INTRODUCTION
The cyclopid copepod Subfamily Halicyclopinae Kiefer, 1927 currently comprises six genera: Halicyclops Norman, 1903, Neocyclops Gurney, 1927; Petkovski, 1986, Troglocyclops Rocha and Iliffe, 1994, Colpocyclops Monchenko, 1977, Sergiosmirnovia Monchenko, 2007, and Prehendocyclops Rocha, Iliffe, Reid and Suárez-Morales, 2000. Halicyclops is the most diverse halicyclopine genus; it contains over 101 valid species (Walter and Boxshall, 2017) with a worldwide distribution (Boxshall and Defaye, 2008; Pesce, 2018). In the Americas, 33 species have been recorded, and up to 19 have been known to occur in the Caribbean region (Reid, 1990; Fiers, 1995; Rocha et al., 1998). The highly diverse Brazilian coast is known to harbor at least ten different species (Rocha, 1983a, Rocha, 1983b; Rocha, 1984; Lotufo and Rocha, 1993). Only four species are known from the United States Gulf Coast (Rocha and Hakenkamp, 1993), five species from Central America, and the Caribbean (Herbst, 1987; Reid, 1990; Fiers, 1995), including a record of H. exiguus Kiefer, 1934 from Costa Rica (Collado et al., 1984, Morales-Ramírez et al., 2014) and three from anchialine caves in Bermuda (Rocha and Iliffe, 1993).
In Colombia the records of this genus are scarce, up to now only four species have been recorded in the country, all ofthem from the northern sector of Colombia, along the Caribbean coast: H. venezuelaensis Lindberg, 1954, H. exiguus Kiefer, 1934, H. gaviriaiSuárez-Morales and Fuentes-Reinés, 2014, and H. hurlberti (Fuentes-Reinés et al., 2013; Suárez-Morales and Fuentes-Reinés, 2014; Fuentes-Reinés and Suárez-Morales, 2015; Fuentes-Reinés and Suárez-Morales, 2018).
During a survey of the plankton community of the Ciénaga Grande de Santa Marta, Colombian coast of the Caribbean, female and male specimens of an undescribed species of Halicyclops were collected. In this work, we describe this new species and compare it with its closest congeners. A key to the known species of Pesce's (2018) group "F' is also provided.
MATERIALS AND METHODS
Plankton samples were taken monthly from the Ciénaga Grande de Santa Marta, Colombia (10° 52'11.25" N and 74° 19'31.64" W) from July 2018, mainly in the littoral areas with vegetation (mangrove) but also from open water. The water salinity was measured with a WTW 3111® conductivity meter. Water samples were collected using a 25 L bucket at both vegetation areas and shallow open water. Samples were filtered with a zooplankton net (45 µm) and then preserved in 70 % ethanol. Copepods were sorted from the original samples and then processed for taxonomical identification. Dissected specimens and appendages were mounted in glycerine and sealed with Canada balsam. Drawings of the mounted appendages were prepared with a drawing tube adapted to an E-200 Nikon compound microscope.
The specimens were measured in lateral a position, from the anterior end of the rostral area to the posterior margin of the caudal rami. The specimens examined were deposited at the Museo de Colecciones Biológicas at the Universidad del Atlántico (UARC), Colombia. Morphological terminology follows Huys and Boxshall (1991). The following abbreviations are used in the description: P1-P6 = first to sixth swimming legs, EXP = exopod, ENP = endopod.
RESULTS
Taxonomy
Order Cyclopoida Burmeister, 1834
Family Cyclopidae Dana, 1846
Subfamily Halicyclopinae Kiefer, 1927
Genus Halicyclops Norman, 1903
Halicyclops gutierrezi sp. n.
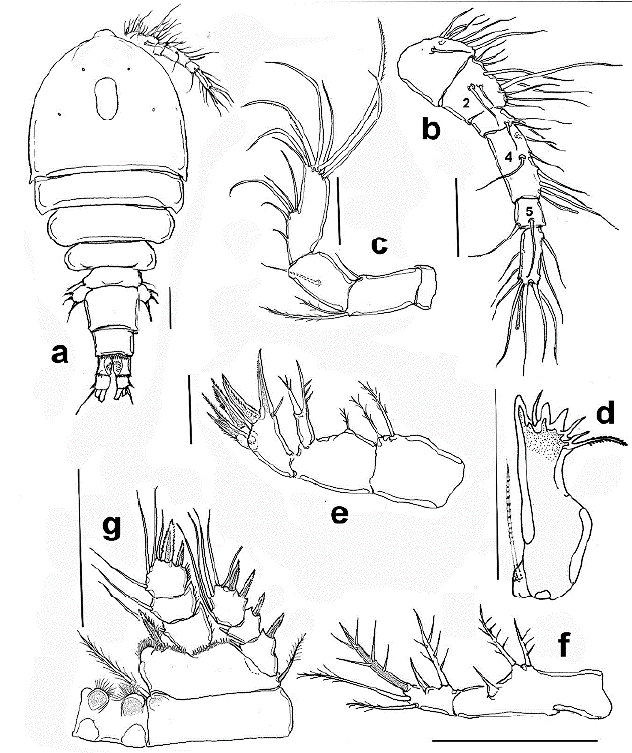
Figure 1 Halicyclops gutierrezi sp. n., adult paratype female from northern Colombia. a. habitus, b. antennule, c. antenna, ventral, d. mandible, e. Maxilla, f. maxilliped, g. P1. Scale bars: a-g= 50 µm.
Material examined. Adult female holotype (UARC333M), and adult male allotype (UARC1157-1164), both partially dissected. Ciénaga Grande de Santa Marta, littoral plankton sample, coll. Juan M. Fuentes-Reinés. Paratypes: three adult females, undissected, ethanol-preserved, vial (UARC334M), plus one dissected female and male, each mounted on slides (UARC344-UARC352M, UARC1165- UARC1172).
Type locality. Ciénaga Grande de Santa Marta, Magdalena, northern Colombia (10° 52'11.25" N; 74° 19'31.64" W).
Description of female. Body robust, cephalothorax widest at first pedigerous somite in dorsal view (Fig. 1a, rostrum subtriangular, wide-based, with rounded apex (Fig. 4a). Body length, excluding caudal setae: 588-616 µm (average = 594 µm, n = 7). Labrum represented by widely rounded plate ornamented with marginal rows of spinules at both sides of medial teeth (Fig. 4b).
Cephalosome with relatively small, an oblong integumental window on dorsomedial position (Fig. 1a). Genital double-somite subquadrate, with an arc-shaped row of five integumental pores plus single medial one on the ventral surface (Fig. 2b, arrowed). Genital double-somite as long as wide with small rounded integumental window on each side of posterior half (Fig. 2a); margins of double-somite straight, smooth, lacking processes. Ventral surface of genital double-somite and succeeding two urosomites (Fig. 2a) with finely denticulate hyaline frill on posterior margins; seminal receptacle with short, slightly curved arms and medial pore (Fig. 2b). Ventral surface of anal somite with pair of "n"symbol-shaped cuticular scars on anterolateral position (arrows in Fig. 2c) and a row of spinules along with insertion of caudal rami (Fig. 2c). Dorsal posterior margin of the fourth urosomite with a hyaline fringe of denticles; medial denticles relatively more extended than the rest of row, forming a weak pseudo-operculum (arrowed in Fig. 2e).
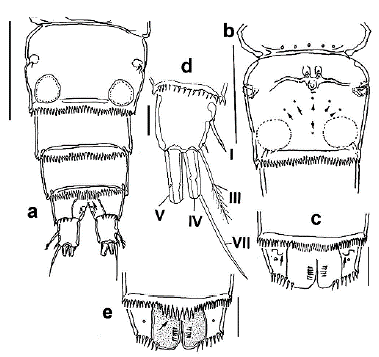
Figure 2 Halicyclops gutierrezi sp. n., adult female paratype from northern Colombia. a. urosome, ventral, b. genital double-somite with pores (arrowed). Ventral view, c. anal somite, ventral view, with n- shaped scars (arrows), d. caudal ramus with caudal setae I-VII. e. anal somite, dorsal view, showing pseudo-operculum (arrow). Scale bars: a, b= 50 µm, c, e= 25 µm: d= 15 µm.
Caudal ramus subquadrate, about 1.1 times as long as wide (Fig. 2d), outer seta III slightly more extended than ramus (1.1 times as long as ramus), apical seta V 1.7 times as long as seta IV, latter caudal seta with heteronomous ornamentation, inner margin spinulated, outer margin furnished with setules. Dorsal, caudal seta (VII) 2.5 times as long as ramus.
Antennule six-segmented, setal formula as follows, s=setae, sp = spine, ae =aesthetasc: 1(8 s), 2(12 s), 3(5 s + sp), 4(7 s), 5(2), 6(10 + ae); fourth segment about 2.8 times as long as wide (Fig. 1b). First segment with row of small spinules.
Antenna four-segmented, with short, unarmed coxa; basis with one plumose and one spinulose seta at the inner corner; short seta representing antennary EXP present. ENP two-segmented. Proximal endopodal segment about half as long as the distal segment, armed with a seta on middle inner margin. Terminal segment of ENP furnished with one proximal and one subdistal row of spines, armed with five inner setae and seven unequally long apical setae. Length/ width ratio of second ENP segment = 3.1 (Fig 1c).
Mandible with well-developed coxal gnathobase, armed with five long teeth, innermost tooth being strongest, accompanied by short seta inserted on the narrow diastema. Dorsal pinnate seta present. Palp reduced, represented by one lightly pinnate seta inserted on small protuberance (Fig. 1d).
Maxillule as usual in the genus, with praecoxal arthrite bearing four strong medial claw-like spines, coxa with two short smooth setae and short medial spine. Palp 2-segmented, first segment armed with one spiniform, one pinnate and one naked inner setae, plus one outer spiniform seta representing EXP. Distal segment representing ENP armed with three naked setae (Fig. 4c).
Maxilla 4-segmented, comprising praecoxa, coxa, basis, and one-segmented endopod. Praecoxa with two endopodal setae. Coxa with a short single seta in proximal half. Basis with two strong unipinnate claw-like basal spines and additional short, slender seta; proximal claw -like element clearly longer than succeeding claw. Palp one-segmented, armed with three robust spines (Fig. 1e).
Maxilliped two-segmented, comprising protopod and ENP. Protopod with three spiniform setae, middle one being 1.3 times as long as proximal seta. ENP was bearing five setae comprising two inner, one apical spiniform seta furnished with few strong setules, and two outer subapical slender setae (Fig. 1d).
P1-P4 exopod and endopod three-segmented (Figs. 1g, 3a-c), armed as in Table 1. Intercoxal sclerite of P1 hairy on the rounded protuberances on posterior margin; basis with a row of long spinules along distolateral margin on the anterior side. Inner basipodal spine stout, reaching 1/3 of the second endopodal segment of P1 (Fig. 1g). EXP/inner spines of P1 basis ratio = 2.21. P2-P4 coxa and intercoxal sclerite smooth, P2-P3 like each other (Figs. 3a, b). P4ENP3 about 1.4 times as long as wide with three pinnate spines and two inner lateral setae, inner apical spine 1.28 times as long as segment and 1.4 times as long as the outer apical spine. Inner lateral spine 1.6 times as long as segment (Fig. 3c).
Table 1 Legs 1-4 setal armature of Halicyclops gutierrezi sp.n. (Roman numerals indicating spines, Arabic numerals representing setae).

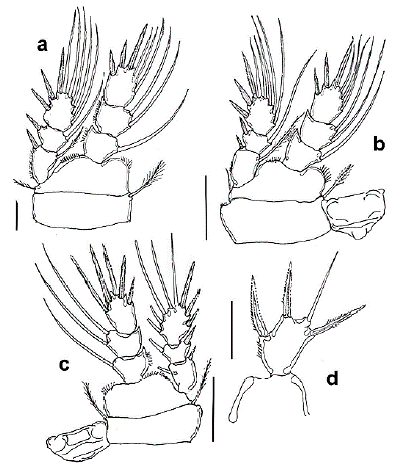
Figure 3 Halicyclops gutierrezi sp. n., adult female paratype from northern Colombia. a. leg 2, b. leg 3, c. leg 4, d. leg 5. Scale bars: a-d= 50 µm.
P5 EXP segment subrectangular (Fig. 3d), about 1.53 times as long as wide, armed with three spines, outer and inner distal spines as long as segment, medial spine shorter than segment, plus one flexible seta about 1.35 times as long as segment; relative length of elements from inner to outer margin as follows 0.71, 1.0, 0.65, 0.71.
Male. Habitus as in female, relatively slender. Body length, excluding caudal setae: 476-504 µm (average = 490 µm; n= 3). Both antennules typically geniculate, 14-segmented (Fig. 4d), geniculation between segments 12 and 13, with aesthetasc on segments 1, 4, 9, 11, 13, and 14. Antenna, maxilla, maxillule, mandible, and maxilliped as in female except for proximal basal claw-like element of the maxilla being shorter than the second one (Fig. 4e, shorter claw arrowed), the opposite pattern occurs in the female in which the proximal claw is longer. (see Fig. 1e). Urosome with six somites as in female but denticle fringes on posteroventral margins of urosomites weaker than in female (Fig. 4f). Caudal rami and P1-P4 as in female. P5 exopod (Fig. 4g) subrectangular, about 1.5 as long as wide, bearing three spines and two setae, relative length of elements from inner to outer margin as: 1.0, 0.33, 0.83, 0.53, 0.4.
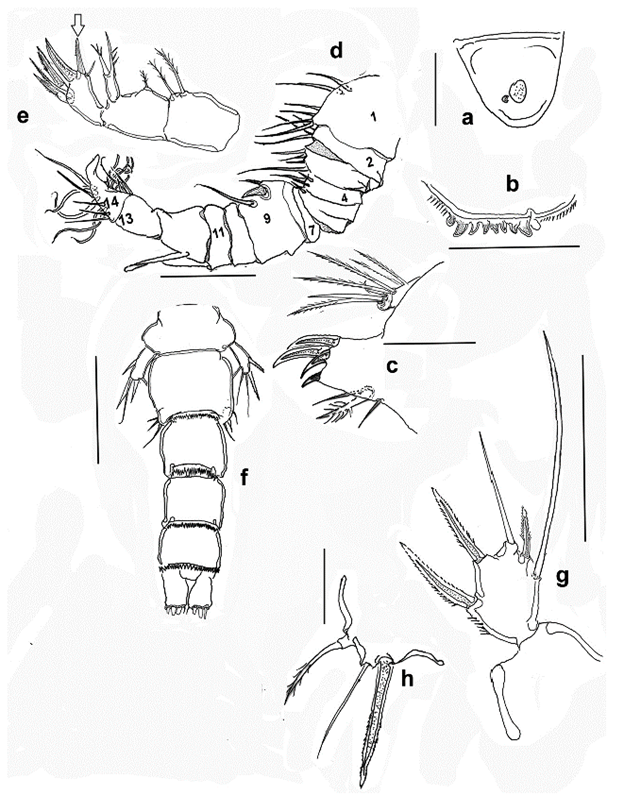
Figure 4 Halicyclops gutierrezi sp. n., adult female paratype from northern Colombia. a. rostrum, b. labrum, c. maxillule. Adult male allotype; d. antennule, e. maxilla, f. urosome, dorsal, g. leg 5, h. leg 6. Scale bars: a= 10 µm, b, c-h=50 µm.
P6 (Fig. 4h) represented by small plate armed with three elements: one long inner serrate spine and two slender setae.
Etymology. The species is named after Dr. José Manuel Gutiérrez Salcedo for his work on Colombian copepods and his leadership in the formation of new generations of planktologists.
DISCUSSION
Karanovic (2006) divided the genus Halicyclops into two subgenera considering the number of inner setae on the male P5: Rochacyclops Karanovic, 2006, with two inner setae and Halicyclops s. str., with one inner seta. The new species, H. gutierrezi and all known species of this genus in Colombia belong to the latter subgenus.
Additionally, Halicyclops species have been separated into eight morphological groups according to their P1-P4 EXP3 spine formula (see Pesce, 2018). Among them, Halicyclops gutierrezi sp. n. is clearly a member of group "F" which includes all species with a 2333 spine formula. Currently, this small group contains five species and two subspecies: H. (H.) canuiLindberg, 1941; H. (H.) brevispinosus brevispinosus Herbst, 1952; H. (H.) brevispinosus meridionalis Herbst, 1953; H. (H.) pusillusKiefer, 1956; H. (H.) tageaeLotufo and Rocha, (1993), and now H. (H.) gutierrezi sp. n. The three former species are poorly described and in need of redescription.
Halicyclops gutierrezi most closely resembles H. canui described from India. Both of them bear long spines and seta in the female P5EXP, but they can be separated by 1) the presence of a strong lateral process in the genital double-somite in H. canui (Lindberg, 1941, Fig. 1b); such a process is absent in the new species, with a genital double-somite having straight, smooth lateral margins (Fig. 2a), 2) in H. gutierrezi the inner setae of P4ENP3 are longer than the segment (Fig. 3c) whereas in H. canui, they are shorter than the bearing segment (Lindberg, 1941, Fig. 1d), 3) the integumental windows on the posterior genital double somite are present in H. gutierrezi (Fig. 1a) but absent in H. canui (Lindberg, 1941, Fig. 1b). Unfortunately, the male of H. canui remains unknown (Lindberg, 1941) and it could not be compared with the male of the new species from Colombia.
Halicyclops brevispinosus brevispinosus described from Germany resembles H. gutierrezi in several characters: 1) the lack of lateral process on the genital double-somite and the length of the inner setae of P4ENP3, longer than the segment. Halicyclops gutierrezi can be distinguished from H. b. brevispinosus by several characters: 1) the inner apical spine of P4ENP3 is longer than the adjacent inner lateral spine in H. gutierrezi (Fig, 3c) whereas in H. b. brevispinosus the opposite condition occurs (Herbst, 1952, taf. 20 q); 2) in H. b. brevispinosus all three spines on the female P5EXP are clearly shorter than the segment (Herbst, 1952, taf. 20r) whereas in H. gutierrezi they are either as long as the segment, or longer (Fig. 3d), 3) the two outer spines on the male P5 of H. b. brevispinosus (Herbst, 1952, taf. 20s) are shorter than the segment, thus diverging from the longer inner spines of H. gutierrezi; both are as long as the EXP (Fig. 4g), 4) the male P6 of H. b. brevispinosus has an inner spine that is shorter than the outer seta (Herbst, 1952, taf. 20t) whereas in H. gutierrezi the inner spine is longer than outer seta (Fig. 4h).
Halicyclops brevispinosus meridionalis, from the North Sea, resembles H. gutierrezi in the length/width ratio of the female fourth antennulary segment: both species diverge in: 1) length/width ratio of caudal rami; it is subquadrate, about as long as wide in H. gutierrezi (Fig. 2d) vs. rectangular (length/width ratio = 1.5 in H. b. meridionalis (Herbst, 1953, taf. 31, Abb. p), 2) the lateral inner seta ofP4ENP3 surpasses the inner apical spine in H. gutierrezi (Fig. 3c), whereas this element is shorter in H. b. meridionalis (Herbst, 1953, taf. l 3, Abb. r), 3) the spines on female P5EXP are shorter than the segment in H. b. meridionalis (Herbst, 1953, taf.31, Abb. s) whereas in H. gutierrezi they are equally long or longer than the P5 segment (Fig. 3d), 4) In the male H. b. meridionalis the two outer spines on P5 are relatively shorter (Herbst, 1953, taf.31, Abb. t) than the corresponding spines of H. gutierrezi (Fig. 4g).
Halicyclops pusillus, described from Madagascar, resembles H. gutierrezi in having the inner setae of P4ENP3 longer than the segment but they can be distinguished by several characters: 1) the length/width ratio of the female fourth antennulary segment (about 1.6 in H. pusillus) (Kiefer, 1956, Fig. 9) vs. 2.1 in H. gutierrezi (Fig. 1b), 2) the spines of the female P5EXP are shorter than the segment in H. pusillus (Kiefer, 1956, Fig. 11) whereas they are longer in H. gutierrezi (Fig. 3d), 3) the distal inner seta of P4ENP3 overpasses the inner apical spine in H. gutierrezi (Fig.3c) whereas in H. pusillus it is shorter (Kiefer, 1956, Fig. 10), 4) the two outer spines and the inner seta of male P5 of H. gutierrezi (Fig. 4g) are relatively longer than in H. pusillus (Kiefer, 1956, Fig 13), 5) in H. pusillus the male P6 has an inner spine about 2.2 times as long as the outer spine (Kiefer, 1956, Fig. 14) vs. 1.6 in H. gutierrezi (Fig. 4h).
Halicyclops tageae, described from Brazil, resembles H. gutierrezi in sharing the length and insertion ofthe inner basal spine of P1. They can be separated by: 1) the length/width ratio of the female fourth antennulary segment (about 2.1 in H. gutierrezi) (Fig. 1b) vs. 3.3 in H. tageae (Lotufo and Rocha, 1993) the number of inner setae in P2ENP2, H. tageae bears one inner seta (Lotufo and Rocha, 1993, Fig. 11) vs. two inner setae in H. gutierrezi (Fig. 3a), 3) the spines on the female P5EXP are shorter than the segment in H. tageae (Lotufo and Rocha, 1993) whereas in H. gutierrezi they are longer or as long as segment (Fig. 3d), 4) the inner distal seta of P4ENP3 is longer than the segment in H. gutierrezi (Fig. 3c), whereas in H. tageae the same element is shorter (Lotufo and Rocha, 1993) the distal inner seta of P4ENP3 is longer than the inner apical spine in H. gutierrezi (Fig. 3c), whereas it is shorter in H. tageae (Lotufo and Rocha, 1993, Fig. 13), 6) the posterior margin of the genital double-somite and succeeding urosomites are more strongly denticulate in H. gutierrezi (Fig. 2a) than in H. tageae, 7) the male P5 of H. gutierrezi bears five setal elements (Fig. 4g) vs. four in H. tageae (Lotufo and Rocha, 1993). In H. tageae the male P6 the inner spine is as long as the outer seta (Lotufo and Rocha, 1993) whereas in H. gutierrezi the same spine is about 1.6 times as long as the outer seta (Fig. 4h), 9) the male antennule is 14-segmented in H. gutierrezi (Fig. 4b) vs. 13-segmented in H. tageae (Lotufo and Rocha, 1993).
Distribution and ecology
Halicyclops gutierrezi sp. n. is currently known from a single locality only, the protected coastal system of Ciénaga Grande de Santa Marta, Colombia. This large lagoon is a shallow water body (depth 0.5-1.5 m), its temperature varies seasonally from 30 to 31.3 °C; pH values during our sampling was 8.9, salinity = 15 PSU and dissolved oxygen =7.86 mg/L. In the surveyed area H. gutierrezi sp. n. was recorded among mangrove.
CONCLUSIONS
In this contribution, we described the sixth known species of Halicyclops belonging to group "F" (see Pesce, 2018). This species can be confused with other congeners of group "F" in the region, but it can be distinguished by some important characters including the length/width ratio of the female caudal ramus, the lack of a lateral processes on the genital double-somite, the relative size of the setal elements of P4ENP3, the number of segments of the male P5, the size and shape of the cephalothorax integumental windows, the details of the male and female P5 setation pattern, the number of inner setae in P2ENP2, and the number of segments of the male antennule. This is the fifth species of the genus recorded in Colombia; this finding also increases the total number of species of Halicyclops known in the Caribbean Sea and the Neotropics.
Key to the species of Halicyclops from Colombia
1A spine formula (outer spines on P1-P4EXP3) 2-3-3-3 ...............................................................H. gutierrezi sp. n.
1B spine formula 3-4-4-3...........................................2
2A Genital double-somite with triangular lateral protuberances, ventral surface of urosomites ornamented with denticles......................H. venezuelaensis Lindberg, 1954
2B Genital double-somite without protuberances, ventral surface of urosomites smooth...........................................3
3A Length/width ratio of terminal segment of antennary ENP about 3.0; P4ENP3 with distal inner seta not surpassing tip of inner apical spine, caudal rami as long as wide.................................................................H. exiguus Kiefer, 1934
3B. Length/width ratio of terminal endopodal segment of antenna lower than 3.0; P4ENP3 with distal inner seta surpassing tip of inner apical spine; caudal rami 1.2 - 1.4 times as long as wide....................................................................................4
4A Inner basipodal spine of P1 reaching at least midlength of P1ENP2; EXP P5 with the three spines longer than segment; distal inner spine of P4ENP3 clearly thicker than apical spines..........................H. hurlberti Rocha, 1991
4B Spine inserted at inner corner of P1 basis reaching distal margin of ENP2; EXP P5 with the three spines shorter than segment, distal inner spine of P4ENP3 as thick as apical spines ...............H. gaviriaiSuárez-Morales and Fuentes-Reinés, 2014.















