INTRODUCTION
Human activity controls spatial heterogeneity in urban and suburban systems, and consequently defines the configuration of flora and fauna (Niemela et al., 2002; Hope et al., 2008; Kropf, 2017; Cilliers et al., 2018). The ecology of mammals in these environments has been studied by looking at their movement using techniques such as telemetry (Vaughan and Hawkins, 1999; Wright et al., 2012), but few studies have tried to understand their patch use behavior and selectivity (Bowers and Breland, 1996; López-Barragán and Sánchez, 2017), which can be studied using models derived from the marginal value theorem. The marginal value theorem (Charnov, 1976) assumes that foragers balance long-term energy gain, and it shows that this happens when foragers leave a feeding patch when instantaneous harvest rate falls to equal the long-term average. Brown (1988) extended the marginal value theorem and included additional costs associated to the perceived risk of predation and costs associated with the loss of alternative opportunities, such as resting, allocating time for breeding or looking for a potential partner. Brown's model predicts that the forager should use a food patch until the benefits from it, indicated by the harvest rate, no longer exceed foraging costs. The point where the forager stops using the patch corresponds to the quitting harvest rate. For a forager that experiences diminishing returns to its harvest rate, the more it exploits a patch, the greater the difficulty in obtaining food per unit of time (Brown, 1988). Thus, the harvest rate depends on the amount of food in the patch, and therefore the amount of food left behind by a forager, the giving-up density (GUD), is associated to the forager's quitting harvest rate (Brown, 1988; 1992). The more food the forager leaves in the patch, the higher are its foraging costs. The GUD technique has been widely applied to evaluate the effects of the perceived risk of predation on a variety of foragers (Brown, 1999; Brown and Kotler, 2007). This is because antipredator responses take place at the expense of time invested in foraging to obtain resources to try to maximize fitness.
We applied the GUD technique to examine the foraging decisions by the Andean White-eared Opossum Didelphis pernigra (Allen, 1900) (also known as fara, chucha, runcho or zarigüeya), in a suburban area of Bogotá, Colombian Andes. This marsupial is one of the most common mammals in the rural and suburban areas of Bogotá, and as far as we know, it is the only Didelphis present in the Sabana de Bogotá (Zúñiga et al., 1990; Calvachi, 2003; Sánchez, 2013; Mendoza and Sánchez, 2014). Previous studies have indicated that microhabitat selection by D. pernigra in suburban environments is conditioned by the risk of predation (Barrera-Niño and Sánchez, 2014; López-Barragán and Sánchez, 2017). This is probably due to the presence of birds of prey that remain around the urban areas such as owls and eagles (ABO, 2000), as well as exotic predators such as cats and dogs (Vanak and Gompper, 2009). Indeed, in the Colombian Andes there is evidence of attacks and killings of wild species by domestic dogs (Martínez-Polanco, 2008), and those carnivores have been suggested among one of the agents of mortality for Didelphis in urban areas (Cáceres, 2000). Indeed, we have observed D. pernigra killed by dogs in the rural-suburban area of Bogotá. Similar to other members of the genus Didelphis, D. pernigra are excellent tree climbers, and they spend considerable amounts of time on trees probably to avoid predators, although they visit the ground regularly (Pérez et al., 1994; Vaughan and Hawkins, 1999; Tirira, 2017). Therefore, we expected the foraging of D. pernigra to be influenced by the location of the food resources in relation to the distance from the ground, which may affect their perception of predation risk (Kotler et al., 1994; Orrock et al., 2004).
Bogotá has a population projection of 8 380 801 inhabitants for the year 2020 (DANE, 2018) and it is considered an example of the chaotic model of urban expansion and growth (Gonzalez, 2015). This has resulted in the loss of natural ecosystems, which in turn has caused several wild species to become threatened or gone extinct (CAR, 2012). Bogotá is at a highland plateau located above 2500 m. a. s. l. and has an average air temperature of 14.18 °C, and minimum temperatures can reach ~0 °C (data from 1989-2010, Meteorological station IDEAM code 2120626). This implies high energy demands to maintain body temperature for opossums, and consequently we expected concentration of energy in the patches and time used to obtain food to affect foraging. Therefore, we examined whether opossums selected food patches with high concentrations of energy but low amount of food versus patches with lower concentration of energy, but more food.
Evaluating the use of space by opossums could help understand the species requirements to move between suitable patches in highly modified environments (Hilty et al., 2019). Moreover, behavioral indicators associated to foraging may provide information on habitat quality and about the sites where foragers could make available benefits for humans, i.e., ecosystem services (Lindell, 2008). Behavioral indicators can be also used to determine places where there is a need to apply ecologically based management strategies. For example, one of the relevant areas for D. pernigra is the Thomas van der Hammen Protective and Productive Reserve in the north of Bogotá (Sánchez, 2010; Acuerdo CAR n° 11 de 2011; Mendoza and Sánchez, 2014). The Reserve is mainly a rural area with natural remnants, and restrictions in land use there should help protecting flora, fauna and other natural resources. Therefore, decision makers need information to preserve ecosystem services associated to this suburban Reserve. Since Bogotá's northern border is a mixture of urban and rural environments, we expected the opossum to perceive the spatial heterogeneity of the area so that its habitat selection will be affected at a ~200 m scale (Nupp and Swihart, 2000), including different types of fences. Thus, in this exercise, we studied the use of space by the opossums along different types of fences, i.e., metallic fences and live fences made of trees and shrubs.
STUDY AREA
This study was done at the University of Applied and Environmental Sciences (UDCA, abbreviation in Spanish), located north of Bogotá; ~2 560 m. a. s. l., 4°35' N - 74°04' W. The University is dominated by grasslands surrounded by scattered trees and/or live fences, mixed with classroom buildings of up to four floors, faculties, cattle fences, and soccer fields. The University is divided into a northern and a southern campus. The northern campus is part of the Thomas van der Hammen Reserve, but the southern campus, where the presence of opossums has been mostly recorded, is not (Sánchez, 2013). Our experiments took place in three linear sections A, B, and C, around a soccer field in the southern campus (Fig. 1). Sections A and C were located at a distance of ~40 m and ~100 m, respectively, from the Reserve/northern campus. Section A (Fig. 1) was a limit of the University and was surrounded by a flower crop outside its boundaries, and had a 2 m high, metallic fence, with barbed wire at the top and was 170 m long. This fence continued with a 10 m long, 1.5 m tall fence, with three lines of barbed wire. Next to section A, toward the soccer pitch, there were planted shrubs of Ricinus communis L. and trees of Sambucus peruviana Kunth, separated ~1.5 m from each other, and these plants then continued with a hedge of Cupressus sp., that contained individuals with their trunks separated ~50-70 cm from each other and their height kept at ~8 m. Section B was also a campus limit, had a ~80 m long, metallic fence made of hexagonal wire netting, with a metallic, horizontal bar at the top at ~2 m of height, and barbed wire. Trees of S. peruviana, Prunus sp., Alnus acuminata Kunth and Lafoensia acuminata (Ruiz and Pav.) DC were planted next to the fence, with a distance of 1-3 m from each other. Section C was within the campus, it has buildings surrounding it, and there was an artificial wetland nearby. Section C had no metallic fence, but had a live fence made of trees: Prunus serotina Ehr., Tecoma stans (L.) Kunth, A. acuminata, Acacia sp., S. peruviana, Pittosporum undulatum Vent., Senna viarum (Little) H.S. Irwin and Barneby, Myrsine guanensis (Aubl.) Kuntze, Escallonia floribunda Kunth, and Abutiloninsigne Planch. These trees were 3-6 m tall, planted 1.5-2 m from each other and their crowns continually overlapped. In June 2012, University officials installed a cable (diameter: 3 cm) to transfer electrical energy from a building to a construction and crossed the live fence of section C at about 2 m from the ground. In all sections, the kikuyo grass, Cenchrus clandestinus (Hochst. ex Chiov.) Morrone dominated the herbaceous stratum; this grass was mowed at least once a month. We identified plant species using Mahecha et al. (2004) and followed the plant taxonomy presented in the International Plant Names Index (INPI.org).

Fig. 1 University of Applied and Environmental Sciences, Bogota, Colombia. A street divides the University in the two campuses (black dotted line). The northern campus (white box) is part of the Thomas van der Hammen Reserve, whereas the southern campus is in the city's urban expansion area (black box). Punctuated, white lines: sections where we evaluated the use of microhabitat and food selection (section B) and the use of space by the opossums (sections A, B and C). Image downloaded from DigitalGlobe © 2012.
FIRST EXPERIMENT - MICROHABITAT USE AND FOOD SELECTION
We did this experiment only in section B (Fig. 1). We recorded the presence of at least eight opossums visiting the southern campus in 2010 by direct observation, using live traps, and video recordings (F. Sánchez, unpublished data). Between November and December 2010, we used feeders (i.e., food patches) designed to generate diminishing returns on the opossums' harvest rates. The feeders consisted of plastic cylindrical containers with a base diameter of 5 cm, a height of 14.5 cm, and with the top open. Each feeder contained 80 glass spheres (diameter: 10 mm) to make it increasingly difficult for the opossums while exploiting the patch (Sánchez, 2006). To confirm that the animals foraging in the feeders were opossums, we placed double-sided tape around them to collect hair samples. All feeders had plastic roofs to protect them from the rain. We examined whether opossums selected food patches with high concentrations of energy but low amount of food versus patches with lower concentration of energy, but more food. We habituated opossums for nine nights using sugar and water mixtures in the feeders. During habituation, we used fresh banana slices between the feeders to attract opossums. After habituation and in all experimental nights, we changed the banana slices for cotton swabs impregnated with 2 mL of banana essence, and we measured for 15 nights the opossums' GUDs. During experiments we offered the opossums two types of food patches, one with 120 mL (~167 g) of a sugar-water mixture with a concentration of 29.41% w/w, weight/weight percent (concentrated mixture), whereas the second had 170 mL (~ 214 g) of a sugar-water mixture with a concentration of 22.72% w/w (diluted mixture). The amounts and concentrations were defined after preliminary experiments done at the study site. We placed the two food patches (concentrated and diluted) next to each other, and we considered them together as a station. There were six stations, with plastic roofs as protection against rain. The stations were located either 0.5 m or 2 m above the ground, interspersing the height between consecutive stations; three stations at 0.5 and three at 2 m; consecutive stations were 20 m apart. The location in the station (left or right) where concentrated or diluted food was placed, was randomly decided. We placed the feeders around sunset, ~ 17:30 h and collected the feeders the next day around sunrise, ~06:00 h; we weighed the amount of food left in the feeders, i.e., the GUDs. We placed the feeders at dusk and collected them at dawn due to the nocturnal behavior of the opossum, and to prevent diurnal animals to make use of the feeders.
SECOND EXPERIMENT - USE OF THE SPACE BY THE OPOSSUMS
We did this experiment in sections A, B, and C. During September and October 2011, and December 2012, we used the same feeders of the first experiment. We kept nocturnal observations from 2011 through 2012, and regularly observed opossums, suggesting similar levels of density in the different sampling periods. In each period, we habituated the opossums for three nights to the use of the feeders, because they quickly started using them from the first night. We arranged individual feeders in four sites in each of sections A, B, and C, i.e., 12 feeders in total placed 2 m above the ground. Consecutive feeders were 25-30 m from each other, and each feeder had 170 mL of sugar-water mixture with a concentration of 29.41% w/w since this concentration of food was the most visited and consumed in previous experiments. During habituation, we used again banana slices as bait, and as in the first experiment, and after habituation we replaced the banana with cotton swabs impregnated with 2 mL of banana essence. During five nights in September and October 2011, and December 2012, we measured GUDs for a total of 15 nights. As in the previous experiment, all feeders had plastic roofs, and we weighed and placed feeders before sunset, ~16:00 h, and we collected and weighed them the next day around sunrise, ~06:00 h, to measure GUDs.
Since opossums are semi-terrestrial marsupials with excellent tree climbing skills (Hunsaker, 1977; Vieira, 1997) and D. pernigra has been reported using trees to forage and as refuge in Bogotá (Mendoza and Sánchez, 2014), we measured several variables related to the trees around the feeding stations. Thus, following to the methods of Matteucci and Colma (1982), we measured: tree height, diameter at breast height (DBH), estimated canopy area, and number of species of trees in a 5 m radius around each feeding site.
DATA ANALYSES
The distance between consecutive stations was relatively short during the experiments, <30 m, and it was possible for an individual to visit several stations during a single night. Therefore, we used run tests to determine deviations from a random pattern in the use of the stations during each one on the nights of both experiments using as cutting point the average, median and mode of the GUDs (Zar, 1999). We found a deviation from randomness only in one night in experiment two, and therefore considered negligible the effect related to the possible use of the same animal of several stations. To examine the data from the first experiment, we applied a general linear mixed model with the logarithm with base 10 of the GUDs as the dependent variable, and experimental night (fifteen nights in total), height (0.5 m and 2 m) above ground, and food type (concentrated or diluted) as fixed factors, and station as random factor (Zar, 1999). We included the experimental night, since variation among nights may affect the opossum's foraging (López-Barragán and Sánchez, 2017). In addition, we used Levene tests and graphical analyses of residuals to examine the assumptions of normality and homogeneity of variance (Zar, 1999), and based on the analyses, we transformed the dependent variable as indicated earlier. We applied Tukey test as multiple comparisons methods for the first experiment.
To examine the data from the second experiment, we applied a generalized linear model with a distribution of gamma probability and link function identity, due to deviations from the assumptions of normality and homogeneity of variance (Marques de Sá, 2007). In the model, the GUDs entered as dependent variable, whereas the experimental night (fifteen nights in total), station, and sampling period (September and October 2011, and December 2012) did it as independent variables (Zar, 1999). Additionally, to examine if the variation in the use of stations was affected by their immediate environment, we did non-parametric correlations between tree height, diameter at breast height (DBH), estimated canopy area and number of species of trees in a radius of 5 m around each feeding site, and the GUDs. We used PASW Statistics v. 18.0.0 and used a = 0.05 as significance level to test all statistical hypotheses. We followed the method proposed by Wilson and Hardy (2002) to include only significant variables in the statistical models.
RESULTS
FIRST EXPERIMENT - MICROHABITAT USE AND FOOD SELECTION
We found that the interaction between the type of food (concentrated - diluted) and height from the ground (0.5 m - 2 m) significantly affected the opossums' foraging (mean squares [MS] = 0.03; F1172= 5.19; p = 0.024). Multiple comparisons indicated that when food was either concentrated or diluted, the opossums preferred to forage at 2m than at 0.5 m from the ground (Fig. 2). Also, concentrated food at 2 m was not significantly different from diluted food, whereas at 0.5 m from the ground opossums preferred concentrated over diluted food. Station (MS = 0.103; F4172= 17.75; p < 0.01) and type offood (MS = 0.240; F1172= 41.42; p < 0.01) independently also influenced the foraging decisions of the opossums. Experimental night and height had no effect, and they were not included in the model.
SECOND EXPERIMENT - USE OF THE SPACE BY THE OPOSSUMS
Station, sampling period and the interaction between station and sampling period significantly affected opossum's foraging, whereas night had not significant effect (Table 1). We used Wald's confidence intervals to examine the interaction between sampling period and station. In September 2011, section C presented higher GUDs compared to section B, except for one station (Fig. 3a), i.e., most of section B was more valuable than section C for the opossums. In this month, stations of section A had GUDs similar to those of section C, and were higher than two stations in section B. In October 2011, section B had lower GUDs compared to sections A and C, and three stations of section A showed GUDs similar to those in the stations ofsection C (Fig. 3a). In December 2012, stations of section A and C presented higher GUDs compared to those in section B, excepting for one station in B and one in C. We found no significant differences between stations in A and C (Fig. 3b). Thus, in general feeding stations in section B tended to have higher value for the opossums than stations in sections A or C. In addition, in December 2012 two stations (nine and ten in Fig. 3b) in section C had in several occasions considerably low GUDs, i.e., stations with GUDs 20-40 g below the average found in previous sampling periods. These low GUDs suggest an increase in the value of the food patch that did not happen in previous sampling periods, but this apparent increase in marginal value was not statistically significant.
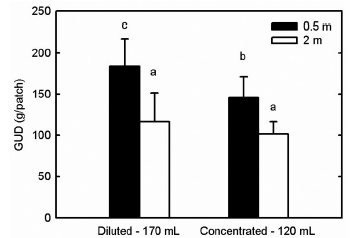
Fig. 2 Giving-up densities (GUD) for opossums in relation of the distance of the feeders to the ground and type of food (concentrated: 120 mL and 29.41% w / w - weight/weight percent - concentration; diluted: 170 mL and 22.72% w/w - weight/weight percent -concentration). The GUDs at the feeders either concentrated or diluted were significantly different at both heights, and food preference was modulated by the height from the ground. We present averages + 1 standard deviation. Bars with different letters indicate significant differences (Tukey test, p <0.05).
Table 1 Results of the generalized linear model using the opossum's GUDs as dependent variable, and station, sampling period, and night as independent variables. The interaction between station and sampling period was significant. All independent variables had significant effects, excepting for night. χ2- Wald= Wald's chi-squared; df= degrees of freedom.
| Source | χ2- Wald | df | p |
|---|---|---|---|
| Station | 631,45 | 11 | <0.001 |
| Sampling period | 25.68 | 2 | <0.001 |
| Night | 8,24 | 4 | 0.083 |
| Station * Sampling period | 164.71 | 22 | <0.001 |
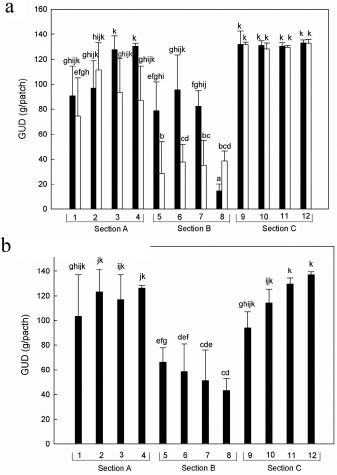
Fig. 3 Variation of giving-up densities (GUD) of Didelphis pernigra along three sections, each with four stations in southern campus in three sampling periods: (a) September 2011 (black bars), October 2011 (white bars), and (b) December 2012. Each feeder had 170 mL of sugar-water mixture with a concentration of 29.41% w/w (weight/ weight percent). We present averages + 1 standard deviation. Bars with different letters indicate significant differences according to Wald's 95% confidence intervals.
Non-parametric correlations between tree height, tree DBH and species richness of trees and GUDs in the three time periods were not significant (all p > 0.05). Only tree cover had a significant negative correlation with GUDs in September (Spearman Rho [S r ] = -0.61, p = 0.035), October (Sr = -0.71, p < 0.01), and December (Sr = -0.73, p < 0.01).
DISCUSSION
In the first experiment, the stations only differed in height and included both types of foods, and the stations were relatively close to each other, which mean that the missed opportunity costs in all stations were very similar (Brown, 1988). Thus, our results suggest that the perception of risk influenced food preferences. In general, the opossums preferred foraging where the risk of predation was low, on the metallic fence, but there was no difference between food preference depending on the height from the ground. This result is in agreement with studies in several species of rodents whose feeding preferences depend on how risky a microhabitat is (Schmidt, 2000; Leaver and Daly, 2003), but contrasts with a study with Capra ibex in which the change in the security level did not affect food selection (Hochman and Kotler, 2006). Microhabitat selection by the opossums in terms of heigh, i.e., foraging more at 2 m above the ground, suggests that opossums perceive lower risk of being predated by aerial predators such as the owls and eagles (ABO, 2000), than by terrestrial predators. This is probably because adult opossums have a body mass which usually is higher than 1 kg (Mendoza and Sánchez, 2014), and they are probably too big for the nocturnal or early morning raptors available in Bogotá, which regularly include prey weighing less than 500 g (Huston and Nelson, 1994; ABO, 2000; Delgado-V et al., 2005; Riaño et al., 2017). It has been suggested that humans are perceived as a threat by D. pernigra, which may explain the preference of places far from the ground to forage (D. Cortés, unpublished data). We consider that using the top of the metallic fence is equivalent to the use of trees by D. marsupialis in rural landscapes in Costa Rica, which has been explained as a strategy to avoid encounters with local predators, including men and dogs (Vaughan and Hawkkins, 1999).
The preference of concentrated over diluted food at particular heights indicates that the opossums can behave selectively. This result is consistent with a previous work showing that this species is able to recognize between protein-rich and carbohydrate-rich food, and prefers the later, but the degree of the preference appears to depend on microhabitat characteristics (López-Barragán and Sánchez, 2017). Thus, despite the fact that Didelphis species are capable of exploiting a wide variety of food items, including prey of animal and plant origin (Pérez et al., 1994; Carvalho et al., 2005), i.e., they are diet generalist, they can behave selectively under particular circumstances (Rosenzweig, 1981), and the GUD technique can be used to detect such behavior.
In contrasts with previous studies showing temporal effects in a daily scale (Barrera-Niño and Sánchez, 2014; López-Barragán and Sánchez, 2017), the experimental night did not affect the opossum's foraging neither in the first nor in the second experiment. In one of the previous studies, such temporal effects were linked to variations in illumination of the moon, but, has also been recognized a high variability in the data. This variation may be linked to abiotic and biotic factors, including for example, movements of the animals. Indeed, D. marsupialis in rural environments may move more than 750 m per night, and they may have more than seven dens in a place (Vaughan and Hawkins, 1999). Unfortunately, such data are not available for D. pernigra, and are needed particular experiments designed to test these ideas, using techniques such as telemetry.
Spatial heterogeneity on campus affected foraging by opossums, and they preferred the metallic fences over the live ones. Furthermore, in December 2012 there was an apparent increase in the marginal value of food in two stations of the section C that coincided with the addition of an electricity cable by the University Maintenance Department crossing the campus, which could facilitate the mobility of the possums in this section. We also found a negative correlation between tree cover and the use of food patches in the months of study. Section C had more tree cover than the other sections, but, had no metallic fence or cable during September and October 2011. Thus, it appears that the negative relationship found here between GUDs and tree cover was not necessarily due to the trees, but because using the metallic fence was easier for opossums in sections A and B. The ability to move depends on the behavior and morphology of the species (Nupp and Swihart, 2000). Although the opossum can avidly move in trees (Pérez et al., 1994), our data and observations on campus indicate that the opossum move on the metallic fence (Section B) and barbed wires quite easily, and probably faster than when moving through the trees (Fig. 4). Escape may thus contribute to the foraging of the opossum and this is probably because the fences appear to provide easy escape from feeding patches (Brown, 1992), thus lowering foraging costs while the path through the trees is more tortuous, and therefore more energetically expensive.
Our results show that the use of the GUD technique can help to examine foraging decisions by the opossum in complex suburban environments in Bogotá. The information about the space more likely to be used by wild species such as the opossum when moving through highly disturbed environments can be used to mitigate the loss of connections between habitat-patches due to anthropogenic intervention (Gilbert-Norton et al., 2010). Our results support the idea that D. pernigra is a versatile species that can take advantage of human-made structures, so that there may be ways to mitigate conflict between humans and opossum in the Reserve. As Rosenzweig (2003) indicates, we humans can change the spaces that we use to allow wild species to persist around us, even if these modifications are not similar to the natural environment. Previous studies suggest that increasing tree-cover may benefit D. pernigra in suburban environments (López-Barragán and Sánchez, 2017). Thus, the use of structures such as metallic fences and the plantation of trees may complement each other to increase the chances of moving for the opossum. Also, to design and implement of ecological corridors this should be taken into account (Hilty et al., 2006).
CONCLUSIONS
This research shows that human decisions can have both positive and negative effects on wildlife, and we identified a positive effect of the presence of metal fences in the foraging and use of space by the Andean White-eared Opossum. Therefore, it is necessary to use our knowledge through environmentally reasonable actions to mitigate the accelerated changes in the environment that impose selective pressures on the species (Palumbi, 2001). Finally, to effectively conserve D. pernigra it is necessary to develop more studies about its response to the challenges posed in environments highly modified by humans, and also more studies on the attitude of the people of the area, including for example dog owners, to achieve their participation in conservation efforts.
















