INTRODUCTION
Solid waste landfill leachates are produced when moisture and rainwater infiltrating and percolating through landfill waste mix with the products of its decomposition (Souto, 2009; Barros, 2013). Leachate composition depends on several factors, such as the diversity and characteristics of the dump, the availability of water and oxygen, the way the landfill is operated, and its age (Muzaini et al., 1995; Aziz et al., 2004). Leachate from younger landfills generally exhibit higher concentrations of organic matter, presenting, for instance, high chemical oxygen demand (COD). Metals are often mentioned as part of the leachate composition; however, the metal content in leachates from old landfills (over 10 years old) is generally low, due to the methanogenic fermentation process in the landfill (Bozkurt et al., 1999; Kulikowska and Klimiuk, 2008). Other common compounds are nitrogen (N) and phosphorus (P), nutrients used for biomass growth. Leachates from old landfills are rich in ammoniacal nitrogen derived from the hydrolysis and fermentation of nitrogenous fractions of biodegradable substrates and, thus, have a low concentration of dissolved oxygen (Kulikowska and Klimiuk, 2008; Wojciechowska et al., 2010). It is therefore important to remove ammoniacal nitrogen from this type of effluent, considering its toxicity to many organisms, like plants (Bernard et al., 1996; Clarke et al., 2002; Arunbabu et al., 2017), microalgae (Bernard et al., 1996; Tam and Wong, 1996), crustaceans (Bernard et al., 1996; Valencia-Castañeda et al., 2018), and fish (Randall and Tsui, 2002; Zhang et al., 2018). In addition, the release of this compound into natural water bodies may cause eutrophication, making it also toxic to the aquatic environment (Aziz et al., 2004; Wojciechowska et al., 2010). Clarke et al. (2002) observed that the growth rate of Typha latifolia was reduced when exposed to 200 mg L-1 of ammonia; and Valencia-Castañeda et al. (2018) estimated that the safe levels of ammonia for the shrimp Litopenaeus vannamei at salinities of 1 and 3 g L-1 were 0.54 mg L-1 and 0.81 mg L-1, respectively.
Due to the wide variety oflandfill leachate composition, it is difficult to determine effective treatment techniques, and not necessarily the method adopted for a particular landfill will be applicable to others (Wojciechowska et al., 2010). Among the methods used to treat urban landfill leachate, it is included physical, chemical and biological treatments. The most common biological treatment methods are the use of microbes (Mehmood et al., 2009; Zhang et al., 2016), and by constructed wetlands (Bulc, 2006; Yalcuk and Ugurlu, 2009; Ogata et al., 2018; Silvestrini et al., 2019). However, when the concentration of ammoniacal nitrogen is very high, direct biological treatment is not recommended, since, as already mentioned, in high concentrations this compound is toxic and inhibits the nitrification process (Aziz et al., 2004).
Conventional physical and chemical methods of effluent treatments usually have a high cost considering the treatment itself, as well as the construction and maintenance of the stations (Mojiri et al., 2013; Pandey et al., 2016). As an alternative, the use of aquatic macrophytes acts as a non-invasive and low cost method for the treatment of effluents. These plants are known for their ability to remove nutrients and for creating favorable conditions in the environment for organic matter degradation (Goldoni et al., 2014). A promising method is the use of floating emergent macrophytes, where plants growin a hydroponic environment, artificially floating in rafts rather than being rooted in the sediment (Headly and Tanner, 2012; Martelo and Borrero, 2012). This method offers the advantage of composing a system of simple operation and low maintenance to the treatment of effluents. Other advantages include the non-generation of sludge, the reduction of energy consumption by the improvement of solar energy, the disappearance of bad odors by their oxygenation, and also, it is not necessary to trim the plants, because the floating system naturally regenerates and the compounds incorporated to the plant return to water by the processes of decomposition of the vegetal tissue (Arcadia Ingeniería del Agua, Aire y Suelo, 2019). Besides, it has been understood that the assimilation of nutrients and other elements, such as metals, may be greater in this type of system when compared to systems in which the plants are rooted in the sediment (Headley and Tanner, 2012). This happens because the roots of the plants are not in contact with the benthic sediment or soil, and thus, they are in contact only with the nutrients limited in the water column (Kadlec and Wallace, 2008). In fact, Zhang et al. (2014) obtained better results for BOD5, COD, nitrate, total nitrogen and total phosphorus in floating macrophyte systems compared to other constructed wetland systems.
Typha species are efficient plants for the removal of ions from effluents compared to other macrophytes due to their aerated internal structure formed by tissues with open spaces, allowing better absorption of pollutants, optimum evapotranspiration, and the transport of oxygen from the atmosphere to the leaves and from the leaves to roots and rhizome, besides its great tolerance to toxic agents (Mannarino et al., 2006; Hegazy et al., 2011; Barros, 2013; Mojiri et al., 2013). Song et al. (2018) observed that Typha angustifolia stood out with the highest potential for landfill leachate remediation compared to other species (Phragmites australis and Scirpus tabernaemontani): in the end of six weeks of treatment, T. angustifolia showed great biomass and high accumulation of sodium, magnesium, phosphor, manganese, iron and zinc. Therefore, the authors conclude that the species should be preferentially used in phytoremediation of landfill leachate.
Typha domingensis Pers., known commonly as southern cattail, is found throughout temperate and tropical regions worldwide, occurs in a wide variety of aquatic habitats from wetlands to totally submerged environments, and has been used to treat the most varied types of effluents (Hegazy et al., 2011; Barros, 2013; Gomber et al., 2013; Schierano et al., 2016), but few studies used the species artificially floating (Mojiri et al., 2013; Goldoni et al., 2014; Luca et al., 2019).
Considering that the reduction of ammoniacal nitrogen concentration is often the most important and the most difficult objective to be achieved in effluent treatments, the study aimed to evaluate the survival and the nitrification potential of Typha domingensis artificially floating in different concentrations of domestic solid waste leachate.
MATERIALS AND METHODS
Leachate was collected in plastic vials from the wastewater outfall of a deactivated domestic solid waste landfill of a southern city in Brazil, at a depth of 15 to 30 cm below the surface. All vials were stored in Styrofoam box with ice until analysis in the laboratory.
Collection, storage, preservation and transport of the samples were performed according to the criteria established by the Brazilian Association of Technical Norms (ABNT/NBR 9898/1987) and Standard Methods for the Examination of Water and Wastewater (APHA et al., 2012). Leachate samples and the control (rainwater with N:P:K [1g L-1]) were analyzed in triplicate for ammoniacal nitrogen by titration; nitrite and nitrate by visible UV spectroscopy, according to criteria established by the Standard Methods for the Examination of Water and Wastewater (APHA et al., 2012). These analyzes were repeated after the plants were exposed to the treatments.
Typha domingensis individuals of one year of age were collected from a cultivation site where they were artificially floating in a nutrient solution (tap water with N:P:K [1 g L-1]). The plants were transferred to plastic containers containing 3 L of each treatment: leachate 50 % (leachate in 50 % dilution with rainwater), 75 % (leachate in 75 % dilution with rainwater) and 100 % (raw leachate), and control. Ten containers were used for each treatment, with five plants in each container, according to the scheme presented in figure 1. The plants remained in flotation for a period of 35 days and twice a week the solution in each container was stirred to avoid decantation of material. The experiment was developed in a greenhouse to avoid influence of rainfall. Immediately before plant exposure, at 14, 28 and 35 days, dissolved oxygen (DO) and pH were measured in each of the treatments using a multi-parameter meter AK88 (AKSO®). To verify the tolerance of the individuals of Typha domingensis exposed to the different treatments, their survival rate after the exposure was calculated.
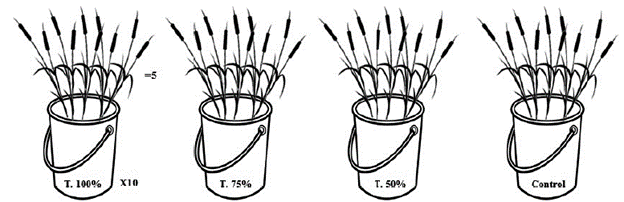
Figure 1 Scheme of the exposure of Typha domingensis individuals to different concentrations of leachate (treatments: 100 %, 75 %, and 50 %) and to control.
Data normality was evaluated using the Shapiro-Wilk test. As the data met the assumptions of normality the analysis of variance (ANOVA) was performed and the mean values of nitrate, nitrite, ammoniacal nitrogen, DO and pH in the treatments and between treatments were compared by the Duncan test, at 5 % probability. For the analysis of nitrate, nitrite and ammoniacal nitrogen means in each treatment, before and after the experiment the Student's t-test of paired samples (5 % probability) was applied. All tests were performed using the SPSS software, version 20.
RESULTS
Before the exposure of the individuals of Typha domingensis to the treatments, the pH of the solutions was neutral, between 7.2 and 7.4. During the experiment, the pH of the leachate samples increased to alkaline (mean of 8.25), while the control's pH remained neutral. When comparing between dates, there was a great variation of pH in all treatments (Fig. 2). DO of the different leachate concentrations and control differed significantly among all treatments before plant exposure, when the control showed the highest value (10.5 mg L-1) and the concentration 100 % the lowest value (3.2 mg L-1). After only two weeks of exposure, DO of the treatments increased twice in the control, three times in 50 % leachate, four times in 75 % leachate, and eight times in 100 % leachate. At 28 and 35 days, there was no significant difference between the treatments, and comparing the same treatment over time, DO concentration presented a significant difference in all the measurements in the control and in 50 and 100 % leachates (Fig. 2).
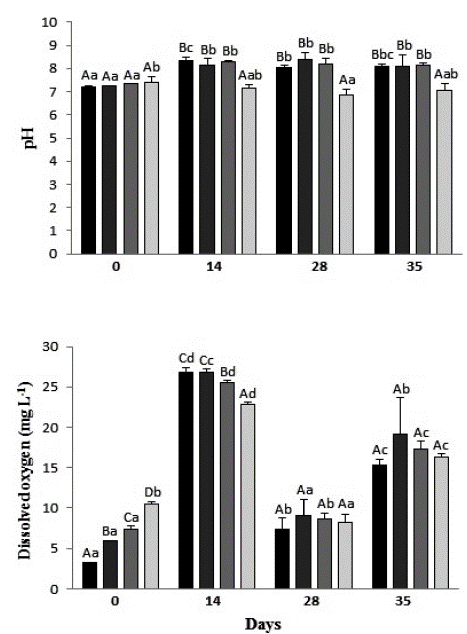
Figure 2 pH and dissolved oxygen mean values of the different treatments during the exposure period of Typha domingensis. Different upper-case letters indicate significant difference between treatments and different lowercase letters indicate significant difference between days for the same treatment, according to the Duncan test, at 5 % probability. DO detection limit: 0 mg L-1.
Before the exposure of Typha domingensis to leachate concentrations and control, all treatments showed significant differences between each other on ammoniacal nitrogen concentrations, and these values were decreasing from the highest concentration of leachate to the lowest, and it was not detected in the control. At the end ofthe experiment, in all treatments this compound was no longer detected (Table 1). Nitrite concentration was significantly higher in the 100 % leachate compared to the other treatments before plant exposure, and it was not detected by the analytical method in the control. In all treatments containing leachate, there was a significant decrease in nitrite concentration after plant exposure. At 35 days, the compound remained undetected in the control, and in 100 % leachate its concentration was again higher in relation to the other treatments (Table 1). At the beginning of the experiment, treatments 75 and 100 % presented higher nitrate concentrations than the control and did not differ significantly from each other. After the exposure of Typha domingensis, as leachate concentration decreased, so did nitrate concentration, and all treatments differed significantly from each other. The treatments containing leachate presented concentrations significantly higher at the end of the experiment in relation to the beginning (Table 1).
Table 1 Nitrate, nitrite and ammoniacal nitrogen concentrations in the different treatments before and after Typha domingensis exposure. Different letters in the line indicate a significant difference between treatments, according to the Duncan test, at 5 % probability. Asterisks in the column indicate a significant difference in each treatment at the end of the experiment in relation to the beginning, according to the paired Student's t-test, at 5 % probability.
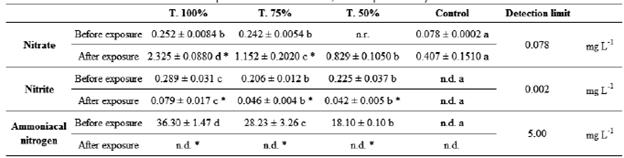
nr.: not rated: n.d.: not detected by the analytical method.
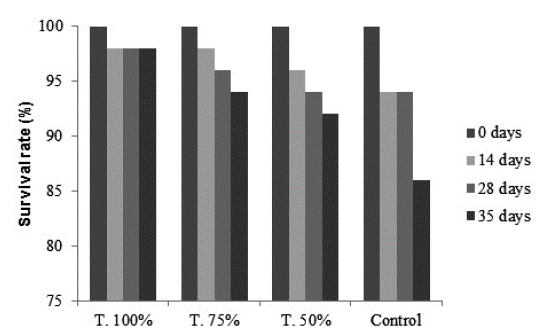
Survival rate at the end of the experiment was higher in those individuals of Typha domingensis exposed to the 100 % treatment and decreased as lower concentration of leachate: 98 %, 94 %, 92 %, and 86 % (control) (Fig. 3).
DISCUSSION
Several factors can lead to changes in a solution's pH, such as the chemical components present in the solution, temperature and gas exchange with the atmosphere (Oliveira, 2007). In addition, the biological activity stimulated by the action ofliving organisms, such as bacteria and plants, influences the maintenance of the organization of the solution constituents. Plant roots, for example, can change the pH of the environment in which they are inserted (Moreira and Siqueira, 2002). As observed, after plant exposure the pH of the treatments increased only in those containing leachate, evidencing that the rhizome of the plants, together with the components present in the effluent, created conditions for the development of biological activity and consequent pH alteration.
The DO results indicate that the species was effective in transporting oxygen to the environment by its root system, as stated by Mannarino et al. (2006), Andrade et al. (2007), and Sales Filho et al. (2015). Typha plants have their internal structure formed by tissues with open spaces through which oxygen transport occurs: from the atmosphere to the leaves, from these to the root system, and then to the area around the rhizosphere, creating conditions for organic matter decomposition and for the growth of nitrifying bacteria (Mannarino et al., 2006). Oxygen is consumed during the process of organic matter degradation per chemical and biochemical oxidation due to respiration of microorganisms (Valente, 1997; Bulc, 2006; Mannarino et al., 2006), which may explain the drop in DO levels in all treatments observed at 28 days.
At the end of the experiment, ammoniacal nitrogen was no longer detected in all treatments. Thus, 35 days were sufficient for ammoniacal nitrogen removal on 30 L of raw leachate with 50 individuals of Typha domingensis in flotation. This result corroborates with several studies that have also demonstrated that Typha species are efficient in the removal of ammoniacal nitrogen from many effluents (Mannarino et al., 2006; Escosteguy et al., 2008; Yalcuk and Ugurlu, 2009; Barros, 2013; Mojiri et al., 2016; Mojiri et al., 2017). Beltrão et al. (2005) also recorded higher nitrate concentrations at the exit than at the entrance of a biochemical system for the treatment of sanitary landfill leachate with the presence of T. domingensis, and Aluko and Sridhar (2014) also observed reduction of ammoniacal nitrogen and increase of nitrate in different chemical and biological combinations of solid waste landfill leachate treatment. Nitrogen is a constituent of proteins, chlorophyll and many biological components, and in organic matter from landfill leachate, it is broken down into amino acids and then reduced to ammoniacal nitrogen. In the presence of DO, ammoniacal nitrogen is oxidized to nitrite and then to nitrate, which explains the increase ofnitrate in the effluents (Aluko and Sridhar, 2014).
The process of oxidation of ammonia to nitrite and then to nitrate is called nitrification and occurs by the action of nitrifying bacteria that grow in aerobic medium (Barbieri et al., 2014). According to the Office of Water Programs (OWP, 2009), nitrification is the most efficient process in reducing ammonia and it is mainly dependent on the availability of dissolved oxygen. In addition, the optimum pH for nitrifying bacteria is alkaline (> 8.0) (Zoppas et al., 2016).
In the present study, it was also observed a decrease in the concentration of ammoniacal nitrogen to the point of being no longer detected, and of nitrite, and an increase in nitrate concentration. Although it was not possible to perform a correlation test between ammonia and nitrate due to the undetected values of ammoniacal nitrogen at the end of the experiment, these results indicate the occurrence of the process of nitrification, since there was also a considerable increase in the DO concentration in the treatments after plant exposure, and also an increase in pH to alkaline, which may have contributed to the growth of nitrifying bacteria in the treatments. The results still corroborate with Yalcuk and Ugurlu (2009), who observed 62.3 % of ammonia nitrification of landfill leachate by Typha latifolia in a horizontal flow wetland (plants rooted in sediment composed of layers of sand, zeolite, and gravel); with Mojiri et al. (2016), that removed 99 % of the ammonia from a landfill leachate mixture with municipal wastewater using Typha domingensis in a constructed wetland (plants rooted in sediment composed of zeolite, activated carbon, limestone, and rice husk ash); and with Mojiri et al. (2017), that used two systems in association (one with Eichhornia crassipes rooted in zeolite and cockle shell, and the other with T. domingensis rooted in powdered bentonite, zeolite and cockle shell), and observed an improvement in ammonia elimination efficiency from 87.7 % to 99.9 %. In fact, these plants do not only extract ions from the environment, but also promote the activity of microbial decomposers of organic and nitrifying compounds, favoring effluents remediation processes since the initial moment of their cultivation (Chen et al., 2015; Ashraf et al., 2018). The greater the oxygenation in effluent treatment systems with plants, more efficient the process of pollutants biodegradation, because the transfer of oxygen from the plants to the solution stimulates the density, diversity and consequent activity of the microbiological community (Zhang et al., 2014).
Survival rate results indicate that the species is tolerant to the 100 % leachate conditions of this experiment, which is an indication of the possibility of its use in the treatment of this effluent without the necessity of its dilution, corroborating with Wojciechowska et al. (2010), who also demonstrated that it is possible to use macrophytes directly in raw domestic waste leachate. In a study to examine the effects of ammonia on emerging macrophytes, Clarke et al. (2002) observed that Typha latifolia reached maximum biomass at 84 mg L-1 concentration of ammonia and had minimal biomass and lower growth rate at 0 mg L-1 concentration. In addition, the growth rate of T. latifolia was also reduced when exposed to 200 mg L-1 of ammonia.
As observed in the present study, Typha domingensis plants exposed to the control, where the concentration of ammoniacal nitrogen was not detected by the analytical method, presented the lowest survival rate, and the plants exposed to 100 % leachate had the highest survival rate, where the concentration of ammoniacal nitrogen was 36.3 mg L-1. This indicates that, at this concentration, ammoniacal nitrogen was not toxic to the plants, but on the contrary, it was important for the survival of the individuals.
Arunbabu et al. (2017) studied the effects of domestic solid waste landfill leachate on Vigna unguiculata. The plants were irrigated with different concentrations of leachate (0, 0.5 %, 1 %, 2 %, 5 %, 10 %, 25 %, 50 %, and 100 % v/v). Above 5 % concentration, root and shoot growth of plants was significantly inhibited, and at 25 % concentration, plants did not survive up to the eighth week. According to the authors, these results suggest that diluted leachate may have produced a positive effect on plant growth, and that the high concentration of ammoniacal nitrogen (2.240 mg L-1 in the raw leachate) showed toxic effects on plants. When ammoniacal nitrogen presents very high concentrations, it is recommended to dilute the leachate, or to perform a pretreatment before plant exposure, thus emphasizing that the method adopted for the treatment of the effluent of a particular landfill will not necessarily be applicable to others.
CONCLUSIONS
The present study demonstrated that Typha domingensis was responsible for the increase of pH in the different concentrations of leachate to the point of making it alkaline. Also, the use of the species artificially floating proved to be efficient in the removal of ammoniacal nitrogen, possibly by the transport of oxygen to the effluent through its roots, which consequently created conditions for the occurrence of the nitrification process.
The species is tolerant to the 100 % leachate conditions of this experiment, which was evidenced by the high survival of plants, and so, it is possible the use of Typha domingensis in the treatment of this effluent without the necessity of its dilution. However, at higher concentrations of ammoniacal nitrogen than the concentration existent in the effluent of the present study, it is recommended to dilute the leachate or to perform a pretreatment on plant exposure due to ammonia toxicity to living organisms.