INTRODUCTION
Technological advances in health, such as cardiac stimulation devices, have allowed to improve the survival rates in people with severe cardiac arrhythmias, making their therapeutic use more frequent 1-5. Despite the positive results in terms of survival, it has been documented that the implanted people have physical, psychological and social difficulties to accept and integrate technology into everyday life 6-10, a fact that does not allow the obtaining of the maximum benefit from the devices in terms of quality of life 11,12.
The "Acceptance" has been a theoretical construct that emerges from the research process and has allowed addressing specifically the issues related to the integration of technology to everyday life. One of the first ones to use the term Acceptance, was Luderitz in 1994, who was interested in the psychological consequences and quality of life issues in the people implanted, defining the Acceptance of the patient as "the perception of the device, the perception of possible discharge, body image changes, changes in lifestyle, perceptions of patients and family members, concerns when returning home, and fear of complications " 13. Later in 1996, Burke, through a qualitative study, was able to identify the acceptance of technology as a central category of the process of living with a cardio-defibrillator, which was defined as "A process characterized by the choice to live with technology, the integration of technology in life, and living life through technology " 14. In 2005 Sears and collaborators defined that "patient acceptance is the psychological accommodation and understanding of the advantages and disadvantages of the device, the recommendation of the device to others, and obtaining benefits in biomedical, psychological functioning and social terms " 15. Likewise, it has been established that Acceptance is an indicator of success of cardiac stimulation therapy, which is why these difficulties are required to be addressed by health professionals in charge of the care of people with this type of device.
However, the review of the literature shows that currently the specific instruments to assess and address the needs of adaptation in people with cardiac devices are few and there is not a version in Spanish for its use in the Hispanic context, which makes it imperative to have this type of instrument. After the bibliographic inquiries and by virtue of their optimal psychometric values, the Florida Patient Acceptance Survey (FPAS) instrument is chosen, which allows to measure the level of acceptance of the patient to the device, being a specific indicator for people with cardiac stimulation devices. The FPAS consists of 18 items with a Likert-type response scale, assessing four categories or dimensions: return to life, distress related to the device, positive assessment and body image concerns. The scale was developed through a psychometric study in 2005, reporting a Cronbach alpha of 0.83 for the whole scale and for each of the categories or dimensions a Cronbach coefficient between 0.74 to 0.89; Likewise, it demonstrated convergent validity with the SF - 36 quality of life scale and divergent validity with the CES - D and STAI scales 15. The scale has been adapted and validated in Danish population, where it was reported that the validity of the instrument is confirmed for the four factors with a Cronbach alpha of 0.73 to 0.85, so the FPAS proved to be a valid and reliable measure of acceptance to the device in patients with cardiac stimulation devices 16,17.
Taking into account that the Florida Patient Acceptance Survey (FPAS) is a specific measure for people with cardiac devices whose original version was developed in the English language and has not yet been applied in the Spanish-speaking population, it is required to perform the process of transcultural adaptation of the instrument to the Colombian population, and thus have a valid tool that allows addressing the acceptance of the patient of the device of cardiac stimulation to everyday life.
MATERIALS AND METHODS
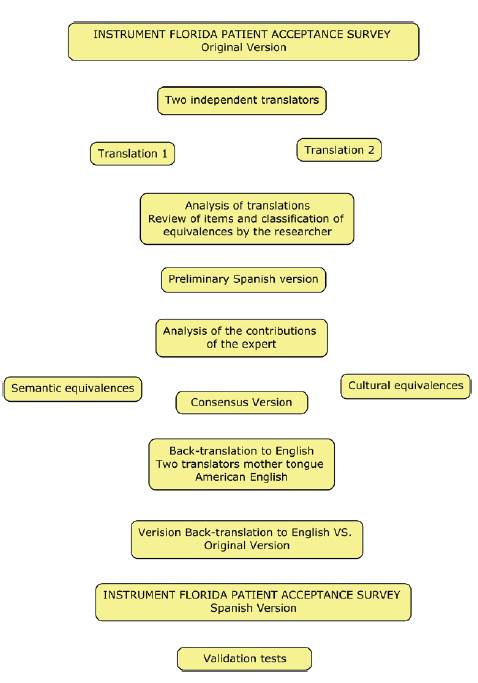
The translation and bilingual back-translation methodology was used to carry out the process of transcultural adaptation of the FPAS instrument 18,19, which seeks to obtain the semantic equivalence of the original instrument to the version obtained for the Colombian context as observed in figure 1, permission was previously requested from author Samuel Sears for use.
Two Bilingual translators of Spanish mother tongue carried out the translation into Spanish of the instrument independently; each translated version was evaluated jointly by the research team and the translators arriving at a preliminary version of the instrument that was sent to 5 experts for carrying out the content and facial validity.
For the selection of experts, the criteria proposed by Skjong and Wentworht were taken into account: 1) experience in making judgments and making decisions based on evidence or expertise 2) reputation in the community, 3) availability and motivation to participate; and 4) impartiality and inherent qualities such as self-confidence and adaptability 20. The group of experts was made up of: 1 expert nurse in cardiac stimulation devices, 2 nurses with experience in validation of measurement instruments and management of patients with cardiac rhythm disorders, 1 psychologist nurse specialist in Mental Health and 1 Physician Electrophysiologist.
Once the result of the evaluation was obtained by the experts, the observations by the researchers and the linguist were reviewed one by one in order to achieve the semantic adaptation to the Colombian context. The final version of the instrument was translated back into the English language by a bilingual translator, a version that was sent to the original author who evaluated it and gave his endorsement, to continue with the psychometric process of reliability and validity.
RESULTS
The FPAS instrument translated into Spanish had some modifications in order to achieve its transcultural adaptation to the Colombian context, considering the observations of the experts, the professional in linguistics and the statistical advice in order to achieve an instrument semantically equivalent to the original. It was taken into account that in the English language a word can have different meanings according to the context of the sentence, when translated literally into the Spanish language it can lose its meaning and affect the sentence that is intended to be evaluated, as is the case of the items 3 and 14 of the original instrument that contained the word "disfigured" that when translated into the Spanish language represents "desfigurado", a word that in the Spanish language is frequently associated with the loss of some part of the body, therefore it does not reflect the condition of having a cardiac device in the body. Initially, a search was made of the meaning in English and Spanish of the word, in order to find the appropriate semantic equivalence, later the synonyms were searched in the English language to cover greater possibilities and the suggestions made by the experts were evaluated. All these results were reviewed by the research team and the linguist, making the modifications, as can be seen in table 1.
Table 1 Process semantic equivalence of the item
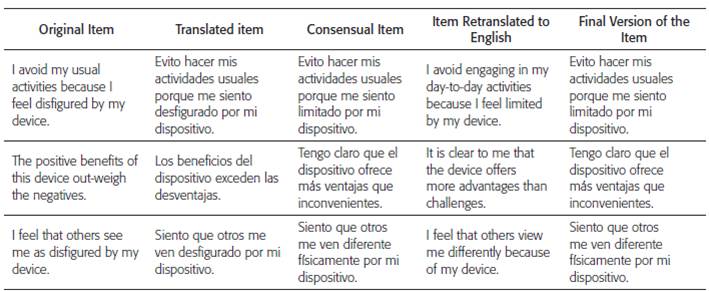
Fuente: Castillo Sierra DM, González Consuegra RV. Intervención De Enfermería Para La Aceptación Del Paciente Al Cardio-desfibrilador Aplicada En El Preimplante.
Similarly, other items to be translated into Spanish, could give an ambiguity to what is being asked, as for example item 10 of the original instrument refers to: "would you receive the device again", in this case the word again is open to an ambiguous aspect, since there is no reference in what time or circumstance this situation could be given to be evaluated by the person who diligently proceeds, requiring to be adjusted in the following manner "if necessary, would you receive the device again" to give clarity; Another aspect that is relevant is the context, given that in Colombia the older adult lacks job opportunities, while in the United States, where the instrument was developed, this population still has a productive opportunity, this is how item 6 in its version original translated into Spanish contemplates: "I have confidence in my ability to return to work, if I wanted to do it", what made the item exclusive for this population group, with the purpose of making it inclusive in our context was generalized to "daily activities", which involves the work aspect.
Finally, as part of the transcultural adaptation of the instrument in the Spanish language, a different ordering of the items was given for two fundamental reasons: the first responds to the observations issued by the experts, who suggested that the first item contemplated in the original instrument "I get depressed when I think of the device" has a negative connotation to start an instrument, which can generate an undesirable impact on the person who respomds, and the second reason, is due to the grouping of items by type of response, taking into account that once the items analyzed by the research team and the statistician, the response of certain items are more consistent with a type of frequency, such as the item "I avoid doing activities that I enjoy by my device"; It is important to clarify that this ordering of the items in the Spanish version does not alter the score awarded on the Likert scale, but the directionality of the answer for subsequent statistical analyzes.
DISCUSSION
Aculturally adapted to the Colombian context Spanish version of the Florida Patient Acceptance Survey (FPAS) is obtained as shown in figure 2, despite the modifications made, a semantic equivalent to the original version is maintained, a circumstance that was validated by the original author of the instrument, who told the research team that the essence of the instrument remained to evaluate the acceptance of people to cardiac devices. This article shows the process that was carried out to obtain an adequate translation and adaptation of the instrument, which are the first steps of the process, since as mentioned by Escobar Bravo "The adaptation of an instrument includes, of course, its translation , its cultural and idiomatic adaptation and the verification of the psychometric characteristics of reliability and validity " 21; the methodology used for the cultural adaptation of the instrument is the one recommended by experts in the field of validation of measurement instruments 18,19,22, the presented version is coherent with the aspects of a cultural adaptation and will allow obtaining psychometric results similar to those of the original instrument.
It is important to subject the instruments of measurement to cultural adaptation and not only to carry out a mere translation of them, before this, Carvajal mentions "The way of asking and the language used are sources of bias, but factors are not less so cultural factors that lead to the same question being valid or not in one language or another, or even in different countries that share the same language " 23, a fact that could be validated through the process of cultural adaptation of the FPAS instrument , where it was evidenced that the English language can have multiple meanings when translated into Spanish and even more so in a specific context such as the Colombian.
Similarly, some authors have described the relevance of carrying out the processes of cultural adaptation of the instruments, not only in terms of semantic equivalence with the original versions, but in terms of decreasing time and costs in the research processes, allowing the strengthening of existing measures, as well as expanding their use in different populations, making empirical indicators more universal in terms of results in health care, which allows to respond to the multicultural needs of a globalized world, which in many cases, demands immediate responses from different health systems 21,23,24.
Finally, it is important to clarify that the fact of having this version in Spanish culturally adapted to the Colombian context does not mean that the instrument has the same psychometric properties of the original instrument, as mentioned above, this constitutes the first steps of the validation, and a following process will define the psychometric properties of this version of the Florida Patient Acceptance Survey (FPAS).
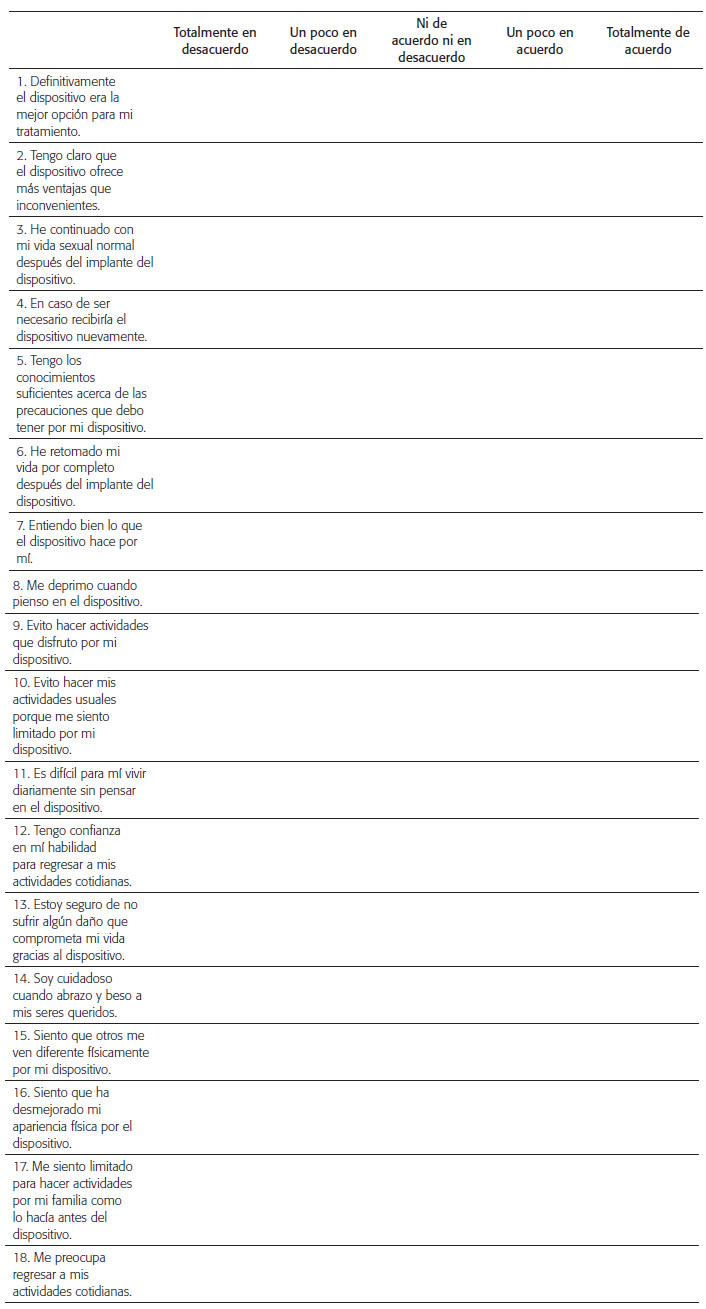
CUESTIONARIO DE FLORIDA PARA LA ACEPTACIÓN DEL PACIENTE (FPAS) VERSIÓN EN ESPAÑOL
Queremos saber qué significa para usted vivir con un dispositivo médico. A continuación, encontrará unas afirmaciones que describen lo que es vivir con un dispositivo médico. Por favor, indique si usted está de acuerdo o en desacuerdo con los enunciados marcando la casilla más apropiada.
CONCLUSIONS
The Spanish version of the instrument "Florida Questionnaire for Patient Acceptance FPAS", is semantically equivalent to the original instrument and adapted to the Colombian context; during the process it underwent minimal modifications that do not alter the essence of the instrument, it is a specific measure to the acceptance of people with cardiac stimulation devices, short and rapid completion in clinical practice and research. In a later phase, psychometric tests will be carried out for the validity and reliability of this version.