INTRODUCTION
Palms (family Arecaceae) are considered one of the most important plant groups for rural communities in the tropics. In Colombia, several species of palms are used to obtain oils, pulp, palm hearts, fibers, and construction materials, as well as other products (Galeano and Bernal, 2010). Palm fruits are a source of food for many human communities (Mesa and Galeano, 2013; Ledezma-Rentería and Galeano, 2014), and about 60 species of Colombian palms with edible fruits have been recorded (Galeano and Bernal, 2010; Mesa and Galeano, 2013; Ledezma-Rentería and Galeano, 2014). Many of these fruits are rich in nutrients and other bioactive molecules, and their consumption can be beneficial to human health (Montúfar et al., 2010; Kang et al., 2012; Li et al., 2016).
Knowledge of phenological cycles and productivity is important in designing effective management plans for fruit-producing palms (Galeano et al., 2015). In tropical America, reproductive phenology and fruit productivity of a wide variety of palm species have been studied. These include Maurítía flexuosa L.f. (Mendes et al., 2017), Acrocomía aculeata (Jacq.) Lodd. ex Mart. (Scariot et al., 1995), Oenocarpus bataua Mart. (Rojas-Robles and Stiles, 2009), Elaeís oleífera (Kunth) Cortés (Moreno and Romero, 2015), Attalea phalerata Mart. ex Spreng. (Fava et al., 2011), Syagrus romanzoffiana (Cham.) Glassman (Mariano and Christianini, 2016), and Butía capítata (Mart.) Becc. (Silva and Scariot, 2013). Tropical palms exhibit annual or multi-year reproductive cycles (Rojas-Robles and Stiles, 2009), and their flowering tends to be synchronized with precipitation (Henderson etal., 2000; Fava et al., 2011; Mendes et al., 2017). Many palms maintain a supply of fruits throughout the year, which represents a key resource for the frugivorous fauna (Adler and Lambert, 2008; Genini et al., 2009; Fava et al., 2011).
The genus Attalea represents a group of palms characteristic of the warm lowlands in Colombia (Gaelano and Bernal, 2010). The taxonomy of this group has been controversial, with no consensus on the number of accepted species, which varies between 29 and 67 (Pintaud, 2008). In Colombia, 16 species are recognized and are found in both humid and dry forest habitats (Galeano and Bernal, 2010; Bernal et al., 2015). Attalea species have either pistillate, staminate, or bisexual inflorescences (Dransfield et al., 2008). Individuals are monoecious, but because pistillate and staminate inflorescences seldom occur at the same time, they are considered functionally dioecious (Moraes et al., 1996; Núñez et al., 2005; Fava et al., 2011; Tucker et al., 2018). Fruits are ellipsoid to ovoid, with one to several seeds, the mesocarp is usually fleshy and fibrous, the endocarp is very hard, and seeds have a solid, homogeneous endosperm (Dransfield et al., 2008). Attalea fruits are rich in oils (Lleras and Coradin, 1988; Devia et al., 2002; Voeks, 2002), and several species have been considered as promising oil sources (e.g., Markley, 1971; Devia et al., 2002; Bernal et al., 2010).
Attalea nucífera H.Karst. is endemic to the Magdalena River basin of Colombia and has been considered threatened due to the transformation and deterioration of its habitat -dry to moderately wet riparian forests (Galeano and Bernal, 2005; Galeano and Bernal, 2010). In the municipality of Guaduas (Cundinamarca), its fruits have been consumed for centuries. During the Royal Botanical Expedition (17831816), José Celestino Mutis described the use of these fruits for the extraction of oil, in addition to their use as food by the rural population of Guaduas (Galeano, 1985). In 1856 the German botanist Hermann Karsten pointed out that the seeds tasted like almonds or walnuts, and farmers sold them as treats in the Guaduas market (Karsten, 1856). Over time, consumption of these nuts has decreased, but the species is still listed as a useful plant with promising potential (Pérez-Arbeláez, 1994; Patiño, 2002). Today, the fruits are no longer marketed, and their consumption is sporadic (Prada, 2018).
In order to assess the productive potential of A. nucífera, we studied its phenology, biometric parameters, and fruit production in Guaduas, Cundinamarca. We asked the following research questions: What is the reproductive cycle of A. nucífera? What is the relationship of temperature and precipitation with the reproductive cycle? How much fruit do A. nucífera palms produce annually? And how are biometric parameters of individual palms related to productivity and fruit biometrics?
MATERIALS AND METHODS
Study species
Attalea nucífera is a palm with a solitary, underground stem (acaulescent). Adults have 7-14 leaves ca. 8 m long, with 92-122 leaflets per side, regularly arranged on the same plane, and apical leaflets are not fused. Staminate flowers are arranged in two rows on the adaxial side of the rachillae and can reach up to 2 cm long. Pistillate inflorescences have flowers arranged directly on the rachis, and fruits are elliptical to almost spherical, 6 to 8 cm long (Galeano and Bernal, 2010).
Study area
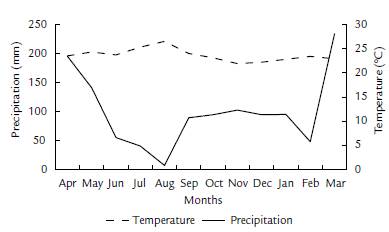
The studied population of A. nucífera occurs in a forest relict on the bank of Quebrada Cucharal (5°07' N, and 74°36' W), in vereda Cucharal, municipality of Guaduas (Cundinamarca). The area has an average elevation of 900 m above sea level, and corresponds to tropical dry forest (Holdridge, 1987), with a vegetation matrix of pastures, tall secondary vegetation, and gallery forest. During the period 2000-2017 average monthly temperature was 23.4 °C, and average monthly rainfall was 105 mm. Between April 2016 and March 2017 monthly precipitation ranged from seven mm to 233.9 mm, with an average of 99.6 mm (Fig. 1). The highest rainfall occurred between March and May, and the lowest rainfall levels were in February and June-August (Fig. 1). The average monthly temperature fluctuated between 21.9 - 26.5 °C, with an average of 23.9 °C. The warmest months were July and August, and the coldest was November (Fig. 1).
Reproductive phenology
Forty adult individuals of A. nucífera were randomly selected and monitored between April 2016 and March 2017. At monthly intervals, the presence, absence and changes in reproductive structures were recorded, considering five phenological phases: (1) bud (from the emergence of the reproductive structure, until the peduncular bract opens); (2) pistillate inflorescence (from bract opening to the beginning of fruit development); (3) staminate inflorescence (from bract opening until all flowers have fallen); (4) unripe infructescence (from the beginning of fruit development until fruits ripen); and (5) ripe infructescence (from the time fruits turn brown until all fruits fall off). The number of leaves, the number of veins on the right side of the youngest leaf and the diameter of the petiole of the youngest leaf, just below the last basal leaflet, were also recorded for each individual. We used these biometric parameters to quantify plant size, as often used for palms (Galeano et al., 2010).
Fruit productivity
Six individuals with ripe infructescences from the 40 that we used to phenology observations were selected for more detailed study. The number of fruits per infructescence was counted, and infructescence length and diameter were measured. For each palm the number of leaves, the number of veins on the right side of the youngest leaf and the diameter of the petiole on the youngest leaf were recorded.
Biometric characteristics of the fruits
Thirty ripe fruits were selected from different positions along the rachis, five from each of the palms used to take productivity data. For each fruit, we recorded length and diameter, fresh weight of the whole fruit, of the shell, the endocarp and the nut. All measurements were made ín sítu since no vegetal material was collected.
Data analysis
The relative frequency for each phenological phase was calculated by dividing the number of occurrences observed during the corresponding month by the total number of occurrences observed throughout the year (Rojas and Cruz, 2004). To test the linear association between phenology variables, Spearman correlation tests (González et al., 2011) were applied as follows: Correlations between the monthly mean of each phenological phase and the values of mean temperature and monthly precipitation for that month, for the previous month and for the two previous months. Additional correlations were calculated between the quantity of total reproductive structures and the biometric data of the individuals, and between the biometric data of the fruits and the biometric data of the individuals.
RESULTS
Reproductive phenology
During the period April 2016-March 2017, 82.5 % of the individuals of A. nucífera in the study area flowered and/or fruited. The entire reproductive cycle, from the appearance of a bud to the dispersion of ripe fruits, lasted 12.6 months. Palms produced buds throughout the year, with a peak between April and June, and another in November (Fig. 2). On average, the development of the buds, it means the time from when we observed a new bud to when it entered anthesis, lasted two months (± 1.07 SD). Staminate inflorescences opened throughout the year (Fig. 2) with the main peak between November-December and another smaller peak in June. Pistillate inflorescences only appeared during two periods of the year, May through July and December through January with the highest proportion of pistillate inflorescences during May-June (Fig. 2). Although we found unripe and ripe fruits throughout the year, the highest proportion of unripe fruits was observed during July-August, and the highest proportion of ripe fruits was between August and January (Fig. 2). The phase of unripe fruits lasted on average 4.2 months (± 1.98 SD) and that of ripe fruits 5.4 months (± 3.11 SD).
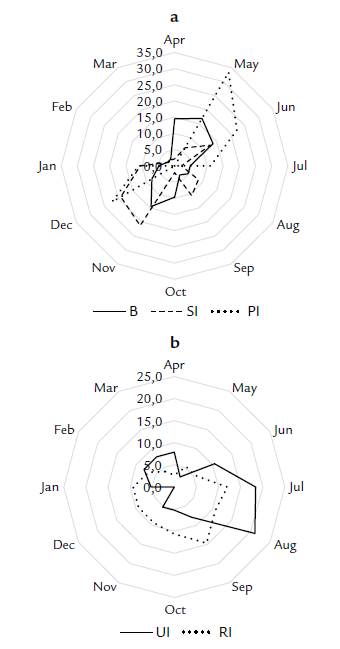
Figure 2 Relative frequency of reproductive structures per month of Attalea nucífera in Guaduas, Cundinamarca, Colombia. a Buds (B), staminate inflorescences (SI), and pistillate inflorescences (PI); b. unripe infructescence (UI) and ripe infructescence (RI).
There was a positive correlation between the occurrence of buds and precipitation in the previous month (rs = 0.667, p = 0.018), but staminate inflorescences showed no correlation with environmental variables (Table 1). In contrast, the occurrence of pistillate inflorescences was correlated with precipitation during the two previous months (rs = 0.787, p = 0.002), that is, at the moment of bud development. The occurrence of unripe fruits had a negative correlation with precipitation in the same month (rs = -0.575, p = 0.050) and a positive correlation with temperature (rs = 0.653, p = 0.048). The occurrence of ripe fruits also showed a negative correlation with precipitation in the same month (rs =-0.639, p = 0.025).
Table 1 Spearman rank correlations among the monthly mean temperature and total precipitation, and the monthly mean of the occurrence of phenological phases of Attalea nucífera in Guaduas, Cundinamarca, Colombia.
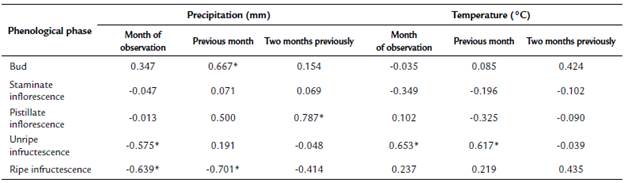
*p < 0.05
We recorded a total of 88 reproductive structures in the 40 palms studied, with a mean of two reproductive structures per individual (± 1.45 SD). Of these reproductive structures, there were 48 staminate inflorescences and 11 pistillate inflorescences (Table S1). We only found a positive correlation between the number of leaves and the total number of reproductive structures (rs = 0.447, p = 0.004), and the number of staminate inflorescences (rs = 0.403, p = 0.010) (Table 2). We found no correlation between the diameter of the petiole and the number of veins in the youngest leaf, and the number of reproductive structures (Table 2).
Table 2 Spearman rank correlations among biometric parameters of individuals and the total number of reproductive structures and the number of staminate and pistillate inflorescences of Attalea nucífera in Guaduas, Cundinamarca, Colombia.
| Number | Number of leaves | Number of veins | Petiole diameter |
|---|---|---|---|
| Staminate inflorescences | 0.403* | 0.251 | 0.069 |
| Pistillate inflorescences | 0.226 | -0.065 | 0.106 |
| Reproductive structures | 0.447* | 0.190 | 0.285 |
*p < 0.05
Productivity and fruit biometrics
On average, each A. nucífera adult palm that reproduced in the study area produced 1.3 ripe infructescences per year (± 0.62 SD). Each infructescence was 18.8 cm (± 1.98 SD) wide and 23.0 cm (± 1.44 SD) long and produced a mean of 16.7 (± 4.94 SD) fruits (Table S2). The fruits measured 76.6 mm (± 6.53 Sd) long and 64.6 mm (± 6.12 SD) wide and weighed 182.9 g (± 45.97 SD). Of this weight, 5 % (9.3 g) corresponded to the nut, 51 % (93.1 g) to the endocarp and 44 % (80.4 g) to the peel (Table S2).
We found that fruit size was correlated with palm size. Specifically, we obtained a positive correlation between the number of leaves in the crown and the biometry of the fruit in general (Table 3). Likewise, we found a positive correlation between petiole diameter and the length (rs = 0.939, p = 0) and width (rs = 0.603, p = 0) of the infructescence, and with fruit biometry in general (Table 3). The number of veins in the youngest leaf show a positive correlation with the width (rs = 0.389, p = 0.033) and weight (rs = 0.431, p = 0.017) of the fruits (Table 3), but was negatively correlated with the width of the infructescence (r s = -0.485, p = 0.007) and the number of fruits per infructescence (rs = -0.809, p = 0).
Table 3 Spearman rank correlations among biometric parameters of individuals and biometric parameters of fruits of Attalea nucífera in Guaduas, Cundinamarca, Colombia.
| Parameter | Width of infructescence | Length of infructescence | Number of fruits | Width of fruit | Length fruit | Weight of fruit | Weight of nut | Weight of endocarp | Weight of peel |
|---|---|---|---|---|---|---|---|---|---|
| Number of leaves | 0.147 | 0.078 | -0.044 | 0.672* | 0.625* | 0.718* | 0.457* | 0.705* | 0.744* |
| Number of veins | -0.485* | -0.329 | -0.809* | 0.389* | 0.231 | 0.431* | 0.321 | 0.548* | 0.311 |
| Petiole diameter | 0.603* | 0.939* | 0.338 | 0.626* | 0.478* | 0.566* | 0.363* | 0.413* | 0.566* |
*p < 0.05
DISCUSSION
The reproductive cycle of A. nucífera corresponded to one of the longest cycles reported for any species in the genus (about 383 days). Núñez (2014) reported a cycle of 306 days for Attalea butyracea (Mutis ex L.f.) Wess. Boer, Attalea ínsígnís (Mart.) Drude and Attalea marípa (Aubl.) Mart., from the appearance of the inflorescence to fruit fall. The production of larger fruits can explain the greater extension of the reproductive cycle in A. nucífera compared to other species. While A. nucífera fruits measure between 6.3 and 8.6 cm long, in other species it can vary between 4 and 7.5 cm long (Galeano and Bernal, 2010).
We observed only staminate or pistillate inflorescences in the individuals studied. However, during the observation period, all palms studied were functionally dioecious, as staminate and pistillate inflorescences never flowered simultaneously on the same individual. This behavior is frequent in species of Attalea (Moraes et al., 1996; Núñez et al., 2005; Fava et al., 2011) and has been related to a transition from monoecy to dioecy (Anderson et al., 1988; Voeks, 2002). Although commonly found in the genus, no androgynous inflorescences were observed (Voeks, 2002; Núñez et al., 2005; Dransfield et al., 2008; Fava et al., 2011; Núñez, 2014). To our knowledge, no androgynous inflorescences have been reported for this species. However, it seems premature to define this condition as typical for A. nucífera, since reproductive behavior of Attalea species is quite fluid and may be influenced by habitat conditions (Tucker et al., 2018).
Attalea nucífera produces buds throughout the year, similar to the patterns observed in other species of Attalea (Voeks, 2002; Núñez et al., 2005; Núñez, 2014; Tucker et al., 2018). The production of buds seems to be influenced by precipitation, as the highest occurrence of buds coincides with periods of increased precipitation. A correlation between the occurrence of buds and precipitation (Table 1) has also been found in Attalea butyracea, A. ínsígnís, and A. phalerata (Voeks, 2002; Núñez, 2014). However, this does not seem to be a generalized pattern, since in A. marípa and A. butyracea the opposite pattern has been reported, that is, buds occur during periods of low rainfall (Olivares and Galeano, 2013; Núñez, 2014; Pires et al., 2016). These variations in phenological cycles may be related to regional annual rainfall patterns (Olivares and Galeano, 2013) or habitat conditions within the same region (Tucker et al., 2018).
The sustained supply of staminate inflorescences throughout the year has been explained as a way to maintain pollinator fidelity, which has been observed as a frequent phenomenon in other palm species (Núñez, 2014). A strategy of this type helps maintain reproductive efficiency in the population (Núñez, 2014), a key aspect for the maintenance of the species in its ecosystem.
In contrast, the increase in the number of pistillate inflorescences towards the end of the rainy season (Table 1) suggests that the pistillate buds are formed during the rainy season. Thus, the reproductive cycle of A. nucífera is synchronized with precipitation, a common feature for many tropical dry forest plants (Pizano et al., 2014), and other palm species (e.g., Fava et al., 2011; Silva and Scariot, 2013).
Synchronization of pistillate inflorescences towards the end of the higher precipitation periods determines that the growth of the fruits and fruit ripening mostly occurs during periods of low rainfall, as evidenced by the negative correlation between these two phenological phases (unripe and ripe infructescences) and precipitation (Table 1). It is worth noting that the occurrence of immature and ripe fruits throughout the year is due to the long duration of fruit formation. In Attalea phalerata, the presence of unripe fruits has been observed throughout the year (Fava et al., 2011), while in Attalea funífera Mart. a low but constant supply of ripe fruits was also reported throughout the year (Voeks, 2002). In addition, the production of staminate flowers and fruits year-round suggests that the species play a key role in the ecosystem, providing a steady supply of food for insects and small mammals. A similar condition has been reported for Attalea funífera (Voeks, 2002) and is probably common in other species of Attalea, which would fulfill the same ecosystem function.
Individuals of A. nucífera with more leaves in the crown produced more reproductive structures as well as a greater number of fruits that are larger. The influence of crown size on the frequency of appearance of the reproductive structures has already been documented in A. phalerata (Tucker et al., 2018) and A. butyracea (Olivares and Galeano, 2013). Thus, larger palms could play a significant role as individual seed sources in the regeneration of the population, as observed in Astrocaryum malybo H.Karst., whose older individuals favor population recovery in relict forests of the Colombian Caribbean (García et al., 2017). The remaining biometric variables, like petiole diameter and the number of veins, do not seem to influence the production of reproductive structures, despite their positive (but weak) correlation with several reproductive parameters.
The average fruit production of an adult A. nucífera (17 fruits/year) is quite low in comparison with other Attalea species for which data are available. Feil (1996) reported production of ca. 8000 fruits/individual/year in Attalea colenda (O.F.Cook) Balslev & A.J.Hend, and Guedes et al. (2015) reported average production of 594 fruit/year/ for individuals of Attalea vítrívír Zona. Although this comparison should be interpreted with care, given the difference in fruit size among these species, the production of few, large-sized fruits in A. nucífera is remarkable. New studies in other acaulescent Attalea species with similar habits, such as Attalea alleníí H.E.Moore or Attalea cuatrecasana (Dugand) A.J.Hend., Galeano and R.Bernal, are needed to broaden our understanding of fruit production in Attalea palm species. Under the conditions of habitat deterioration where A. nucífera grows in Guaduas, low fruit production is probably a disadvantage, since there are fewer opportunities for the successful dispersal and germination of fruits. Preliminary observations made by the authors indicate that animals disperse only 30 % of the fruits with an average dispersal radius of only four meters around the source individual. These conditions could explain the population decline found by Prada (2018) for this species in the same area of Guaduas.
CONCLUSIONS
Phenology and fruit production of Attalea nucífera is described in this study for the first time. The reproductive cycle of A. nucífera took 12.6 months from bud emergence to fruit ripening, and during the period of observation palms produced both staminate and pistillate inflorescences, although they were functionally dioecious. All palms observed produced buds throughout the year with highest production levels during periods of increased precipitation. The population had a sustained supply of staminate inflorescences year-round, however, pistillate inflorescences were only observed at the end of the rainy season. In contrast, the growth and ripening of the fruits occurred mainly during the periods of low rainfall.
On average, an adult palm of A. nucífera produce around 17 fruits/year, and only 5 % of each fruit consists of an edible nut. This indicates a low potential for promoting the widespread use of A. nucífera fruits and highlights this species vulnerability to habitat disturbance and forest conversion to other uses.