INTRODUCTION
Plukenetia L. (Euphorbiaceae) is a pantropical genus with 37 species of lianas and scrambling vines. Several species of Plukenetia have traditionally been cultivated in their native range, e.g., Plukenetia volubilis L. (Sacha Inchi), Plukenetia huayllabambana R. W. and Plukenetia conophora Müll. Arg. (Tropicos.org, 2020). It is found in the Lesser Antilles and South America, primarily in the northern and western regions and margins of the Amazon Basin in Suriname, Venezuela, Colombia, Ecuador, Peru, Bolivia and Brazil (Wang et al., 2018). It is known as the Inca peanut because the Inca Civilization used it as early as 3000 years ago. In the past decade, P. volubilis has become increasingly popular in the horticultural, pharmaceutical, cosmetic and food industries (Kodahl, 2020).
P. volubilis seeds are an excellent source of lipids; 77.584.4 % are polyunsaturated fatty acids (o 3, o 6 and o 9), 8.4-13.2 % are monounsaturated fatty acids, and 6.8-9.1 % are saturated fatty acids (Maurer et al., 2012). In addition, its seeds have essential amino acids and are used as dietary supplements (Sathe et al., 2012). Food enriched with omega polyunsaturated fatty acids can play an important role in preventing and treating coronary heart and neurodegenerative diseases, diabetes, rheumatoid arthritis, pulmonary diseases and other inflammatory autoimmune diseases, making P. volubilis oil highly attractive to the food and pharmaceutical industries (Kerdiles et al., 2017; Watanabe and Tatsuno, 2017; Tenenbaum and Fisman, 2018). Furthermore, its seeds contain antioxidants, including vitamins A and E, which may explain the oil's increasing popularity in the cosmetic industry (Chirinos et al., 2016). All the potential uses of P. volubilis oil in different industries make this species a promising crop for farmers involved in oilseed production for food and non-food markets (Wang et al., 2018).
P. volubilis has a considerable degree of morphological variation and high genetic diversity between different populations. It is an allogamous species usually propagated by seeds. Therefore, its offspring is heterogeneous (Cachique et al., 2018). Moreover, its seeds have poor seed viability, a lower germination rate, lower disease resistance and delayed seedling rooting (Solis et al., 2016). Vegetative propagation allows maintaining 100 % of superior genotypes' identity, ensures germplasm conservation and increases genetic gain in short periods by using both additive and non-additive components of genetic variance (Solis et al., 2019).
The method of asexual propagation through cuttings has limited establishing commercial plantations because genotypes with desired traits cannot be propagated in masse. One strategy to overcome these limitations is in vitro tissue culture, which allows regeneration via organogenesis and/or somatic embryogenesis. In vitro regeneration offers a significant opportunity for mass propagation and genetic enhancement of plants. It can be performed in reduced spaces and makes it possible to keep a stock of disease-free plants.
Some studies have been developed for the in vitro propagation of P. volubilis. Seed germination times have been shorter in in vitro germination than in nurseries. However, some are still long (de la Rosa and Quijada, 2013; Cardoso et al., 2018; Kodahl et al., 2018). Some studies used apical meristems obtained from in vitro plantlets. Viegas Rodrigues et al. (2014) achieved shoot formation but did not report the rooting phase and Solis et al. (2018) also achieved shoot formation and rooting but did not reach the ex vitro phase.
Restrepo-Osorio et al. (2020) and Bordignon et al. (2012) used different hypocotyl and epicotyl segments of seeds germinated in vitro to obtain plants from induced shoots. Millones and Vásquez (2008) and Thuy and Nhung (2019) used hypocotyl segments and obtained shoots and rooting. However, Patthanajuck and Bunnag (2017) used the same explant they did not attend to, which was root formation in shoot regenerates. Dong et al. (2016) used cotyledon leaves to obtain shoot regeneration, but lacked rooting. Viegas Rodrigues et al. (2014) performed a field evaluation of in vitro regenerated plantlets, which showed an increase in productivity and no genetic variability compared to plantlets propagated by seeds.
Considering the results above, this study had two objectives: to evaluate plant production via germination and vegetative propagation by ABD. The following were evaluated in terms of seed germination: two culture media (MS and 1/2 MS), two seed coat presence/absence conditions and two culture temperatures (18 °C and 28 °C). ABD was evaluated at different times, first using different types and concentrations of cytokines: KIN, 6-BAP and 2-ip between 0.1 to 0.8 mg L-1. CAL was subsequently evaluated as an unwanted response. Second, the effect of modified MS in 453 mg L-1 CaCl2 and 351.62 mg L-1 MgSO4 supplemented 0.4 mg L-1 KIN on ABD and shoot survival was assessed. Third, different treatments with 10 % coconut water, 0.6 mg L-1 2-ip and 0.4 mg L-1 KIN were evaluated to optimize ABD and shoot leaf formation. Therefore, this investigation made it possible to develop an efficient protocol for in vitro plant production via seed germination and vegetative propagation through nodal explants. Acclimatization was also addressed in a preliminary manner. The results contribute to standardizing the P. volubilis micropropagation protocol for commercial purposes, since it includes both the rooting shoots and acclimatization stage.
MATERIALS AND METHODS
Plant Material, Seed Disinfection and Culture Conditions
The research was performed in the Plant Tissue Culture Laboratory at Universidad de Antioquia (Antioquia, Colombia). The fruits were obtained from Colombiana de Biocombustibles S.A. (Santa Fe de Antioquia, Antioquia, Colombia) and seeds were stored at 24 °C for a maximum of 5 days prior to their introduction to the laboratory. Fruits in an advanced stage of physiological maturation were used (Fig. 1a-d) and manually processed to remove the pericarp and isolate the seeds for further disinfection (Fig. 1b-c). Seeds were disinfected with a 70 % v/v ethanol solution for 15 min followed by a 10 % v/v iodine solution (Merck) for 5 min in a laminar flow cabinet. They were subsequently immersed in a 3 % v/v sodium chloride and 10 % v/v acetic acid solution for 30 min and then rinsed with sterile distilled water. Seeds were manipulated and inoculated in the culture media (establishment phase) using sterilized tweezers and scalpels. Glass flasks (109.3 mm x 43.7 mm, 68.3 mm x 57.5 mm and 117 mm x 100 mm), each containing 20 -50 mL of culture medium, were used in all phases. Uniform culture conditions were maintained in all experiments. All culture media were supplemented with 20 g L-1 sucrose and 2.7 g L-1 gelling agent (Phytagel®), and medium's pH was adjusted to 5.8 using 1M NaOH or HCl with a pH meter (Metrohm) prior to autoclaving at 121 °C and 15 psi for 15 min.
In Vitro Germination
The establishment phase basically considered three factors' effect on seed germination: culture media evaluated considering a Murashige and Skoog basal medium (MS) (Murashige and Skoog, 1962) and a half basal medium MS (½ MS), seed coat presence or absence determined by manually removing coats with a scalpel under sterile conditions, and the effect of temperature under two conditions: 18 °C and 26 °C. Combinations of these factors contributed to a total of eight treatments as follows: MS + coat 18 °C, MS + coat 28 °C; MS - coat 18 °C; MS - coat 28 °C; ½ MS + coat 18 °C; ½ MS + coat 28 °C; ½ MS - coat 18 °C; ½ MS + coat 28 °C.
One seed was cultured in a single glass flask (109.3 mm x 43.7 mm) and, after eight days, the number of seeds with radicle development in each treatment mentioned in the previous paragraph were recorded. Each treatment was replicated twice. In order to calculate the percentage of germination, the count performed in groups of 20 seeds was taken as a unit of measurement.
In vitro seeds were kept in complete darkness until radicle development occurred (Fig. 2c). After epicotyl extension, seedlings were transferred to glass flasks with MS medium and gelling agent (Phytagel®) (117 mm x 100 mm), where plantlet development was completed (Fig. 2f-g). Plantlets were kept at 23 ± 2 °C and with continuous light at an intensity of 2000 lux from fluorescent light tubes. The culture medium was renewed every three weeks.
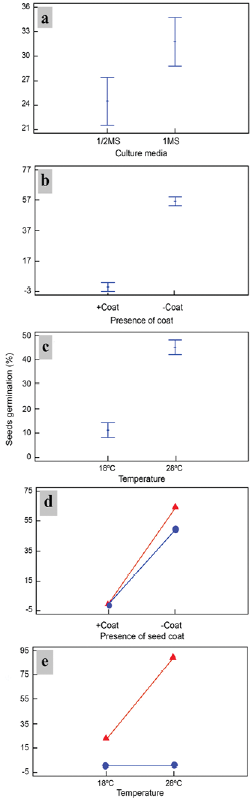
Figure. 2 The independent effect of culture medium, a) seed coat presence b) and temperature c) on in vitro seed germination, as well as interactions between the effect of basal culture medium concentration [MS (triangle), 1/2 MS (circle)] and seed coat presence or absence d) and seed coat [presence (circle) or absence (triangle)] and temperature e) on the germination of Plukenetia volubilis seeds. Differences were significant at p < 0.05 using multiple Fisher LSD rank comparisons.
In Vitro Vegetative Propagation by Axillary Bud Development (ABD)
The Effect of KIN, BAP and 2-ip concentrations on ABD
In the first experiment, the effects of different KIN, 2-ip and BAP concentrations (0.8 mg L-1, 0.6 mg L-1, 0.4 mg L-1, 0.2 mg L-1, 0.1 mg L-1) on ABD and callogenesis (CAL) were evaluated in the multiplication phase as an unwanted response. In previous experiments with MS basal medium and without growth regulators, explants did not establish or develop. Therefore, these features were not considered for this experiment.
Nodal segments (2.0 - 2.3 cm long) were collected from two- to three-month-old seedlings (Fig. 2c). The nodal segments were planted vertically with almost 30 % of their surface in contact with the MS basal medium and gelling agent (Phytagel®), using two nodal segments for glass flasks (68.3 mm x 57.5 mm). The count was performed in groups of 12 nodal segments, which were taken as experimental units to estimate ABD percentage. The size of the formed callus (CAL) was measured with millimeter paper under sterile conditions. Callus formation was an unwanted response and was avoided. Fifteen treatments were performed with six replications per treatment. Culture conditions were the same as plantlet maintenance for in vitro germination.
Effect of Calcium and Magnesium Modifications to the MS Basal Medium on ABD and Explant Survival
In the second experiment, modifications were made to calcium and magnesium concentrations to counteract the chlorosis and necrosis of young leaves after some weeks of culture. These modifications were performed to analyze the soil of a commercial plot of P. volubilis provided by Colombiana de Biocombustibles S.A. It was observed that Ca and Mg macronutrients and Cu and Fe micronutrients were lower in the MS medium's soil (Appendix 1). Consequently, Ca, Mg, Fe and Cu were the nutrient concentrations that should have been changed, but only macronutrient concentration was considered in this study. The effects of the following treatments on ABD and survival percentage were evaluated: (a) control full MS (332.2 mg L-1 CaCl2 and 180.7 mg L-1 MgSO4); (b) MS with 453 mg L-1 CaCl2 (Kao and Michayluk, 1975); (c) MS with 351.62 mg L-1 MgSO4 (White, 1964). Three treatments were performed, with three replications per treatment. The count was performed in groups of 25 nodal segments, which were taken as experimental units.
The Effect of Different PGR's and Coconut Water on ABD and Leaf Formation
In the third experiment, the effects of different treatments on ABD percentage and number of leaves were tested in order to optimize axillary bud growth in MS, modified according to the results above: (a) MS Control; (b) 0.4 mg L-1 2-ip; (c) 0.4 mg L-1 KIN; (d) 10 % coconut water; (e) 0.4 mg L-1 2-ip supplemented with 10 % coconut water, (f) 0.4 mg L-1 KIN supplemented with 10 % coconut water. In this case, six treatments were performed to estimate ABD percentage, with three replications per treatment. The count was performed in groups of 20 explants, which were taken as experimental units. Finally, the leaves formed in each explant were counted.
Statistical Analysis
The data was analysed using parametric analyses of variance (ANOVA) to test significance with One-way and Multi-way analyses. The Shapiro-Wilk Test was used for normality and Bartlett's test for homoscedasticity. Fisher's Least Significant Difference (LSD) comparison was used to determine significant differences in averages. All tests were performed at a 5% level of significance (p < 0.05). The data was analysed using Statgraphics Centurion XVII software.
RESULTS
In Vitro Germination
Culture media, seed coat presence and culture temperature had a significant effect on the percentage of germinated seeds during seed germination (p < 0.05). Germination was higher in the full MS medium (31.75 %) than the 1/2 MS medium (24.5 %) (Fig. 2a). It was 56.25 % when the seed coat was removed and 0 % when keeping the coat (Fig. 2b). When the seeds were kept at 28 °C, germination was 45 %, and it was 11.25 % when kept at 18 °C (Fig. 2c).
Interactions between the different factors corresponding to culture medium-seed coat presence (Fig. 2d) and culture temperature-seed coat presence (Fig. 2e) were found to be statistically significant (p < 0.05). These results indicated that all factors (culture media - seed coat and temperature - seed coat) are related and, therefore, the best culture conditions for seed germination are MS medium without coats (66.5 %) and 28 °C without coats (91.6 %) (Fig. 2d-e). It was observed that radicles appeared within two days after in vitro introduction.
In Vitro Vegetative Propagation by Axillary Bud Development (ABD)
Effect of KIN, BAP and 2-ip Concentrations on ABD and Callogenesis
The independent effect of KIN, BAP and 2-ip concentrations on the percentage of axillarybud development was significant (p ≤ 0.05). The highest percentage of ABD was obtained with 0.6 mg L-1 2-ip (43.33 %). Nevertheless, there was no difference with 0.4 mg L-1 KIN (42 %) (Fig. 3b). None of the BAP concentrations induced ABD, but they reached the highest values of CAL (4.84 cm2) with 0.8 mg L-1 BAP (Fig. 3a). The desired result was the lowest CAL, which was obtained at 0.1 mg L-1 (0.52 cm2), 0.2 mg L-1 (0.62 cm2) and 0.4 mg L-1 (0.47 cm2) 2-ip following 0.4 mg L-1 (0.86 cm2) and 0.2 mg L-1 (1.07 cm2) KIN (Table 1). The lower values of CAL, at 0.47 cm2, matched the better ABD results with 0.4 mg L-1 KIN. However, axillary bud development after 20 days of cultivation presented chlorosis and necrosis in young leaves (Fig. 3c).
Table 1 The effect of different plant growth regulators (PGR) (KIN, BAP and 2-ip) on axillary bud development (ABD) and callogenesis (CAL) in Plukenetia volubilis.
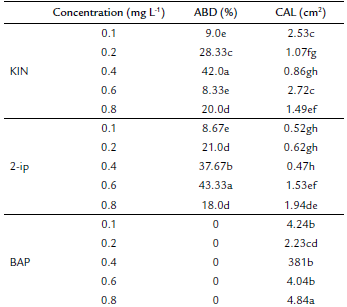
Values followed by the same letter and in the same column are not significantly different (p < 0.05) using an LSD multiple rank analysis.
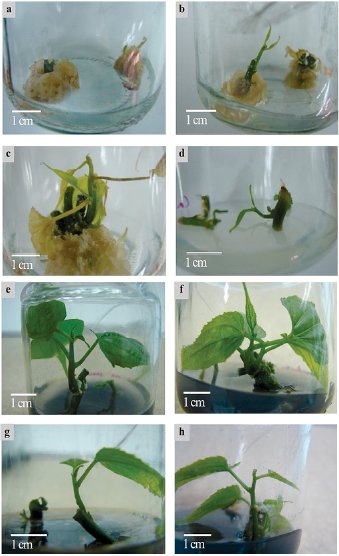
Fig. 3 Plukenetia volubilis. callogenesis with 0.1 - 0.8 mg L-1 BAP (a), axillary bud development (ABD) in 0.8 mg L-1 KIN (b), explant chlorosis and necrosis after 20 days of culture (c), ABD in 0.4 mg L-1 KIN and MS modified in 453 mg L-1 CaCl2 in (d), ABD after 15 days of culture in MS modified with 453 mg L-1 CaCl2 and supplemented with 0.6 mg L-1 2-ip and 10% coconut water (e-f), ABD after 15 days of culture in MS modified with 453 mg L-1 CaCl2 and supplemented 0.4 mg L-1 KIN and 10% coconut water (g-h).
Effect of Calcium and Magnesium Modifications in the Basal MS Medium on ABD and Explant Survival
As mentioned earlier, various modifications were made to Ca and Mg concentrations in MS basal medium to counteract chlorosis and necrosis during ABD. It was observed that macronutrient modifications in the MS basal medium significantly improved ABD and explant survival (p ≤ 0.05). The rise in CaCl2 concentration to 453 mg L-1 increased ABD up to 76 % and explant survival was 82 %, with significant differences with respect to MgSO4 (21 % for ABD and 39 % for explant survival) (Appendix 2). The buds developed in the medium modified for Ca concentration demonstrated more vigor, which means health and strength. Leaves showed normal development and leaf-fall and necrosis events decreased (Fig. 3d). Nevertheless, the bottom of the explants in contact with culture media presented oxidation. Activated carbon was used in culture media to avoid this oxidation (1 g L-1).
Effect of Different PGR's and Coconut Water on ABD and Leaf Formation
The effect of different treatments with different PGR's and coconut water on ABD and leaf formation was significant (p ≤ 0.05) (Table 2). Ninety-five percent ABD was the best result obtained from culture media supplemented with 0.6 mg L-1 2-ip and 10 % coconut water (Fig. 3e-f) and 0.4 mg L-1 KIN and 10 % coconut water (Fig. 3g-h), without a significant difference between them. Activated carbon (g L-1) was added to media to counteract oxidation from using coconut water. Moreover, the highest number of leaves was 2.45 and 2.75 leaves, respectively, for the same treatments mentioned above, without a significant difference between them (Table 2). ABD only required 15 days in both treatments.
Table 2 The effect of different culture media supplemented with coconut water on axillary bud development (ABD) and leaf formation in Plukenetia volubilis.
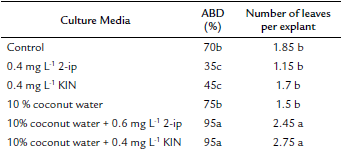
The culture media in all treatments were MS modified with 453 mg L-1 CaCl2 and supplemented with 1 g L-1 activated carbon.
a Values followed by the same letter and in the same column are not significantly different (p < 0.05) using an LSD multiple rank analysis.
Root formation was achieved by removing PGR from the basal culture medium (KIN and 2-ip) for 8 - 15 days. Plantlets were subsequently transferred to ex vitro conditions using a fertilized soil substrate. Regenerated plants with at least five internodes and a 5 - 10 cm stem length were transferred into nursery conditions. The plants were washed with tap water to remove excess culture media from roots and transferred to a plot (5L) with sterilized soil. Each plant was completely covered with a bag to avoid dehydration and the bags were punctured two days later to favor gas exchange. Bags were removed on day 5, and a ½ MS culture medium was used for irrigation (data not shown).
DISCUSSION
The highest seed germination results were obtained in full MS basal medium, without seed coats and at 28 °C. Some studies have hypothesized that low nutrient concentrations facilitate the germination of mature zygotic embryos (Hesami et al., 2018). However, this cannot be generalized because each species has specific physiological requirements. Cardoso et al. (2018) only obtained 70 % seed germination in a full MS medium with P. volubilis, but did not remove seed coats. Germination took 20 - 25 days. The results obtained in the study are consistent with Kodahl et al. (2018), who attained 100 % germination when the testa was removed. They observed the radicle emerge 7 days after culture, while Restrepo-Osorio et al. (2020) reported 30 days. Nevertheless, we observed radicle extension two days after in vitro introduction, not only considering the MS culture medium and the absence of testa, but also keeping seeds at 28 °C in darkness. This result is most likely due to the resemblance to natural environmental conditions. P. volubilis grows in dry tropical forests, where an increase in temperature can trigger biochemical responses required for germination. Likewise, according to Azcón and Talón (2008), the type of seed coat can be responsible for controlling impermeability and the movement or imbibition of water. In addition, temperature, soil moisture and light conditions are the most important factors that determine the end of seed dormancy. Also, the absence of a seed coat greatly favors germination, and metabolic activity is immediately reactivated. These culture conditions (MS, without testa, 28 °C), which allowed obtaining 91.6 % germination, are recommended for improving the propagation process of P. volubilis through seeds.
The positive results obtained for ABD with 2-ip (0.8 mg L-1) and KIN (0.4 mg L-1) show that cytokinins must be added to media to stimulate axillary bud development in P. volubilis. It has been widely reported that axillary bud proliferation in in vitro cultures is achieved by supplementing media with cytokinin to avoid apical dominance and stimulate bud sprouting (Gupta et al., 2020). Furthermore, it was observed that low concentrations of cytokinins promote sprouting and decrease the formation of calli. These results may be attributed to interactions between exogenous plant growth regulators and the endogenous contents of other phytohormones in plant tissue (Almeida et al., 2012).
Several researchers have reported using BAP and IBA for leading shoots from stem segments in P. volubilis.Bordignon et al., (2012) used explants hypocotyl segments. After nine weeks of in vitro culture with 0.5 mg L-1 BAP or 1.0 mg L-1 BAP + 0.1 mg L-1 IBA, they obtained shoot induction. In addition, Patthanajuck and Bunnag (2017) obtained shoot induction with hypocotyl segments using 1 mg L-1 BAP after 4 weeks. Furthermore, Thuy and Nhung (2019) regenerated shoots with 1.0 mg L-1 BAP + 0.1 mg L-1 IBA after 4 weeks using segments between the first and second leaves considered mature leaves. On the other hand, ABD was obtained in the study without any of the BAP concentrations, but there was callus formation with BAP. This is like the mentioned authors' results. ABD induction from nodal segments using 2-ip (0.8 mg L-1) and KIN (0.4 mg L-1) without callus formation was addressed for the first time in this study. Conversely, other works have used the apical meristem as an explant for shoot induction. Viegas Rodrigues et al. (2014) used 1.0 mg L-1 BAP for six weeks of culture and Solis et al. (2018) used 0.1 mg L-1 BAP and 0.05 mg L-1 NAA for sprouting 14 days afterwards. This last 14-day response time was similar for this study, where axillary development was observed at 15 days.
The increase in Ca concentration, which changed from 332.2 mg L-1 to 453 mg L-1 CaCl2, favored axillary bud development and prevented chlorosis in young leaves and subsequent leaf-fall. Several studies reported that high calcium concentrations prevented sprout tip necrosis without affecting growth and regeneration (Bairu et al., 2009). Tuteja and Mahajan (2007) propose two possible hypotheses for avoiding chlorosis and young leaf abscission: the first is that a deficiency in calcium affects cell division, and the mitotic index is high in these tissues. Secondly, the middle lamella is formed between two daughter cells, which is primarily calcium pectate, and a deficiency can alter its formation. Furthermore, it has been demonstrated that calcium is required as a second messenger for some hormones and environmental responses to function (Xu et al., 2009).
In this work, ABD response was optimized by adding coconut water (10 %). This result indicates P. volubilis may require additional complementary components to successfully develop axillary buds. Adding organic compounds to basal culture media is an alternative used routinely for the micropropagation of various species (Rai 2019; Agustini et al., 2020; Sembiring et al., 2020). The effect of coconut water on tissue culture mainly occurs due to the high complexity and content of sugars, minerals, vitamins, amino acids, enzymes, volatile aromatic compounds and other biochemical compounds (Burns et al., 2020).
The effect of coconut water as a growth factor on the micropropagation process occurs due to the singular or synergetic effect of its components, such as auxins and cytokinins. Furthermore, some studies reported that medium supplementation with organic compounds increases ex vitro survival, which is one of the most challenging steps after in vitro culture (Moreno Martínez and Menchaca García, 2007). However, oxidation at the base of explants was a recurring problem when using coconut water in culture media. It was overcome using activated carbon (data not shown). Activated carbon is mainly used to control oxidation problems associated with tissue culture, such as the release of phenolic compounds that induce oxidation and tissue death. Therefore, activated carbon absorbs chemical substances and prevents them from interacting with tissue (Thomas, 2008).
CONCLUSIONS
In conclusion, the protocol developed in this study was successful and facilitated the in vitro germination of P. volubilis seeds. High percentages of in vitro seed germination were achieved by removing their coats, using MS culture media and keeping them at 28 °C for 2-3 days. The response in germination time improved compared to other works, shortening the seedlings' production times. These results could help overcome seed dormancy and expedite the germination process when rapid multiplication is desired. Furthermore, the protocol developed for the micropropagation of P. volubilis plantlets is feasible through axillary bud induction of nodal explants and represents a significant improvement in differentiating axillary buds, developing shoots and forming leaves on the plantlets, with respect to the previously reported works. The best axillary bud development was obtained when the culture medium was supplemented with 453 mg L-1 CaCl2, 0.6 mg L-1 2-ip or 0.4 mg L-1 KIN, 10 % coconut water for 15 days of culture. This work contributes to improving the efficiency of the propagation of P. volubilis to improve its propagation and complement traditional vegetative propagation methods in nurseries. With a perspective towards the future, studying the genetic stability of micropropagated plantets and examining the role parameters, such as age, cultivar genotype, liquid media and the concentrations of plant growth regulators play in regeneration efficiency in detail is proposed.
















