INTRODUCTION
Reptiles, like many other biological groups, are strongly threatened by various anthropogenic factors, such as land-use changes, wildlife trafficking, pollution and global warming and catastrophic events (floods, fires, droughts; Díaz et al., 2006). Loss of habitat due to changes in vegetation cover and land use is a generalized cause that negatively affects most species in many different ecosystems (Martínez-Meyer et al., 2014). In particular, reptiles have been affected because the abundance and diversity of species vary according to changes in the composition and number of types of microhabitats (homogeneity-heterogeneity) present in the environment (Manzanilla and Péfaur, 2000; Magno-Benítez et al., 2016). However, some species can adapt to these modified environments, exploiting as many resources as possible in these environments, and consequently their populations may be favored (Germaine and Wakeling, 2001).
Some species of reptiles that have suffered a significant decrease in their population densities are iguanas of the genera Iguana and Ctenosaura (Valenzuela, 1981). Population declines of these species are, in most cases, caused by disturbance to their habitat (landscape fragmentation) and microhabitats (transformation of their shelters, trees, rocks, among others) within their distribution area, as well as exploitation by humans for commercial purposes (meat and eggs), handicrafts such as use of their skin for purses and belts, and other negative factors (Morales-Mávil et al., 2016). Historically, iguanas have been part of the human diet in marginalized communities in Central and South America. In addition, consumption of the meat of these species is common because it is rich in proteins; therefore, its commercial value is high, and it represents a source of income for people living in rural communities (Valenzuela, 1981; Pough et al., 1998). Young iguanas also are captured and sold to the pet trade (Valenzuela, 1981). These are some of the negative factors that individuals of these species face (Valenzuela, 1981). However, Fitch and Henderson (1978) and Lee (1996; 2000), among others, mention that some species of the genus Ctenosaura can be favored in sites disturbed by man.
Ctenosaura similis Gray (1831) is an endemic species to Mesoamerica (Mora, 1986; Lee, 2000). The distribution of this species is discontinuous in southern Mexico, Central America and in some Caribbean islands, at elevations from sea level to 800 m (Garrido and Sandoval, 1992; Lee, 1996). The species inhabits open areas of arid zones in Mexico, where its populations generally occur in disturbed places by humans (Fitch and Henderson, 1978). Individuals of this species live in burrows that they construct in the ground or in those that are already present among rocks or in logs, which they use as refuges to avoid predators and thermal stress (Burger and Gochfeld, 1991). With regards to the feeding habits of this species, hatchlings are initially insectivorous but gradually shift to a more omnivorous diet (Lee, 1996). At an age of six months, this species undergoes an ontogenetic change in its color pattern, becoming darker, and assuming a more robust body shape, including a distinctive crest (Lee, 1996; 2000). This information on the natural history of the species is based on isolated anecdotal accounts obtained from throughout its distribution area (Henderson, 1973; Fitch and Henderson, 1978; Mora, 1986; Burger and Gochfeld, 1991; Mora, 1991).
Therefore, considering the scarce data on the natural history of the species, we felt it was important to complement this information with more reports on basic aspects of the ecology of a population of the species. The goal of this study was to document aspects of the natural history of a population of the black iguana, C. similis, on Isla Contoy in the state of Quintana Roo, Mexico. This study focused primarily on feeding habits of the population, as well as the use of habitat and microhabitats by each sex and age class, and on evaluating some environmental factors, such as temperature and humidity, that may influence activity patterns of individuals.
MATERIALS AND METHODS
Study area
This study was carried out in the Parque Nacional Isla Contoy (PNIC; 21°29'N, 86°47'W), which is located in the northern part of the state of Quintana Roo, Mexico. The PNIC has a total area of 238.18 ha, of which 230.18 ha are mainland and small islets, and 8 ha consist of inland lagoons. The elevation ranges from zero to 12 m. a. s. l. The climate is warm subhumid with rain in summer. Mean annual temperature is 27.7 °C with little monthly variation, and a mean annual precipitation of 980 mm (INE, 1997); the vegetation type is coastal scrubland. In the PNIC there are four areas that are visited by tourists: (1) Buildings of the Parque Nacional Isla Contoy (BPNIC), an area where tourists arrive daily. This area is approximately 6 ha. The vegetation is a coastal scrubland association with sea grape (Coccoloba uvifera Linneo, 1759) and ciricote (Cordia sebestena Linneo, 1753). (2) Mangrove areas; this habitat is widely distributed on the island over an area of approximately 112 ha, that contains black mangrove (Avicennia germinans Linneo, 1764). (3) Rocky areas, located on the east (windward) side of the island, with an area of approximately 33 ha, exposed to strong waves from the open sea and the prevailing southeasterly winds. (4) Coastal dunes (CD), an area which is characterized by the presence of coastal scrub association with rocky substrate that occurs along almost the entire west coast of the island, with an area of approximately 79 ha.
Habitat use, microhabitats and feeding habits
Data were recorded while walking, and from a boat sailing along the shores of the island, to register sites occupied by iguana, and thus describe habitat use according to the frequency of observations in the four different habitat types described above, as well as to identify the type of microhabitat within each habitat used by the observed individuals. In each habitat, the following types of potential microhabitats were distinguished; (1) BPNIC: tree, bush, concrete wall, grass or wood plank, (2) mangrove areas: tree, bush, sand or leaf litter, (3) rocky areas: rock, and (4) CD: bush, grass or sand. The observations were made during two weeks each month from May 2001 to May 2002 in a schedule from 0600 h to 1800 h. Observations were made during two-hour data collection periods with intervals of one hour between them, enabling documentation of peak activity periods of the iguanas over the course of the day. For each iguana, we recorded its sex, size class (hatchling, juvenile, or adult), as well as environmental and microhabitat temperatures where the iguana was perching, using a fast reading thermometer (Taylor, 0-50 °C), percent humidity, time, and weather conditions (cloudy, sunny, windy). When iguanas were observed foraging, the type of food they were consuming was recorded, classifying it as vegetable matter (fruits, seeds, leaves, and flowers) or animal matter (insects and vertebrates). In addition, some iguana feces were collected to supplement information about diet items. To determine items in the diet, for vegetable matter a catalog of the vegetation of Isla Contoy was used, supplemented with a floristic summary for the island of Cozumel, Quintana Roo (Téllez and Cabrera, 1987), to attempt to identified plant diet items to the species level. For animal diet items, insects were identified to the order level (Leyte-Manrique and Ramírez-Bautista, 2010), while terrestrial vertebrates were identified to the genus level, and when possible, to the species level, using a National Geographic field guide (1999) and comparing specimens with studies by Lee (1996; 2000).
Iguanas were not captured, but was estimated how many were resident in each place, and they were identified based on color patterns and scars or other marks on the body; sex was determined based upon the larger, more conspicuous dorsal crests of the males (Lee, 1996; 2000). Individuals were classified into age (size) classes based on estimated snout-vent lengths (SVL); those that measured 5-15 cm SVL (approx.) were considered to be hatchlings, 16-25 cm (approx.) juveniles, and larger individuals (> 26 cm SVL) adults. In addition, this classification was complemented with information on the color patterns of the iguanas, as the pale grayish-brown individuals with dark brown reticles were considered hatchlings (some hatchlings were also green), those with a bright green color without dark markings or very small spots were considered juveniles, and those with a grayish pattern with black bands were recognized as adults (Lee, 1996; 2000).
Analyses
The difference in frequencies of habitat and microhabitat use between sexes and age classes was evaluated using contingency tables and Chi square tests. For feeding habits, the frequencies of the types of prey consumed by adults, juveniles and hatchlings were recorded, and date information was used to show how these frequencies changed throughout the year (spring, summer, autumn and winter). With this information, contingency tables and Chi square tests were performed (Zar, 1996). In addition, differences in environmental and substrate temperatures, and percent humidity at the time when males and females were observed to be active, were evaluated using U Mann-Whitney tests (Zar, 1996). Differences in values for these variables among age classes were analyzed using Kruskal-Wallis tests, and subsequently a post-hoc test was applied when there were significant differences (Zar, 1996).
RESULTS
Habitat and microhabitat use
Ctenosaura similis individuals inhabited the entire area of the island; however, individuals were more concentrated in mangrove habitat (35 %), followed by BPNIC areas (25.8 %), rocky zones (23 %), and coastal dunes (17.7 %). The frequency of habitat use suggested that each age class used a particular habitat more frequently (X2 = 30.39, d.f. = 6, p = 0.00003, Table 1). Adults and juveniles were more frequent in the mangrove zone, while the hatchlings were more common near the facilities of the BPNIC (Table 1). A chi-square test also showed differences in habitat use between sexes (X2 = 15.13, d.f. = 3, p = 0.017, Table 1), with males found more frequently in the facilities of the BPNIC, while females were seen more in the mangrove zone (Table1).The frequencyin the use of the microhabitats was also different among age classes (X2 = 34.84, d.f. = 14, p = 0.0015, Table 2). However, all three age classes used rocks and trees more frequently, while the least used microhabitat type was grass (Table 2). Comparing the sexes, there seemed to be no difference in the frequency of use of microhabitat types (X2 = 10.91, d.f. = 6, p = 0.090, Table 4). Both males and females more frequently exploited rocks and trees, and were seen less frequently in bushes, litter, or on concrete walls (Table 2).
Table 1 Frequency of habitat use (percentage) by age class and sex (adult males and females) of Ctenosaura similis from Isla Contoy, Mexico. n = total number of observations.
| Habitat | Age classes | Sex (only adults) | |||
|---|---|---|---|---|---|
| Adults (n= 344) | Juveniles (n=151) | Offspring (n= 36) | Males (n= 162) | Females (n= 182) | |
| BPNIC | 27.9 | 15.8 | 47.2 | 35.1 | 21.4 |
| Coastal dunes | 15.4 | 19.2 | 33.3 | 11.7 | 18.6 |
| Mangrove area | 32.5 | 40.30 | 13.8 | 25.3 | 39 |
| Rocky zone | 24.12 | 24.5 | 5.5 | 27.7 | 2.8 |
Table 2 Frequency of microhabitat use (percentage) by age class and sex (adult males and females) of Ctenosaura similis. n = number of observations.
| Microhabitat | Age classes | Sex (only adults) | |||
|---|---|---|---|---|---|
| Adults(n= 332) | Juveniles(n= 146) | Offspring (n= 34) | Males (n= 159) | Females (n= 173) | |
| Tree | 25.60 | 34.24 | 14.7 | 21.38 | 29.48 |
| Bush | 1.5 | 4.1 | 2.9 | 1.26 | 1.73 |
| Sand | 12.04 | 8.9 | 14.7 | 13.21 | 10.98 |
| Concrete fence | 6.3 | 2.7 | 11.76 | 10.06 | 2.89 |
| Leaflitter | 4.8 | 10.95 | 5.8 | 3.77 | 5.78 |
| Rock | 35.8 | 33.5 | 32.35 | 34.59 | 36.99 |
| Wood plank | 13.8 | 4.7 | 14.7 | 15.72 | 12.14 |
| Grass | 0 | 0.68 | 2.9 | 0 | 0 |
Table 3 Frequency (percentage) of observation by age class of Ctenosaura similis that were seen feeding on any kind of food in Isla Contoy, Mexico. n = Number of observations.
| Type of food | Age class | ||
|---|---|---|---|
| Adults (n=49) | Juveniles (n=28) | Offspring (n=13) | |
| Flowers | 6.12 | 21.42 | 30.76 |
| Fruits | 32.65 | 35.71 | 23.07 |
| Leaves | 34.69 | 25 | 0 |
| Invertebrates | 0 | 17.85 | 46.15 |
| Vertebrates | 26.53 | 0 | 0 |
Table 4 Observed frequency (percentage) of food consumption by individuals of Ctenosaura similis during the four seasons of the year in Isla Contoy, Mexico. n = Number of observations.
| Type food | Seasons | |||
|---|---|---|---|---|
| Spring (n=20) | Summer (n= 24) | Autumn (n= 22) | Winter (n=24) | |
| Flowers | 10 | 12.5 | 4.54 | 29.1 |
| Fruits | 45 | 33.33 | 45.45 | 8.33 |
| Leaves | 40 | 20.83 | 22.72 | 25 |
| Invertebrates | 5 | 0 | 18.18 | 25 |
| Vertebrates | 0 | 33.33 | 9.09 | 12.05 |
The environmental temperature at the time when males (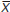 = 26.9 ± 0.15 °C) and females were found (
= 26.9 ± 0.15 °C) and females were found (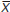 = 27.07 ± 0.13 °C) did not differ (U = 13605, p = 0.865), nor did the temperatures of substrates (U = 13595, p = 0.99) used by the sexes (males, X = 28.8 ± 0.18 °C, females, X = 28.9 ± 0.19 °C), nor did percent humidity while active (U = 1362, p = 0.9527)) differ between males (
= 27.07 ± 0.13 °C) did not differ (U = 13605, p = 0.865), nor did the temperatures of substrates (U = 13595, p = 0.99) used by the sexes (males, X = 28.8 ± 0.18 °C, females, X = 28.9 ± 0.19 °C), nor did percent humidity while active (U = 1362, p = 0.9527)) differ between males (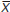 = 52.64 ± 0.68 %) and females (
= 52.64 ± 0.68 %) and females (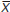 = 52.6 ± 0.63 %). The same pattern held when comparing age classes, with no differences in environmental temperature (H = 4.35, p = 0.114) during the times when adults (
= 52.6 ± 0.63 %). The same pattern held when comparing age classes, with no differences in environmental temperature (H = 4.35, p = 0.114) during the times when adults (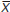 = 27.01 ± 0.09 °C), juveniles (
= 27.01 ± 0.09 °C), juveniles (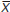 = 27.38 ± 0.19 °C) or hatchlings (
= 27.38 ± 0.19 °C) or hatchlings (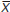 = 27.35 ± 0.25 °C) were active. However, significant differences were found (H = 8.24, p = 0.016) in the substrate temperature used by individuals. A post-hoc test indicated that hatchlings (
= 27.35 ± 0.25 °C) were active. However, significant differences were found (H = 8.24, p = 0.016) in the substrate temperature used by individuals. A post-hoc test indicated that hatchlings (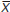 = 30.10 ± 0.48 °C) differed in this variable from juveniles (
= 30.10 ± 0.48 °C) differed in this variable from juveniles (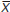 = 29.27 ± 0.24 °C, p = 0.043), and from adults (
= 29.27 ± 0.24 °C, p = 0.043), and from adults (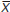 = 28.85 ± 0.13 °C, p = 0.0032). In addition, differences were registered (H = 7.95, p = 0.01) in percent humidity at the time when iguanas were active, differing between hatchlings (
= 28.85 ± 0.13 °C, p = 0.0032). In addition, differences were registered (H = 7.95, p = 0.01) in percent humidity at the time when iguanas were active, differing between hatchlings (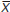 = 50.55 ± 1.35 %) and juveniles (
= 50.55 ± 1.35 %) and juveniles (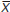 = 53.55 ± 0.79 %; p = 0.0098). Although a difference was observed between the means of hatchlings (
= 53.55 ± 0.79 %; p = 0.0098). Although a difference was observed between the means of hatchlings (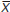 = 50.55 ± 1.35 %) and adults (
= 50.55 ± 1.35 %) and adults (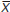 = 52.83 ± 0.45 %), these were not significant (p = 0.058).
= 52.83 ± 0.45 %), these were not significant (p = 0.058).
Feeding habits
Based on observed feeding bouts, Ctenosaura similis consumes several species of plants and animals (in some cases, dead animals, Appendix 1). The analysis showed that this species has an ontogenetic change in its diet (X2 = 40.66, d.f. = 8, p = 0.000002, Table 3). Hatchlings and juveniles fed on invertebrates (insects), flowers, fruits, and less frequently on leaves; while adults ate leaves, fruits and some vertebrates, such as birds (several species), turtles, iguanas (cannibalism), and fishes (Table 3, Appendix 1); for example, adult iguanas were observed on two occasions feeding on newly hatched sea turtles (Eretmochelys imbricata Linnaeus, 1766). Moreover, three adult individuals were observed practicing cannibalism, two of them consuming hatchlings, and another individual eating an adult that was smaller in SVL.
Analyzing all the diet data, it was also apparent that the frequency with which these iguanas consume certain types of food items differed by season of the year (X2 = 30.64, d.f. = 12, p = 0.0022, Table 4). In spring, iguanas consumed fruits and leaves more frequently, in summer there was a higher consumption of fruits and vertebrates, while in autumn, fruits and leaves were again the most consumed food items, and in winter there was a greater frequency of feeding on flowers, leaves and invertebrates (Table 4).
DISCUSSION
The habitat in which an individual lives directly influences on its growth, survival and reproduction, and therefore its life history (Stearns, 1992; Ramírez-Bautista, 1995). Factors such as the availability of food, shelter, or the risk of predation, among others, influence variation in foraging success, survival, and reproduction of animals (Ramírez-Bautista, 1995). Therefore, the choice of the a good habitat is important for the activities (feeding and growth, among others) of individuals (Ramírez-Bautista and Vitt, 1997). This being so, although C. similis was distributed throughout the study island, individuals used mangroves and BPNIC habitats more frequently, perhaps because these places offered more shelters and feeding areas. This pattern varied somewhat between age and sex classes, since adults and juveniles were seen more frequently in the mangrove zones, while hatchlings used the BPNIC area more frequently (Table 1). Overall size classes, males used the BPNIC more frequently; in contrast, females used the mangrove zone more (Table 1). These results are similar to those reported by Rogel (1979), Fitch and Henderson (1978), and Burger and Gochfeld (1991), who noted that both adults and juveniles inhabit mangrove areas, indicating that there are microhabitats in these areas that iguanas can exploit, such as the hollows of trunks of the black mangrove (Avicennina germinans), rock crevices, and holes in the sand that they dig themselves. In addition, Fitch and Hackfort-Jones (1983) stated that individuals of Ctenosaura similis can adapt to different types of habitat, including areas disturbed by humans, such as vacant lots, fences and walls, peripheral zones around crops field, and grazing areas. Fitch and Henderson (1978) noted that sites disturbed by humans may provide shelters that favor survival, and therefore increase population densities of the black iguana.
The higher frequency of iguanas (C. similis) recorded in the mangrove zone and the BPNIC may be directly related to the greater amount of potential microhabitats registered in the mangrove zone (4) and the BPNIC (5) compared to rocky areas (1) and CD (3), or the higher frequencies of these iguanas in certain areas of the island could be a consequence of the quality of the microhabitats offered by each habitat, necessary for feeding, breeding, and for protection against predators, or for avoiding thermal stress. This idea is supported by the fact that although the BPNIC covers a smaller area than the other three environments, the BPNIC recorded a higher frequency of iguanas than rocky areas and CD, but with a frequency slightly lower than the mangrove zone; however, the BPNIC is 19.5 times smaller than the mangrove zone. Therefore, it can be assumed that the BPNIC offers better quality habitats to the iguana, because this environment offers the best conditions to meet the iguana's requirements. However, it is necessary to quantify the quality of the habitats in order to generate more robust conclusions. The results show that juveniles and adults used mangrove zones more frequently, while hatchlings were seen more frequently in the BPNIC. These results may be related to the fact that these iguanas practice cannibalism, in which larger iguanas eat smaller ones (see below), and therefore small iguanas would avoid living with larger iguanas (Mora 1991; Mora et al., 2015). However, this result should be interpreted with caution, since the observation of iguanas depends heavily on their detectability in different environments, and therefore, there is a risk that some iguanas are more easily detected in one or another environment.
The most used microhabitat type for the different age classes and sexes were rocks and trees (Table 2). Hatchlings and adults used rocks more frequently, perhaps due to the fact that this resource maintains temperatures necessary for thermoregulation, although rocks also have dark colors similar to the bodies of older iguanas, perhaps helping them pass unnoticed by predators; whereas juvenile iguanas that are green use trees more frequently, as this microhabitat is effective for allowing them to blend in with the branches and leaves; nevertheless, the second most used microhabitat by juveniles was the rocky areas. In this regard, Lee (1996) mentioned that in the Yucatan Peninsula, black iguana individuals perch on constructions or rock walls, although it is also common to find them perching on trees. In spite of these observations, this species often is described as being of terrestrial or saxicolous habit (Lee, 1996; 2000).
The temperature of the environment and substrate, as well as the percent humidity registered during the times of greatest activity of the iguanas, did not differ significantly between sexes. This may be due to the fact that both males and females perch in similar places (rocks and trees) or that their activity schedules are very similar and they are therefore exposed to the same thermal constraints (Lara-Reséndiz et al., 2014), or their thermoregulatory behaviors are very similar (Lara-Reséndiz et al., 2014). On the other hand, there were differences in the temperature of the substrate and percent humidity during activity of hatchlings vs. juveniles, and between hatchlings vs. adults (for the latter comparison, the only difference was in the temperature of the substrate). These differences may have been due to the type of microhabitats that were used most often by hatchlings (rocks) and juveniles (trees), although it also could indicate that the hatchlings have different activity schedules from juveniles and adults (Lara-Reséndiz et al., 2014).
The diet of C similis in Isla Contoy is omnivorous, as has been reported by other authors for other populations (Fitch and Henderson, 1978; Mora, 1991; Garrido and Sandoval, 1992; Lee, 1996). However, the frequency with which they consumed certain types of prey items changed with the age of the individual. For example, hatchlings and juveniles fed mainly on insects, flowers and fruits, while adults consumed leaves, fruits and some vertebrates more often. In this regard, Lee (1996; 2000) noted that black iguanas are insectivorous at hatching, but while growing, the consumption of vegetable matter increases, although insects remain as an important part of the diet; in addition, larger individuals are able to consume vertebrates.
These ontogenetic changes in the frequency with which certain food types are consumed may be a consequence of the morphological and physiological restrictions for each age class. For example, it has been reported in some groups of lizards that small individuals consume small, soft prey in comparison to adults (Gadsden et al., 2011; Ngo et al., 2015). However, the nutritional requirements of each age class also could result in changes in diet, since it has been recorded that not all types of prey contribute same amount of nutrients (Leyte-Manrique and Ramírez-Bautista, 2010; Cruz-Elizalde et al., 2014). Durtsche (2000) noted that the consumption of insects provides a more effective source of nutrients and proteins for juveniles, which results in rapid growth that helps small lizards to transition more rapidly out of the period of greatest predation risk. On the other hand, adults stop consuming insects and increase the consumption of plant material, such as flowers, fruits and leaves of different species of plants (Appendix 1), as well as vertebrates (Appendix 1). Authors such as Fitch and Henderson (1978) and Lee (1996; 2000) reported that this iguana species may eat birds, small lizards, eggs of other lizards, or other iguanas and some rodents; which suggests that the individuals of this species are generalists and opportunists, as are the individuals of C. pectinata Wiegmann, 1834, which also feed on some vertebrate species (Rodríguez-Juárez and Osorno-Cepeda, 1988). We also observed in the field (but did not report in the results section) the fact that on three occasions hatchling were seen feeding on feces of adult iguanas. Burghardt and Rand (1985) mentioned that coprophagy in hatchling of Iguana iguana Linnaeus, 1758 may be a mechanism to strengthen their intestinal microflora, which favors their digestion of the vegetative food they consume.
In this species of iguana, it is well known that individuals can practice cannibalism. For example, in the genus Ctenosaura many cases of cannibalism have been previously reported by Mora (1991), who recorded five incidents of cannibalism in C. similis and later, Mora et al. (2015) reported one more event where an adult female ate a juvenile of the same species. The latter case occurred at a time when the availability of food was limited; therefore, the authors suggested that this may be a strategy of adults to withstand times of drought and low food production. On the other hand, Sánchez-Hernández et al. (2017) reported a cannibalism event of a juvenile C. pectinata towards a neonate of the same species. This behavior could be due to the fact that this species is opportunistic, and also because cannibalism might serve to reduce future competition (Mora, 1991; Robbins et al., 2013; Mora et al., 2015). Although there are different types of predators (snakes and crocodiles) that eat iguanas, C. similis is a relatively abundant species on this island; therefore, cannibalism might be expected to emerge as a feeding strategy to exploit high densities, as well as to reduce competition for food and space. However, together with cannibalism and the fact that these iguanas consumed dead animals, this behavior could possibly be a consequence of the limited availability of food on the island (Mora et al., 2015; Sánchez-Hernández et al., 2017).
In addition to the ontogenetic changes registered in the diet of these iguanas, there were also changes in the diet of all individuals throughout the year. During the spring and summer, the consumption of invertebrates (insects) was low because recruitment of hatchlings still had not occurred. The hatchlings emerge at the end of the summer (Lee, 1996; 2000); therefore, a greater consumption of invertebrates was observed during autumn and winter because of the presence of these individuals in the population. The frequency with which the iguanas consumed vegetable matter (flowers, fruits and leaves) was constant throughout the year, probably due to the fact that adult individuals are present throughout the year, and they mainly consume vegetable matter. However, despite the fact that throughout the year there are adult organisms, consumption of vertebrates occurred more frequently in the summer. This could be a consequence of the emergence of sea turtle hatchlings (Eretmochelys imbricata), and of iguana hatchlings, which are part of the diet of adult iguanas. These changes in the diet can be attributed to the fact already mentioned that C. similis is opportunistic, in addition to the availability of food in the environments being variable and depending on various factors, such as temperature and precipitation (Gadsden-Esparza and Palacios-Orona, 1995; Zamora-Abrego and Ortega-León, 2016).
CONCLUSION
Our results indicated that the mangrove zone and the BPNIC area were the habitats most used by the black iguana C. similis, while rocks and trees were the most commonly used microhabitats. The diet of these organisms is omnivorous, showing ontogenetic changes in the consumption of food types, as well as changes in the frequency of consumption of certain food items throughout the year. This work provides data on the natural history of C. similis, which complements the scarce information previously available in the literature. This information helps our understanding of how black iguana populations may respond to environmental stress; however, more systematic studies that address different aspects of the general biology of this species throughout its distribution area are still needed.














