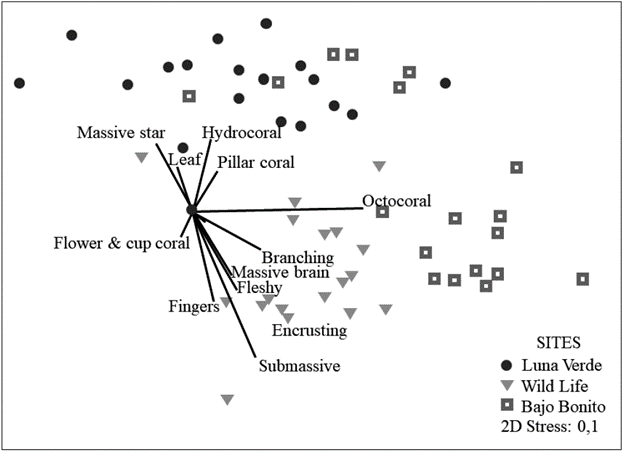
Figure 2 Bray-Curtis based non-metric multidimensional scaling (nMDS) of the benthic reef organisms at the three sampling sites. The relative contribution of the morpho-functional categories of corals to the variation in the structure by sites using Spearman correlation.
Between years, species richness was similar [N1 (2013) = 11.5; N1 (2014) = 9.06], without appreciable variations in beta diversity (βw2013= 2.10; βw2014= 2.14). MacAlg and ArtCAlg increased, while crustose coralline algae (CruCAlg) and turf algae (TurAlg) decreased between 2013 and 2014. During this period, CALP and Oct also increased.
Regarding environmental variables, Wild Life site was the deepest, followed by Bajo Bonito and Luna Verde. The temperature was similar at all the sites with minimum variations from the average of 0.4 degrees. Regarding climatic periods, the dry season was slightly warmer (mean= 27.6 ± 0.37 SD) than the rainy season (mean= 27.5 ± 0.49 SD). The temperature correlated slightly with the abundance of adult Lutjanusapodus (r= -0.23; p<0.07) and with the depth of juvenile Ocyurus chrysurus (r= -0.24; p<0.06).
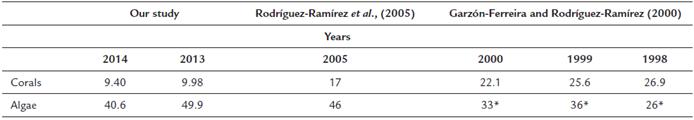
Finally, we observed in the comparison of the coverage of live coral (hard coral) and algae, that since the year 1998 there had been a decrease in live coral cover and an increase in algae (Table 1).
Fishes
A total of 96 Lutjanids belonging to two genera and four species were registered, 69 % were juveniles, and 31 % were adults. L. apodus (5.8 ind/500 m2) and Lutjanus mahogoni (1.8 ind/500 m2) were the most abundant; Lutjanus jocu and O. chrysurus showed the density of 0.2 ind/500 m2 each. L. jocu and O. chrysurus were only observed during the rainy season of 2013 at low abundance. The density of Lutjanids was similar in all the sites; Wild Life (8.75 ind/500 m2), followed by Luna Verde and Bajo Bonito with 7.75 ind/500 m2 and 7.5 respectively. However, the adults of L. mahogoni (r= -0.38) and L. apodus (r = -0.67) were correlated with the axes of the nMDS, indicating that these species have high densities in Bajo Bonito area in octocoral zones, while juveniles have higher density in Luna Verde and Wild Life, areas dominated by calcareous substrate (Fig. 3).
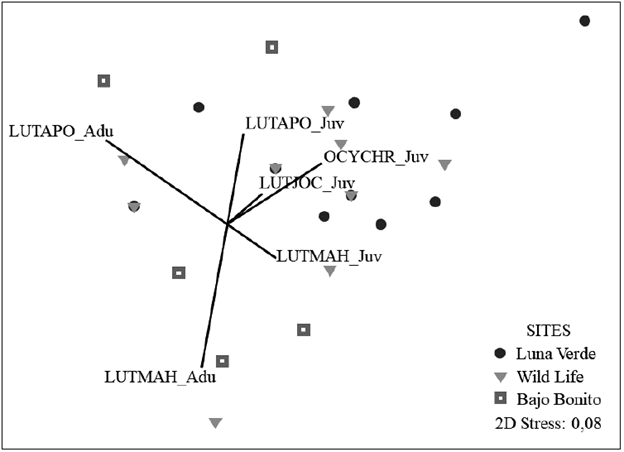
Figure 3 Bray-Curtis based non-metric multidimensional scaling (nMDS) of benthic morpho-functional cover in the leeward reefs of the San Andrés Island. The relative contribution of the Lutjanids found by size to the variation of the structure of the benthic community with Spearman correlation was included. Species and sizes of the Lutjanids: LUTAPO = Lutjanus apodus, LUTJOC = Lutjanus jocu, LUTMAH = Lutjanus mahogoni, OCYCHR = Ocyurus chrysurus; Adu = Adult, Juv = Juvenile.
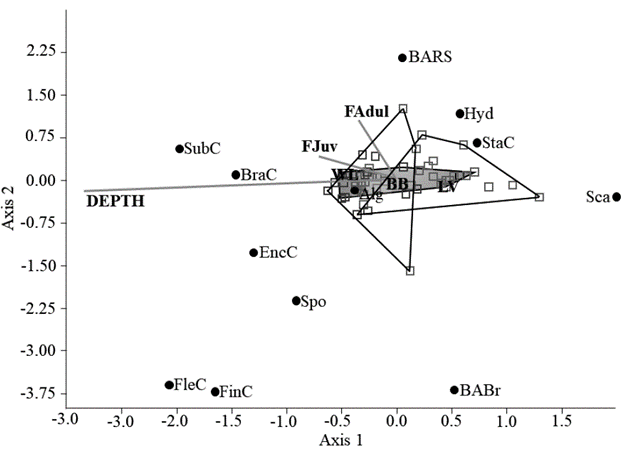
Finally, in the canonical correspondence analysis, a relationship was observed between environmental variables, coral coverage and Lutjanids (Fig.4). Apparently, the SubC and BranC tend to grow deeper, and these were associated with the presence of juvenile lutjanids, while StaC and Sca were found in shallow areas. Mature adults were constant in BARS. Other categories did not show a greater relationship with environmental variables. Depth was a determining variable in the structure of the reef in the studied area, and it had a high correlation with axis 1.
DISCUSSION
Our results indicate that the structure of the reef on the leeward side of San Andrés Island has deteriorated over the last 20 years (Table 1). In this area, only three types of cover dominate 60 % of the reefs: algae, octocorals and inert substrates. Although the taxonomic structure of the corals is like that observed in most tropical Caribbean reefs (Alevizon and Porter, 2015), the functional structure is possibly being altered by anthropogenic actions, mainly aquatic activities that are increasing with tourism.
The increase in macroalgae cover has been considered an indicator of reef degradation (Tanner, 1995; Mccook et al., 2001; Hughes et al., 2007). The death of corals, due to the effects of sedimentation and fragmentation, is one of the reasons for which the cover of macroalgae has increased. Macroalgae have faster growth rates than corals and colonize substrates of dead coral. We found that the algae (Turf algae, calcareous form, crustose coralline algae, and macroalgae) occupy more than 45 % of the reefs, an increase compared to the 40 % observed almost two decades ago (Garzón-Ferreira and Rodríguez-Ramírez, 2000; Garzón-Ferreira and Rodríguez-Ramírez, 2010). Additionally, algae are also favored by the enrichment of the marine waters caused by the wastewaters of the island, which have increased with the growth of the islands resident and tourist population (Gavio et al., 2010; James-Cruz and Márquez-Calle, 2011). This waste is discharged through a marine outfall that drains into the leeward zone of the island. Another factor that favors the proliferation of macroalgae is the low presence of herbivores (Mumby, 2006) and the dominance of coral-eating fish (e.g., some species of Pomacentridae). On the island, the coral-eating fish are one of the dominant groups, and their abundance is increasing due to the low presence of piscivorous fish such as snappers (Sierra-Rozo et al., 2012).
In more than a decade, reefs went from being dominated by stony corals (Garzón-Ferreira and Rodríguez-Ramírez, 2000) to possibly being shaped primarily by octocorals and inert substrates. Apparently, octocorals are more resistant to the constant changes produced by anthropogenic factors. Our results indicate that octocorals are a negative factor about some groups of stony corals. For example, they increased when corals resistant to fragmentation decreased. Octocorals have a great capacity to adapt to environments deteriorated by sedimentation, and they have higher growth rates than other corals (Velásquez and Sánchez, 2015). This may facilitate its colonization of the degraded reefs of San Andres Island.
The decrease in the cover of stony corals around San Andres Island in the last decades has been caused, among other things, likely by the resuspension of particles caused by tourist activities. Stony corals die when their polyps become clogged causing the zooxanthellae to be expelled (Birrell et al., 2005). Also, we observed that these reefs are affected by corals breaking, which manifested itself in many fragments of dead coral on calcareous substrates. This study suggests that the leeward reefs of San Andrés are more exposed to sedimentation because this area has a lower incidence of winds, which is why most of the sites for diving activities were established there (James-Cruz and Márquez-Calle, 2011).
Although the taxonomic structure is similar between sites, the morpho-functional structure is different. It is important to study additional functional features to the taxonomy in the reefs, which will help to identify patterns of coral loss or recovery better; especially for being susceptible to tourists essentially diverse. To the south of the island, snorkeling and diving are common activities, where tourists rarely touch the bottom (LV), while towards the central area (WL), in addition to the previously mentioned activities, tourists also take part in underwater walks (James-Cruz and Márquez-Calle, 2011). Probably the greatest amount of underwater activities is carried out in WL than in LV and BB, which have the most significant effect on massive starlet corals, which are more sensitive to sedimentation (Castro-Triana and Pereira-Chaves, 2016). The presence of stony corals benefits other species, such as juvenile snappers, which tend to take refuge in their cavities (Díaz-Pulido et al., 2004; Sierra-Rozo et al., 2012; Huijbers et al., 2015). This is the reason why there are more juvenile snappers in LV.
We found that coral reef cover in the leeward zone of San Andres has decreased drastically in the last two decades. We estimated that in the last 15 years, 1.2 % of the cover had been lost per year. This indicates that the reefs of San Andrés are going through a process of biota deterioration and alteration of their functioning. Based on this scenario, our results indicate that Agaricia agaricites and Porites astreoides are the species that have contributed most to the degradation process in this area of the island. A. agaricites is a generalist species that can survive impacts, as they have a high colonization rate and a phenotypic plasticity which allows them to adapt to several substrates (Díaz-Pulido et al., 2004; Vidal et al., 2005) and P. astreoides is a coral that is resistant to fragmentation and abrupt changes in the system (Darling et al., 2012).
On the other hand, other species may be at greater risk, because they are more vulnerable, as is the case of Porites porites and Madracis mirabilis, corals susceptible to fragmentation and bleaching (Nugues and Roberts, 2003). These two species are still found in the reefs of San Andres Island, but, if the current impacts persist, in the future they could follow the same path as Acropora palmata. A. palmata occupied 55 % (Díaz-Pulido et al., 2004) of the reefs of the island and currently we only find 0.1 %.
The loss of cover of these and other species implies low structural and topographic complexity which influences the decrease of organisms at important stages of their development (Kimirei et al., 2013; Mora et al., 2016; Richardson et al., 2017). It is important, therefore, to protect not only species, but also coral groups that provide morphological diversity to coral reefs. In this case, the WL site, which has the largest number of coral functional groups, should receive special conservation attention, along with the LV site, which reports snappers at an early stage of development and provides good habitat conditions for the fish. However, a low density of snappers was found in our study (1 ind/ 500 m2), like that found in other Caribbean reefs (Alevizon and Porter, 2015; Alvarez-Filip et al., 2015). However, research on the assessment of fishery resources, traditional and industrial fishing carried out in Seaflower, shows the year-on-year decrease in catches of snappers in both fisheries (Santos-Martínez et al., 2013). Also, the type of fishing with harpoon which is not registered to the authorities on the island and that is carried out in the zones of our study is one of the factors of reduction of snappers. This low presence of snappers can reduce the biological control of herbivorous, which can damage the coral, thus allowing the establishment of algae (Wilkinson, 2000; Weil, 2006).
Moreover, around the island, several important relationships between the benthic components of the reefwere found. Corals were susceptible to sedimentation correlated positively with articulated calcareous algae, sponges and dead coral. This was possibl because: calcareous algae have strategies to avoid herbivory, sponges are more abundant in environments with more nutrients, and dead coral is possibly derived from coral that is sensitive to fragmentation (Barrios et al., 2003; Zea et al., 2007). Therefore, all the above were where there were mainly brain and massive corals, which are more resistant to damage. This shows the importance of finding relationships among all the biotic components of the reef, which can be inferred from the system and about which we still need to know more.
Finally, the environmental variable that influenced the structure of the community was depth. Although the inherent characteristics of the corals influence the depths they occupy (Díaz et al., 2000), it is possible that the frequent presence of tourists in shallow areas, more than in the deep areas of the reef, influences the permanence of the corals due to the negative effects of the anthropogenic intervention. It should be noted that during our study there were no typical environmental events in the Caribbean (storms, hurricanes) that could have influenced the results. Although bleaching and climate change are important factors in the loss of coral, it was not possible to evaluate them in this study.
Due to disturbances that affect the reefs, such as intensive tourism and the other factors described above, and to preserve the biodiversity of the marine area of the archipelago as a tool for the protection of marine ecosystems, the Seaflower protected marine area was created in 2005 (CORALINA, 2016). However, it is necessary that protection regulations are in line with tourism and that the actors that influence them to carry out tourist awareness programs so that their visit will be responsible, especially when the object is to observe the coral reefs.
CONCLUSIONS
San Andres is an island that has experienced several impacts due to the excessive use of its natural resources, especially its marine systems. Nautical activities, overfish, constant contamination from the discharge of wastewater and the disturbances through which it has passed year after year has homogenized the biota existing in these systems. Organisms such as algae (especially macroalgae and crustose coralline algae) and octocorals are dominant in all the reefs of the leeward zone of the island, while other forms of coral growth are in low percentages. Hard coral cover is mainly composed of species that have life strategies that allow them to overcome the unfavorable and generally sudden conditions of the system. The variations in the structure of the leeward reefs of the island seem to be influenced by depth. The snappers were scarce, and although they did not directly influence the coral structure, they are an indicator of coral reef degradation; fishing, and human intervention is probably the most important cause of this low abundance. Tourists have greatly influenced this area since it is a recommended area for diving, a factor contributing to the deterioration and low reef complexity of these ecosystems. It is recommended that changes in the structure of benthic organisms and inert substrates over the years are monitored, taking into account additional factors such as the frequency of coral diseases, fisheries, negative natural events and the frequency of tourists visiting these areas, in order to make an integral monitoring system of this protected marine area effective, which will allow not only protect coral reefs but also, among others, fish of commercial interest such as snappers.