INTRODUCTION
The recent attention given to the systematic of the nectarivorous tailless bats of the genus Anoura (Glossophaginae: Anourina) has resulted in taxonomic additions in the subfamily Glossophaginae, with seven species newly recognized in the last four decades (Molinari, 1994; Muchhala et al., 2005; Mantilla-Meluk and Baker, 2006; Mantilla-Meluk and Baker, 2010; Pacheco et al., 2018). A significant portion of these additions corresponds to the elevation of diversity previously documented as subspecies in the A. caudifer and A. geoffroyi complexes (Mantilla-Meluk and Baker, 2006; Mantilla-Meluk and Baker, 2010; Pacheco et al., 2018). Most of the recognized species in the genus have relatively wide distributions (Gardner, 2008), that include contrasting environments, and natural barriers that have potentially restricted the genetic flow among subpopulations (Mantilla-Meluk and Baker, 2006). Interestingly, Clare et al. (2011) reported high genetic divergence between samples of A. caudifer recovered from the Guianan shield and Ecuador (maximum intraspecific distance: 16.5 %). These authors also report considerable genetic differences between samples of A. geoffroyi from the Guianan shield and Central American populations that include samples from Guatemala, Honduras and Panama (maximum intraspecific distance: 7.8 %).
Central American populations of Anoura, putatively assigned to A. geoffroyi and historically identified as A. g. lasiopyga (Anderson, 1957), have been subjected to taxonomic debate. On the other hand, A. caudifer extend its distribution from Southern Brazil into the Pacific coast of Colombia and Ecuador, on both versants of the Andean system, identified as a geographic barrier preventing gene flow among bat populations in other phyllostomid taxa (Gardner, 2008), suggesting a potential genetic divergence associated geographic structure among populations within this taxon.
Currently, to there is not phylogeographic hypothesis for the genus Anoura derived from the analysis of molecular data that allows for a quantification of potentially vicariant events within the genus. The objective of the present study was to further examine the degree of divergence among A. geoffroyi and A. caudifer populations from several localities in Central and South America, based on sequence variation in the barcoding gene COI (cytochrome oxidase subunit I). Sequences examined here were obtained from specimens collected across a significant portion of the geographic range of both A. geoffroyi and A. caudifer.
MATERIALS AND METHODS
Molecular information and alignment of sequences
Sequences of A. caudifer and A. geoffroyi from several localities in Central and South America, including both East and pacific slopes of the Andes, were obtained from the GenBank database. Geographic coordinates associated with analyzed sequences were taken from the Barcoding of Life Database (Appendix I). To further examine phylogenetic relationships within the genus Anoura, we also included COI sequences of A. latidens and A. cultrata. Finally, sequences of Glossophaga soricina, Lonchorhina orinocensis, Choeroniscus minor and Phyllostomus discolor (Appendix 1) were selected as outgroups following Baker et al. (2016).
Alignments were performed in the MEGA7® software (Kumar et al., 2016), implementing the Muscle algorithm with predetermined values. The evolutionary model was obtained according to the AICc information criterion, selecting the HKY+G model.
Haplotypes and gene flow among populations
Haplotype diversity and gene flow among populations were analyzed through pairwise genetic distances and Fst estimation in DNASP v5 (Librado and Rozas, 2009; Peña-Cruz et al., 2015). We performed a permutation test in GenAlEx 6.5 based on i) a matrix of genetic distances from Fst values obtained, and ii) geographic distances, infer from locality coordinates (Peakall and Smouse, 2012). Amid the construction of our haplotype network, based on the Median-Joining Network algorithm in PopART v1.7 (Leigh and Bryant, 2015), we excluded localities from Panamá and Venezuela, represented by only one individual.
Phylogenetic analysis and time of divergence
Phylogenetic relationships were inferred in MrBayes 3.2 (Ronquist et al., 2012). We executed the MCMC analysis (Markov Chain Monte-Carlo) during 50 million generations, samples procured every 1000a generation, and 25 % of the initial trees were eliminated to obtain the consensus tree.
Divergence times among clades were estimated in Beast v 1.7.5 (Drummond and Rambaut, 2007), for which we incorporated external calibration points with priors of normal distribution, based in the divergence times estimated by Rojas et al. (2016) for the nodes of each outgroup: Choeroniscus minor (17.5 ma), Glossophaga soricina (19 ma), Lonchorina orinocensis (23 ma), Phyllostomus discolor (27 ma). The MCMC analysis was executed during 50 million generations and samples procured every 1000a generation. The sampling of the parameters (ESS < 200) was verified in Tracer v1.6. We eliminated 25 % of the first trees to obtain the consensus tree in TreeAnnotator v.1.7.5, later edited and visualized in Figtree v.1.3.1. (Gernhard, 2008).
RESULTS
Anoura geoffroyi.- We obtained 27 haplotypes among A. geoffroyi samples, that grouped into two haplogroups: a) Central America and b) South America (Fig. 1). Maximum intraspeciic distance values were 7.023 %, and average intraspecific distance was 1.141 %, with nucleotide diversity ofn = 0.0142. South American A. geoffroyi samples from Guiana, Suriname and Venezuela share eight haplotypes, with low genetic divergence. Samples from east and southwestern Suriname share a haplotype with the Roraima mount population in northwestern of Guiana. The sampling localities that comprise the Central American haplogroup (Panama, Chiriqui; Guatemala, Zacapa; and El Salvador, Santa Ana) do not share haplotypes among them (Fig. 1).
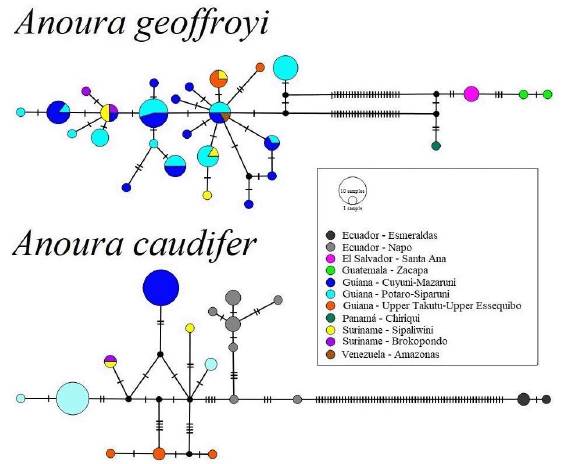
Figure 1: Haplotype networks for the mitochondrial cytochrome oxidase subunit 1 (COI) gene sequences of Anoura caudifer and A. geoffroyi, distributed from Guiana to Ecuador and from Guiana to Guatemala respectively, under the MJ approach (median-joining network) and plotted in Popart v 1.7.
Anoura caudifer.- Eighteen haplotypes were identiied for analyzed samples within A. caudifer, subdivided into two haplogroups: i) Surinam, Guiana, and eastern Ecuador, and ii) Western Ecuador. Values of maximum intraspecific distance were 15 %, with an average intraspecific value of 2 %, and a with nucleotide diversity of n = 0.043. The first haplogroup showed a marked genetic structure, with only populations from Sipaliwini and Brokopondo in Suriname sharing haplotypes among them (N = 1). Samples from eastern Ecuador and Guiana comprised a unique haplotype (Fig. 1). The second haplogroup, conformed by samples from Esmeraldas Province, northwestern Ecuador, accumulated 64 mutational steps from the rest of the analyzed samples.
Mantel test. - For Anoura geoffroyi populations, the Mantel test shows a signiicant relationship between genetic variation and the geographical distance (Fig. 2), in addition, an index of Fst > 0.8 among haplogroups reveals a decrease in gene flow as geographical distance increments. On its part for A. caudifer, the Mantel test estimated a lower R2 value between genetic divergence and distance among location, with a Fst value > 0.9. This result is primarily associated with higher genetic differentiation between populations on the eastern and western slopes of the Andes in Ecuador, separated by a relatively short geographic distance (approximately 400 Km).
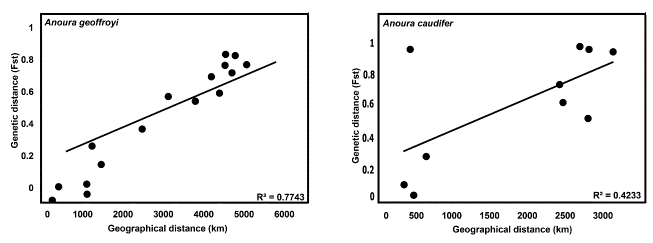
Figure 2: Permutation tests of genetic distances (Fst) and geographic distances for Anoura caudifer and Anoura geoffroyi sequences. The values of R2 are shown.
Phylogenetic analysis. - Our phylogenetic analysis recovered the A. caudifer haplogroups as polyphyletic: with the Pacific Ecuadorian haplogroup as the sister of A. cultrata, and the Ecuadorian piedemonts and Guiana haplogroups as the sister ofthe A. latidens + A. geoffroyiclade (Fig. 3). The sequence JF447477, from Suriname (identified as A. caudifer), was recovered outside of the Guiana/Suriname caudifer clade but recovered inside the group in the haplotype network. In our phylogeny, A. geoffroyi appears as independent from A. latidens, with two clades, representing: i) Central American and ii) South American populations with an estimated divergence time 3.57 ma. On its part, A. cultrata + pacific A. caudifer clade split from the other clades ca 7.56 ma., and separated from Guiana + eastern Ecuador ca 0.72 ma.
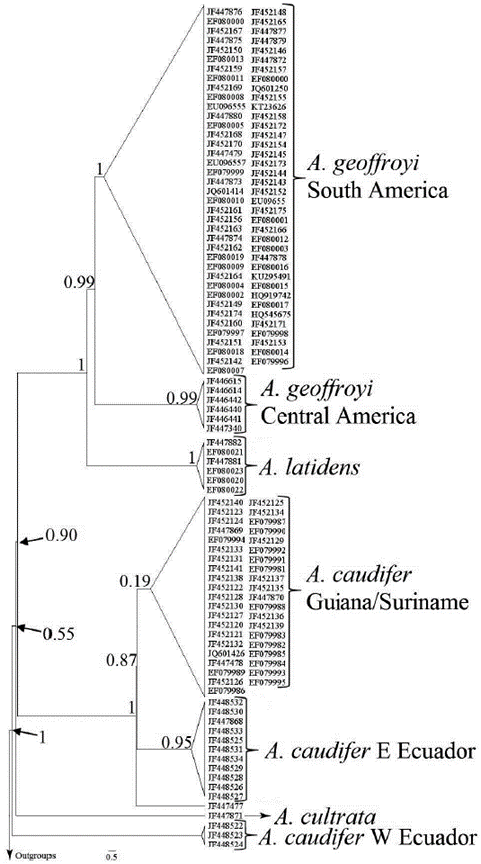
Figure 3 Phylogenetic tree constructed in MrBayes 3.2 (Ronquist et al., 2012), using the concatenated dataset of COI sequences published for the genus Anoura, including sequences of A. latidens and A. cultrata. Bayesian posterior probabilities of relevant nodes are shown.
DISCUSSION
South American A. caudifer. - The taxonomy of A. caudifer has been the subject of a broad debate. Having its type locality in Brazil, this taxon has a wide distribution in South America across areas with contrasting geologic origins and ecologies including the Cambric Brazilian and Guianan shields, the lowlands of the Amazon and from the piedmonts to high elevations in the northern Andes (Mantilla-Meluk and Baker, 2006; Griffiths and Gardner, 2007). Lomberg (1921) recognized Anoura (Lonchoglossa) aequatoris for western Ecuador and Peru, as different from the rest of South American populations of A. caudifer.Mantilla-Meluk and Baker (2006) treated A. caudifer as a complex of species from which A. aequatoris, A. luismanueli, A. fistulata, and A. cadenai have been derived. However, Jarrin-V. and Kunz (2008) discuss the lack of consistency in the morphometric analyses supporting the propose novelties within this taxon and explaining the observed difference as a result of morphological clinal variation among the populations. Recently, Pacheco et al. (2018) described a new species belonging to the A. caudifer complex (A. javieri), restricted to the upper montane forests of Manu Biosphere Reserve, Cusco, Peru, and possibly Bolivia.
Although the geographic coverage in our study was limited by the availability of sequences, we recovered several divergent clades, some of them supported by a substantial amount of genetic differentiation. Anoura caudifer sequences grouped into four phylogroups (Fig. 3). The most divergence clade of A. caudifer representing populations from the western slope of the Andes was placed as the sister of the rest of the samples, including A. cultrate and A. caudifer sequences from eastern South America, and representatives of A. geoffroyi from Central and South America. Two of the currently recognized species in the genus occur in western South America: A. cadenai and A. fistulata. On the eastern versant of the Ecuadorian Andes, only A. fistulata and A. caudifer have been reported. The observed divergence is interpreted as a result of a vicariant event associated with the Andes uplifting, geographic feature that operates as a geographic barrier (Gregory-Wodzicki, 2000; Graham et al., 2001; Baker et al., 2003; Ghosh et al., 2006; Mantilla-Meluk and Baker, 2006; Garzione et al., 2008; Leier et al., 2013). This result is also supported by the haplotype network, and the low value of R2 (0.42) obtained in the permutations test, since cis and trans-Andean Ecuadorian haplogroups, with low geographical distance (≈ 300 km) have a high genetic distance (> 60 mutational steps). When we compare the Guianan and trans-Andean Ecuadorian haplogroups (separated by ≈ 2272 km), we found a significantly lower genetic distance (five mutational steps).
Without examining voucher specimens, we cannot identify the species from which the western Ecuadorian A. caudifer haplogroup belongs, and whether the Esmeralda series (Appendix 1) represents a new species or one of the already described species within the A. caudifer complex. In Ecuador, Anoura is represented by five species: Anoura aequatoris (Lönnberg 1921) distributed in the western, eastern and low Amazon piedmonts (Tirira, 2017); Anoura caudifer Geoffroy (1818), distributed mainly on the north coast, low Amazonas and piedmonts of the Andes (Tirira, 2017); Anoura cultrata Handley(1960) distributed in western, eastern and low Amazon piedmonts (Tirira, 2017); Anoura fistulataMuchhala, Mena-Valenzuela and Albuja (2005), distributed in the piedmonts on both sides of the Andes (Tirira, 2017); and Anoura peruana Tschudi, 1844, distributed at the south coast, Sierra and piedmonts on both sides of the Andes (Tirira, 2017). Tirira (2017) highlights the lack of resolution of the diversity and taxonomy of Anoura in Ecuador, recommending an exhaustive review. It should be noted that the species' distributions mapped in Tirira (2017), do not include the populations of A. caudifer of the Pacific Coast of northern Ecuador (Esmeraldas), a situation that adds to the overlook of the A. caudifer complex in Ecuador. This situation validates the importance of the conclusions provided in this study. Therefore, it is imperative to review the taxonomy of the A. caudifer specimens from which the sequences analyzed here belongs, taking a step further to clarify the taxonomy of the A. caudifer complex.
Central and South American A. geoffroyi.- The taxonomy of Central American populations of Anoura has been the subject of extensive discussions (Arroyo-Cabrales and Gardner, 2003; Mantilla-Meluk and Baker, 2010; Solari and Martinez-Diaz, 2014; Calderon-Acevedo and Muchhala, 2018; Pacheco et al., 2018). Saussure (1860) identified Anoura ecaudata based on an individual collected in the central region of Mexico (Arroyo-Cabrales and Gardner, 2003); this individual was lately assigned by Peters (1868) in a new genus and species, Glossonycteris lasiopyga, based on his interpretation of the dental formula (Arroyo-Cabrales and Gardner, 2003). Thomas (1893) considered G. lasiopyga as a junior synonym of A. geoffroyi, being Sanborn (1933) the first author using the name combination Anoura geoffroyi lasiopyga. Recently, Mantilla Meluk and Baker (2010) proposed the elevation of A. g. lasiopyga to the specific level, based on cranial differences in the snout angle between the holotype of Glossonycteris lasiopyga (MHNG 515.88 S) and typical and A. g. peruana from South America. Besides, these authors discuss on coat color pattern differences between Central American specimens from Hidalgo Mexico (TTU 15474 and 1547, Light-Gray to Light-Drab, Ridgeway, 1912). Until 1957, A. g. lasiopyga was known only from 41 specimens, all from localities north of Guatemala (Goodwin, 1934). Anderson (1957) extended the distribution of A. g. lasiopyga into Costa Rica based on one specimen (KU 39249) from San Rafael, Canton de Tarrazu (misspelled on tag as Terrazi), 30 km S from San Jose (1300 m). Anderson (1957) compared specimen KU 39249 with a series of 13 specimens (KU 60635-46, KU 60978-61076) from Teopisca, Chiapas, and mentioned that despite differences in external color and cranial measurements, specimen KU 39249 was within the ranges proposed for A. g. lasiopyga. Also, specimen KU 39249 was paler than typical A. g. peruana from South America and both the Costa Rican and the Mexican specimens averaged smaller than those of A. g. peruana (Anderson, 1957). The above paragraph points on the difficulties in determining the southern limits of Central American variants of Anoura. On the other hand, the nominal subspecies A. g. geoffroyi has its type locality in "Rio de Janeiro, Brazil" (Vieira, 1942: 324), and up to date, the northern limits of its distribution remain unclear. Although Albuja (1983) and Ortega and Alarcon (2008) suggested the presence of A. g. lasiopyga into Ecuador and Colombia, respectively, Mantilla Meluk and Baker (2010) restricted its distribution to Central America medium and high elevations, from Mexico to Costa Rica.
This work represents the first approach to quantitatively determine the genetic degree of differentiation between Central and South American populations putatively assigned to A. geoffroyi (Fig. 3). Our results document the existence of an independent evolutionary destiny for Central American representatives of the genus, supporting the taxonomic and geographic delimitation proposed by Mantilla-Meluk and Baker (2010), and the consideration of A. lasiopyga as a full species. Our results also suggest the potential presence of A. lasiopyga for Panama. On the other hand, the genetic structure evidenced in our analysis for Central American haplogroups suggest the existence of three divergent population entities with low genetic flow between them (Fig. 1), potentially derived from vicariant events between Panama, Guatemala and El Salvador (Almendra and Roger, 2012). Globally, our analyses document a South American origin for the genus and a historical geographic expansion of South American Anoura through the Isthmus of Panama into Central America during the Pliocene (Almendra and Roger, 2012; Cuadrado-Ríos and Mantilla-Meluk, 2016; Rojas et al., 2016), accompanied by a latitudinal decrease in terms of gene flow across the subcontinents (Central and South America), supported by our mantel test (R2 = 0.77, Fig. 2).
Finally, our results also support the independence between A. latidens from the Guianan region and the northern Amazon populations of A. geoffroyi. The absence of available sequences from the Andean system prevented us to evaluate the phylogenetic placement of the subspecies A. g. peruana and A. carishina, described from specimens previously identified as A. geoffroyi. We are conscious with the fact that the number of sequences in our analysis is low, which could lead to an underestimation of the genetic flow rate and the average and maximum values of genetic distances (Larsen et al., 2007). We suggest a more detailed scrutiny with a greater geographic coverage, including samples from other localities in both Central and South America, mainly from or nearby type localities of identified subspecies within the so-called A. geoffroyi complex (Mantilla-Meluk and Baker, 2010; Clare et al., 2011); we also suggest the use of sequences from other molecular markers to perform population comparisons with a lower underestimation.














