INTRODUCTION
Insects are involved in several ecological and parasitic processes and can become pests under favorable conditions in the environment (Singh and Kaur, 2018). In Brazil, annual losses from pests in the field are estimated at US$ 17.7 billion, corresponding to 25 million tons of lost agricultural and wood products, poisoned by agrochemicals (Oliveira et al., 2014).
Termites belong to the order Blattodea and are the main pest of wood, being firstly forest plantations to urban spaces the most affected, with the degradation of wooden houses and buildings (Oliveira et al, 2014). In Brazil is estimated that just for the city of São Paulo, losses can reach around US$ 10 to 20 million a year (Oliveira et al., 2011). The main causes of economic losses are termites of the family Termitidae, belonging to the genus Nasutitermes (Banks) (Constantino, 1999). Nasutitermes termites are common in urban areas, considered an opportunistic pest that attacks structural wood and also in the countryside, through tunnels built in host tree, maintaining communication with the soil (Boulogne et al., 2017; Souza-Neto et al., 2018).
The main method for termite control involves the application of organophosphate pesticides, carbamates and pyrethroids (Nascimento and Melnyk, 2016). These products can leave residues in the environment, in addition to contaminating surface and underground water, used for consumption and irrigation (Oliveira et al., 2014). It also induces natural selection, increasing the number of resistant pests (Blackburn et al., 2016). This alternative also reports levels of toxicity to humans, producing an estimated cost of approximately US$ 29 million spent on treating intoxicated patients (De Rezende Chrisman et al., 2009).
Biological control emerges as an alternative to reduce the population of pests using populations of natural enemies present in the agrosystem, without causing damage to the environment (Begg et al., 2017). In this context, entomopathogenic fungi are interesting for having dispersal structures such as spores, showing the ability to spread among the invasive population. Thus, the spores show persistence in the field, favoring host infection, and later release of more spores after infection and host death (Mascarin and Pauli, 2010).
Previous studies reported the biocontrol of pests by entomopathogenic fungi, such as the genus Metarhizium sorokin, with potential to control the leafhopper (Mahanarva fimbriolata Stal) (Barbosa et al., 2015). Beauveria bassiana (Bals.-Criv.) Vuill. has also been reported to control several pests in Brazil, such as the red mite (Tetranychus urticae Koch), the whitefly (Bemisia tabaci Genn) and scale insect (Dactylopius coccus Costa) (Faria and Magalhães, 2001). Other fungi used for biological control are species of the genus Paecilomyces bainier, which have been effective against numerous pests, such as Hedypathes betulinus Klug (Coleoptera: Cerambycidae) (Leite et al., 2011) and Bemisia tabaci (Hemiptera: Aleyrodidae) (Potrich et al., 2011).
Although the existence of these fungi in the biological control market, their efficiency varies according to the genetic variability of the strain, as well as abiotic factors that determine differences in the degree of virulence and pathogenicity, directly affecting the percentage of insect mortality (Kin et al., 2017). Thus, it is essential to search for new isolates with virulence characteristics for use in agriculture.
The Amazon biome is an interesting environment, as the tropical climate and extensive vegetation form the ideal ecosystem for the study of microbial diversity. It is estimated that for every gram of soil there are 100 million microbial cells. Thus, the biome becomes the perfect environment to search for new strains and bioactive molecules for agriculture, with emphasis on termite control (Pylro et al., 2014; Mendes et al., 2015). Therefore, to test the hypothesis of pathogenicity of these fungi, the objective of this work was to verify if there is virulence of native fungi from Amazonian soil (Tolypocladium endophyticum, Metarhizium anisopliae and M. marquandii) in termites Nasutitermes sp. (Blattodea: Termitidae).
MATERIALS AND METHODS
Origin of fungi
The tested fungi are native to the Amazonian soil of the Catuaba Secondary Forest (geographical coordinate: 10°4'36"S 67°37'0"W), located in the municipality of Senador Guiomard, in the State of Acre. The strains were preserved in distilled water and mineral oil in the Collection of Microorganisms of the Microbiology Laboratory of Universidade Federal do Acre and were reactivated in January 2019 (UFAC).
Molecular characterization
One fungus from the genus Tolypocladium (4.439) and two from the genus Metarhizium (4.443, 4.472) were reactivated from the microbiological collection using Potato-Dextrose-Agar (PDA) medium incubated at 28 ± 1 ° C for 14 days. The extraction of genomic DNA was made using the Quick-DNA mini fungal/bacterial preparation kit (Zymo Research) following the manufacturer's instructions. Amplification of ITS rDNA was done in a 50 μL reaction mix with 2 μL of DNA template (1-20 ng), 0.4 μM ITS1 primers (5'-TCCGTAGGTGAACCTGCGG -3') and ITS4 (5'-TCCTCCGCTTATTGATATGC -3 '), 1.5 mM MgCl2, 0.2 μM dNTPs, 5 μL Taq buffer and 1.25 U Taq DNA polymerase (Qiagen) (White et al., 1990). PCR amplification was performed on a PCR cycler machine (Bio-Rad) with initial denaturation at 95 °C for 2 min, followed by 35 cycles of amplification (95 °C for 30 sec, 55 °C for 30 sec and 72 °C for 1 min) and an extension step at 72 °C for 7 min. PCR products from 600 to 700 bps were purified using the QIAquick PCR purification kit (Qiagen) and quantified on a 2 % agarose gel. Forward and reverse sequencing reactions were performed on a 7330 x l DNA analyzer (Applied Biosystems). Forward and reverse reads for each isolate were paired to generate a consensus sequence. Consensus sequences were constructed using BLASTn at the NCBI (The National Center for Biotechnology Information).
Preparation of conidial suspensions
For the preparation of suspensions, the evaluated strains were cultivated in PDA medium at 28 ± 1 °C for 14 days. Subsequently, the conidia were scraped from the surface of the colonies and suspended in 10 mL of sterile distilled water with Tween 80 (0.01 %) (Alves and Lecuona, 1998). The suspensions were adjusted to concentrations of 1 05, 1 06, 1 07 and 108 conidia/mL by quantification in a Neubauer chamber using an optical microscope (Remadevi et al., 2010). An aliquot of 108 conidia/mL suspension of each fungi was inoculated in a Petri dish containing PDA medium and incubated at 28 ± 1 °C and relative humidity > 80 % for 24 h to assess conidial viability (Alves and Lecuona, 1998).
Obtaining termites Nasutitermes sp.
Termites of the genus Nasutitermes sp. were collected manually from a tree nest at the Parque Zoobotanico Park of UFAC. The morphological characteristics of termites were observed under a stereomicroscope and compared with the structures described in the specific literature (Constantino, 2002; Boulogne et al., 2017). Termites were kept in plastic containers at 28 °C and 80 % relative humidity with corrugated cardboard fragments as a source of shelter and food until the time of testing (Denier and Bulmer, 2015).
Bioassay
The virulence of Tolypocladium (4.439), Metarhizium (4.443) and Metarhizium (4.472) strains was evaluated in suspensions at concentrations of 105, 1 06, 1 07 and 108 conidia/mL against termites, following the method of Remadevi et al. (2010) with modifications. For each fungus, five replicates were performed with 16 termites (workers and soldiers), which were transferred to Petri dishes containing fi lter paper with an aliquot of 1 mL of the conidia suspension, allowing direct contact between the termites and the fungal inoculum for a period of 3 minutes. The negative control consisted of treating termites with 1 mL of distilled water + Tween 80 (0.01 %). After infection, termites were transferred to Petri dishes containing filter paper and corrugated cardboard fragments as a source of shelter and food. The samples were stored in constant scotophase at 28 ± 1 ° C and relative humidity ≥ 80 %. Termite mortality was assessed every 24 h for 10 days (Remadevi et al., 2010).
Reisolation of entomopathogenic fungi from infected termites
The colonized termites were superficially disinfected with 2 % hypochlorite for 1 min and transferred to Petri dishes containing PDA medium with antibiotic (chloramphenicol 0.05 %). The cultivated mycelium was transferred to test tubes, and its morphological aspects were analyzed after 14 days to confirm the analyzed fungi (Alves and Lecuona, 1998).
RESULTS
After the DNA sequences were obtained and compared with the data available in GenBank, the fungi were identified 4.439 as Tolypocladium endophyticum (Gazis, Skaltsas & P. Chaverri), 4.443 as Metarhizium anisopliae (Metschn.) Sorokin and 4.472 as M. marquandii (Massee) Kepler, SA Rehner & Humber (Table 1).
Table 1 Molecular identification table of Tolypocladium (4.439) and Metarhizium (4.443 and 4. 472) fungi.
| Origin of the strain | Fungi N° | GenBank Accession N° | Tipo de marcador Closest match in GenBank | identity |
|---|---|---|---|---|
| SFC | 4.439 | OL442683 | Tolypocladium endophyticum (KF747260.1) | 94.25 |
| SFC | 4. 443 | MN639710 | Metarhizium anisopliae (KP294313.1) | 99.05 |
| SFC | 4. 472 | MN63971 1 | Metarhizium marquandii (MH483912.1) | 100 |
SFC: Secondary Forest of Catuaba (Amazon/Brazil)
Termite mortality was 100 % in all conidia/mL dilutions for T. endophyticum (4.439) on the Fourth day of treatment, characterizing it as the most virulent strain for Nasutitermes sp. (Table 2, Fig 2). M. anisopliae (4.443) caused the death of all termites tested on the seventh day for a 107 conidia/mL dilution and on the sixth day for a 108 conidia/mL dilution. For this same strain, concentrations of 105 and 106 conidia/ mL killed all termites on the eighth day of treatment (Fig 1 and 2). Treatments with M. marquandii (4.472) did not differ from the control from the fourth to the seventh day, showing virulence from the eighth day on for all tested concentrations. However, no treatment had 100 % mortality for the termites tested (Table 2, Fig 1 and 2).
Table 2 Average mortality percentage of termites Nasutitermes sp. exposed to concentrations of conidia/mL of the fungi Tolypocladium endophyticum, Metarhizium anisopliae and M. marquandii in different days of treatment.
| Fungus | Control | 105 | 106 | 107 | 108 | |
|---|---|---|---|---|---|---|
| 4° day | T. endophyticum (4.439) M. anisopliae (4. 443) M. marquandii (4. 472) | 6,6 ± 3,39 a 6,6 ± 3,39 a 6,6 ± 3,39 a | 100 ± 0 b 58 ± 13 b 16 ± 3,58 a | 100 ± 0 b 63 ± 12 b 40 ± 11,58 a | 100 ± 0 b 96 ± 2 b 22 ± 7,69 a | 100 ± 0 b 96 ± 4 b 26 ± 5,9 a |
| 7° day | T. endophyticum (4. 439) M. anisopliae (4. 443) M. marquandii (4. 472) | 14 ± 2,97 a 14 ± 2,97 a 14 ± 2,97 a | 100 ± 0 b 88 ± 11 b 57 ± 15,9 a | 100 ± 0 b 99 ± 1 b 43 ± 13,6 a | 100 ± 0 b 100 ± 0 b 63 ± 15,4 a | 100 ± 0 b 100 ± 0 b 75 ± 13,1 a |
| 10° day | T. endophyticum (4. 439) M. anisopliae (4. 443) M. marquandii (4. 472) | 28 ± 6,39 a 28 ± 6,39 a 28 ± 6,39 a | 100 ± 0 b 100 ± 0 b 92 ±4,38 b | 100 ± 0 b 100 ± 0 b 88 ± 10,7 b | 100 ± 0 b 100 ± 0 b 83 ± 12,1 b | 100 ± 0 b 100 ± 0 b 88 ± 6,7 b |
* Means followed by equal letters on the same line do not differ at 5 % significance level (p > 0,05).
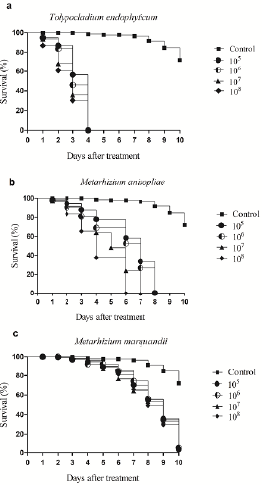
Figure 1 Termite survival percentage Nasutitermes sp. against the fungi Tolypocladium endophyticum (4.439), Metarhizium anisopliae (4.443) and M. marquandii (4.472). a. Termite infected with T. endophyticum. b. Termite infected with M. anisopliae. c. Termite infected with M. marquandii (4.472).
DISCUSSION
Fungal strains of Tolypocladium endophyticum, Metarhizium anisopliae and M. marquandii isolated from Amazonian soil showed virulence against termites Nasutitermes sp. Previous studies report the efficiency of biocontrol using fungi in experimental tests, mainly targeting termites of the species Coptotermes formosanus Shiraki, Reticulitermes flavipes Kollar (Meikle et al., 2005; Hussain et al., 2010) and Heterotermes tenuis Hagen (Sterling et al., 2011).
The strain Tolypocladium endophyticum (4.439) had the greatest potential for control (Table 1), infecting and killing all termites in the shortest time and at the lowest concentration evaluated, presenting one of the ideal characteristics for commercial fungicide formulation, rapid virulence and mortality (Oliveira, 2009). Potential entomopathogenic fungi for the control of insects are considered effective when they present mortality values greater than 40 %, and this fact is observed for all tested fungi (Lopes, 2007). This is the first report in the literature about the fungal species T. endophyticum for the biocontrol of insects. However, there is evidence of T. cylindrosporum in the biocontrol of important insect vectors of diseases in plants, mainly in larval stages and flies (Barson et al., 1994; Scholte et al., 2004). This mechanism may be related to the presence of chitinolytic enzymes and hydrolases in this fungus (Scorsetti et al., 2012). Important characteristics of tolerance to abiotic factors such as heat, cold and UV-B were also observed, which are fundamental in the persistence of the spore in the field (Santos et al., 2011).
The strain Metarhizium anisopliae (4443) also showed promise in the control of Nasutitermes sp., causing 100 % mortality on the sixth day of treatment (Fig 1). In previous studies, different varieties of the same species, such as Metharizium anisopliae var. anisopliae (Metschn.) Sorokin, showed greater potential to control N. coxipoensis when compared to Metharhizium anisopliae var. acridum Driver & Milner (Albuquerque et al., 2005). The virulence of M. anisopliae var. dcjhyium was also evidenced against the subterranean termite Odontotermes formosanus Shiraki, being highly susceptible to the fungus, causing 100 % mortality 3 days after inoculation (Dong et al., 2009). Other studies with M. anisopliae also demonstrated virulence on the termites Odontotermes obesus, Coptotermes curvignathus and C. formosanus (Keppanan et al., 2018; Francis, 2019; Syazwan et al., 2021). The pathogenicity and virulence of the species is explained by enzymatic activities and by the induction of metabolic alterations in the host, as seen for the mite species Psoroptes ovis var. cuniculi, which induced the antioxidant substances glutathione S-transferase (GST), superoxide dismutase (SOD), peroxidase (POD) and catalase (CAT) (Gu et al., 2020). The proteins involved in the process can also be extracted and used together with the strains, a fact that was verified for the insecticidal protein serine protease produced by M. anisopliae JEF-279, which accelerated the development of pathogenicity against adults of sawdust beetles (Monochamus alternatus) assisting in the penetration of the insect's body by conidia (Kim et al., 2020).
There are no reports in the literature regarding the use of the species M. marquandii for biological pest control. It is a recently identified species (last five years), frequently found in the soil and even in the marine environment, which has been reported to have active natural compounds, such as three groups of polyketides (1, 2, 4, 5, clavatol and penilactone A), alkaloids (3, viridicatin, cyclopenol and dehydrocyclopeptin) originated from phenylalanine and anthranilic acid, as well as butenolides (El-kashef et al., 2019; Mongkolsamrit et al., 2020). In general, the Metarhizium fungus is widely distributed in nature and is found mainly in the soil in association with plant roots and also in arthropod corpses, as a result of natural biological control (Barelli et al., 2016). This fungus can behave as a saprophyte or parasite, according to the conditions to which it is subjected (Schrank and Vainstein, 2010; Brito et al., 2019). As a parasite, Metarhizium has the ability to secrete acid trehalase into the insect's hemolymph. Trehalose is known to be the main sugar present in the hemolymph of insects, therefore, by producing enzymes that degrade this sugar, the fungus is able to reduce its availability, preventing the host's nutrition (Jin et al., 2015). This fungus may have different mechanisms of action being related to the high molecular diversity of the strains, making it possible to distinguish several isolates of Metarhizium spp. recovered from the environment through technologies such as microsatellite markers (Iwanicki et al., 2019).
Therefore, our results present new findings of virulent strains native to the Amazon, such as Tolypocladium endophyticum (4.439), Metarhizium anisopliae (4.443) and Metarhizium marquandii (4.472) for the control of termites Nasutitermes sp. All of them have the potential to carry out bioformulations, however new experiments are needed to verify the persistence of virulence of the strains in a natural environment, in order to enter the biological control market. Furthermore, future studies involving the bioprospecting of molecules from these strains can generate explanations about their virulence, as well as new applications in agriculture.
CONCLUSIONS
Tolypocladium endophyticum (4.439), Metarhizium anisopliae (4.443) and Metarhizium marquandii (4.472) are native strains of the Amazonian soil and have the ability to cause virulence in termites of the genus Nasutitermes sp. showing potential for the biological control of this pest. Furthermore, this is the first report of strains of the species T. endophyticum and M. marquandii causing virulence in the agroforestry pest termite Nasutitemes sp.
















