Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Ingeniería e Investigación
versión impresa ISSN 0120-5609
Ing. Investig. v.28 n.3 Bogotá sep./dic. 2008
Flavio Humberto Fernández Morales1, Julio Enrique Duarte2 y Joseph Samitier Martí3
1 Ingeniero electrónico, Universidad Distrital Francisco José de Caldas, Colombia. Ph.D., en Ingeniería Electrónica, Universidad de Barcelona, España. Profesor asociado, Universidad Pedagógica y Tecnológica de Colombia, Seccional Duitama, Colombia. Grupo de Energía y Aplicación de Nuevas Tecnologías GEANT. flaviofm1@gmail.com
2 Licenciado en Física, Universidad Industrial de Santander, Colombia. Ph.D., en Física, Universidad de Barcelona, España. Profesor titular, Universidad Pedagógica y Tecnológica de Colombia, Seccional Duitama, Colombia. Grupo de Energía y Aplicación de Nuevas Tecnologías GEANT. julioenriqueduarte@latinmail.com
3 Ph.D., en Física, Universidad de Barcelona, España. Profesor catedrático, Departamento de Electrónica, Universidad de Barcelona, España. samitier@el.ub.es
ABSTRACT
Common dielectrophoresis (c-DEP, i.e. neutral matter motion induced by non-uniform electric fields) has become a basic phenomenon of biochips intended for medical, biological and chemical assays, especially when they imply bioparticle handling. This paper deals with modelling and experimental verification of a castellated, c-DEP-based, microelectrode array intended to handle biological objects. The proposed microsystem was developed employing platinum electrodes patterned by lift-off, silicon micro-machining and photoresin patterning techniques. Saccharomyces cerevisiae were used as test bioparticles for experimental verification. Yeast cells were repelled toward electrode bays and toward interelectrodic gaps tor frequencies around 20 MHz where there is minimum electric field strength, corresponding to a negative dielectrophoretic phenomenon. Yeast cell agglomerations were observed around electrode edges for frequencies of around 2 MHz where there is maximum electric field strength, thereby verifying the positive dielectrophoretic phenomenon. Bioparticles were separated from the electrode edges when the working frequency was reduced and they were dragged towards the electrode centre, remaining there while the frequency was low enough. Such atypical pattern may be explained due to the occurrence of positive dielectrophoresis overlap with electrohydrodynamic effects (i.e. the viscous drag force acting on the particles was greater than the dielectrophoretic force at frequencies where positive dielectrophoresis should occur). The experiments illustrated microsystem convenience in microhandling biological objects, thereby providing these microarrays possible use with other cells. Liquid motion resulting from electrohydrodynamic effects must also be taken into account when designing bioparticle micromanipulators, and could be used as a mechanism for cleaning electrode surfaces.
Keywords: dielectrophoresis, cell handling, biochip.
RESUMEN
La dielectroforesis común (c-DEP), es decir, el movimiento de materia eléctricamente neutra inducido por campos eléctricos no uniformes, se ha convertido en un fenómeno fundamental dentro de los biochips dedicados a ensayos médicos, biológicos y químicos, especialmente cuando ellos implican la manipulación de biopartículas. El presente artículo describe el modelado y la verificación experimental de un arreglo de microelectrodos interdigitados, basado en c-DEP y destinado a manejar objetos biológicos. El microsistema propuesto se desarrolló empleando técnicas como lift-off para el grabado de electrodos de platino, micromecanizado de silicio y moldeado de resinas fotocurables. La verificación experimental se realizó utilizando Saccharomyces cerevisiae como biopartículas de prueba. Para frecuencias cercanas a 20 MHz se observó que las levaduras son repelidas hacia las bahías de los electrodos y hacia el espaciado interelectródico, donde el campo eléctrico es mínimo, lo cual corresponde al fenómeno de dielectroforesis negativa. Para frecuencias cercanas a 2 MHz se observó la aglomeración de levaduras en el borde de los electrodos, donde el campo eléctrico es máximo, verificando así el fenómeno de dielectroforesis positiva. Al reducir la frecuencia de operación, las biopartículas se desprenden del borde de los electrodos y son empujadas hacia el centro de los electrodos, permaneciendo allí mientras la frecuencia sea lo suficientemente baja. Este comportamiento atípico se puede explicar porque la dielectroforesis positiva se traslapa con los efectos electrohidrodinámicos, o sea que la fuerza de arrastre viscoso que actúa sobre las partículas es mayor que la fuerza dielectroforética, a frecuencias en donde la dielectroforesis positiva debería ocurrir. Los experimentos ilustran la conveniencia de los microsistemas como micromanipuladores de objetos biológicos, abriendo la posibilidad de utilizarlos con otro tipo de células. Adicionalmente, el movimiento del líquido, como resultado de efectos electrohidrodinámicos, debe ser tenido en cuenta cuando se diseñan micromanipuladores de biopartículas y podría ser utilizado como mecanismo para la limpieza de los electrodos.
Palabras clave: dielectroforesis, manipulación de células, biochips.
Recibido: febrero 25 de 2008
Aceptado: octubre 28 de 2008
Introduction
Much effort has been invested in developing new bioparticle-microhandling microtools involving manufacturing devices whose size roughly matches that of bioparticles (being normally between 1 to 100 μm or less if viruses or DNA macromolecules must be handled) (Talary et al., 1998; Figeys and Pinto, 2000; Dalton and Kaler, 2005; Wälti et al., 2007). Nowadays, these dimensions and resolution levels may be achieved by using microsystem technologies. The advantages of miniaturisation include reducing required sample volume and test times, cost reduction by mass production, the possibility of integrating multiple analytical functions in the same chip and minimising problems such as solution becoming heated due to the voltages being applied (Fuhr and Shirley, 1998; Hoettges et al., 2003).
Common dielectrophoresis (c-DEP) has become a basic occurrence in microchips intended for medical, biological and chemical assays, especially when they imply bioparticle manipulation (Müller et al., 2003). Furthermore, c-DEP has been employed as an additional mechanism in developing new materials requiring the precise allocation of micro- and nano-particles (Velev and Kaler, 1999; Rosenthel and Voldman, 2005).
This research was conceived for exploring the possibilities of dielectrophoresis-based microsystems aimed at bioparticle handling. A microsystem was thus designed and fabricated by means of microsystem techniques. A castellated microelectrode array was tested using yeast cells. Negative DEP was observed for frequencies of around 20 MHz. Positive DEP could also be verified for frequencies below 2 MHz but particle behaviour could not be explained by the classical dielectrophoretic theory for values lower than 1 MHz. In other words, positive DEP could not be experimentally verified for frequencies lower than 1 MHz because (usually unconsidered) electrohydrodynamic effects were observed as the dominant phenomena when working at low frequencies. This shows that liquid motion resulting from electrohydrodynamic effects must be taken into account when designing bioparticle micromanipulators and could be used as a mechanism for cleaning electrode surfaces, this being one of the most important problems related to these kinds of devices.
This paper also describes some theoretical aspects concerning c-DEP (i.e. the physical phenomenon hinging on non-uniform electric-field-mediated forces on which the microdevice tested here is based). Modelling and the numerical calculation of the electric field above an interdigitated castellated microelectrode array is also reported, as is the technological approach used for manufacturing the microsystem.
Methods and Materials
Basic phenomena
When an uncharged body is placed in a non-uniform electric field it induces electrical charges upon the particle surface. Such charge distribution will have equal quantities of positive and negative electric charges. The electric field will cause alignment on the dipoles in relation to itself. As the field is non-uniform, one dipole end will be in a weaker region than the other thereby originating a net force acting on all permanent or induced dipoles and causing them to be constrained to move towards or away from the region having the highest field density.
Such a lateral motion imparted to uncharged particles resulting from non-uniform electric field-induced polarisation is termed dielectrophoresis (DEP) (Pohl and Schwar, 1959), which may be towards (positive DEP) or away from (negative DEP) the maximum electric field depending on the particles electrical properties and suspending medium, respectively (Tang et al., 2003)
The time-averaged dielectrophoretic force is given by the equation below for a non-ideal insulating spherical particle having radius r (Pohl and Crane, 1972):
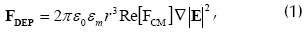
where ε0 = 8.854x10-12 (F m-1) is free-space permittivity, εm is effective medium permittivity,  is the gradient operator, E is the electric field strength and Re[FCM] denotes the real part of the Clausius-Mossotti factor defined by:
is the gradient operator, E is the electric field strength and Re[FCM] denotes the real part of the Clausius-Mossotti factor defined by:
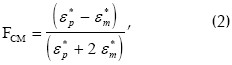
where subscripts p and m stand for particle and medium and ε* is complex dielectric permittivity given by:
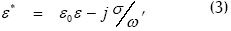
ε being relative effective permittivity, σ effective conductivity and ω the applied fields angular frequency.
From equation (1) it can be seen that FDEP depends on particle size and electric field magnitude which is related to the electric potential applied. The gradient operator stands for the spatial non-uniformity of the electric field applied which is a geometrical factor depending on the electrode layout.
Equations (1) and (2) also reveal that FDEP magnitude and polarity depend on the frequency of the field applied and relative values of particle conductivity and permittivity and the surrounding medium in quite a complicated way.
Thus, if particle polarizability exceeds that of the surrounding medium, i.e.  , then the arrangement of the induced charges produces a dipole moment with the same direction as the applied field, thereby inducing p-DEP. Nevertheless, if
, then the arrangement of the induced charges produces a dipole moment with the same direction as the applied field, thereby inducing p-DEP. Nevertheless, if 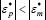 , then the induced dipole moment is opposite to the field, originating n-DEP.
, then the induced dipole moment is opposite to the field, originating n-DEP.
Electric field simulation
In view of the critical role played by field inhomogeneities in DEP, suggested by the ruling equations, calculating the field distribution is always of interest and provides insight. Thus, finite element method (FEM) numerical calculation was extensively used for gaining insight into electric field profiles over diverse electrode shapes.
An interdigitated castellated microelectrode array was considered for simulations. The 3-D model shown in Figure 1 was studied by means of commercial ANSYS (Swanson Analysis Systems, Inc.) software for assessing and visualising electric field distribution (this being FEM method-based, general purpose software for solving physical problems).
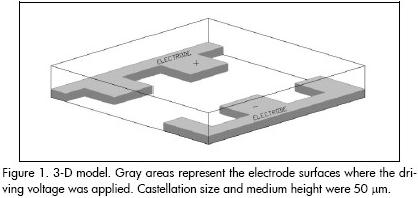
Figure 2 represents electric field distribution corresponding to a pseudo-topographical colour-coded field surface. It can be seen that points located near the electrode tips would experience the highest electric field strength, whilst this strength diminishes as the observation point moves towards the central part of the interelectrodic gap, towards the bays or onto the electrodes metallic surface.
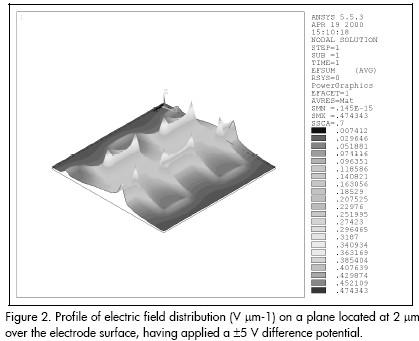
Computer analysis predicted that p-DEP would result in particle agglomeration at electrode tips whilst n-DEP would cause particles to accumulate on electrode bays and electrode surface, as well as in the interelectrodic gap. Of course, final particle position depends upon polarisability function value.
Proposed microsystem
When designing dielectrophoretic-based microsystems intended for bioparticle microhandling one must bear in mind that this techniques typical read-out is mainly performed with optical tools (microscopes, image analysers, etc.) rather than with electrical apparatus. An effort must be made to form a true microchamber by patterning the walls of a cavity with a known volume, i.e. a micropool should be fabricated to guarantee a constant volume of the suspending medium over the electrodes, thereby avoiding possible experimental fluctuations due to this item. A whole microsystem was thus designed and fabricated taking these considerations into account, as described below.
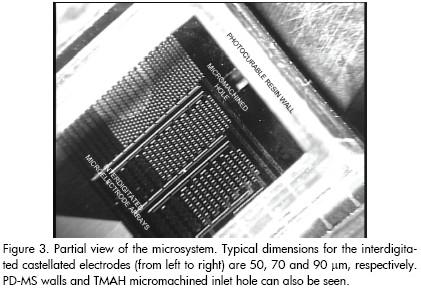
The technological process at wafer level can be roughly divided into three stages: platinum microelectrode patterning in which they are defined onto a 300 μm thickness silicon wafer by lift-off; the wafer being drilled by silicon micromachining to shape inlet and outlet holes; and UV-curable polymer (polydimethylsiloxane, PDMS) photolithographic structuring onto the component side of the wafer (this process moulds the microchamber walls). Figure 3 gives a partial view of the resulting microdevice.
Results and Discussion
This section deals with the experimental results obtained whilst testing the microdevice, using Saccharomyces cerevisiae as test bio-particles; they correspond to a widely-studied yeast strain whose electrical properties are well-known (Paul and Harrison, 1997; Krommenhoek. 2005; Zakhem 2006; Abidin, et al., 2007).
The yeast cells were kindly provided by Barcelona Universitys Microbiology Department. All measurements required using particles suspended in very low conductivity solutions to reduce the risk of heating-related problems. Bioparticles were suspended in de-ionised water; final suspension was adjusted to a 5x106 cells mL-1 concentration. Solution conductivity was adjusted between 20 and 30 ?S cm-1. The sample was micropipetted using a 100 ?L Eppendorf micropipette dispenser onto the electrodes active area limited by the photoresin walls.
The results described below were obtained with interdigitated 70 ?m castellated microelectrodes. Such structures are well-suited for studying c-DEP phenomena because they are only driven by two signals inverted in phase.
Common dielectrophoresis
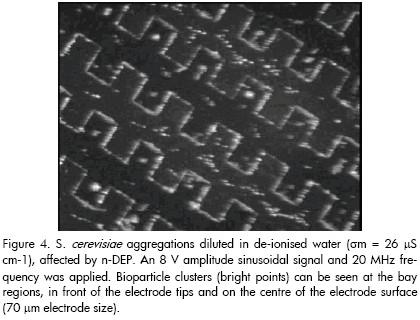
Having dropped the yeast dissolution into the micropool, 70 ?m electrodes were energised with an 8 V sinusoidal signal in 20 MHz amplitude. Bioparticles became concentrated after a few minutes, forming aggregations located at the electrode bay regions, in front of the electrode tips and on the centre of the electrode surface (i.e. particles were driven towards and agglomerated at regions corresponding to electric field minima, as predicted by previously described electric field simulations). Figure 4 illustrates the aforementioned pattern (i.e. yeast cells affected by n-DEP). A partial view of the microelectrode array can be seen with trapped particle clusters on it (bright points).
When applied signal frequency was decreased (voltage amplitude remaining unaltered), bioparticle motion was observed towards the electrode corners. In other words, the previously described cell aggregations were now attracted towards the highest electric field regions (electrode edges), thereby verifying the p-DEP phenomenon. This pattern was evident for frequencies below around 2 MHz. Interestingly, particles remained attached to the electrode corners until the frequency had risen again to a value in which n-DEP could stably occur (i.e. if frequency was increased, particles were repelled towards their original positions at the electrode bays, meaning that n-DEP and p-DEP occurrence could be successively achieved by simply changing and selecting the appropriate applied frequency signal values). Figure 5 proves the occurrence of p-DEP (observation area being the same as in Figure 4). Particles were attached to castellated microstructure corner tips, edges and the rear side of bays. Cells initially settled on the electrode centre remained attached at the surface and could not be moved by the effect of the dielectrophoretic force.
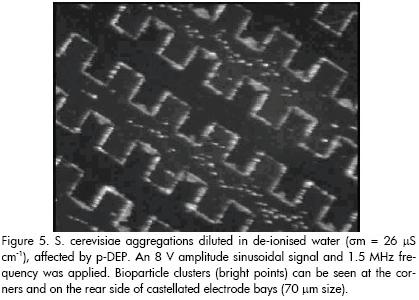
Frequency variations produced particle motion towards well-defined regions on the microelectrode array. Frequencies above 20 MHz compelled particles to concentrate at the electric field minima in our experiments (i.e. n-DEP was induced onto them). On the other hand, frequencies lower than 2 MHz were observed to produce particle attraction towards the electrode edges when electric field strength was at its maximum (i.e. p-DEP had occurred). These observations agreed with results obtained by other researchers for non-viable yeast cells (S. cerevisiae) and have been explained according to the two-shell particle model (Huang et al., 1993; Wang et al., 1993; Talary et al., 1996).
One can say that c-DEP occurrence in non-viable yeast cells was experimentally verified in view of the experimental results. However, we would like to point out that more experimental work should be carried out for accurately measuring the actual dielectrophoretic spectrum and characterise the crossover frequency at which n-DEP commutes to p-DEP.
Electrohydrodynamic effects
Once yeast cells had been collected by p-DEP at 1.5 MHz, the applied frequency was gradually reduced to 2 kHz. Particles were observed to detach from electrode edges as frequency was decreased (electric field maxima points of the structure) and move towards the electrode centre, agglomerating to form diamond-shaped clusters. Bioparticles returned to their initial positions when frequency was raised. Figure 6 shows a sequence from this pattern; the absence of cells at the interelectrodic gap during this regime was noticeable.
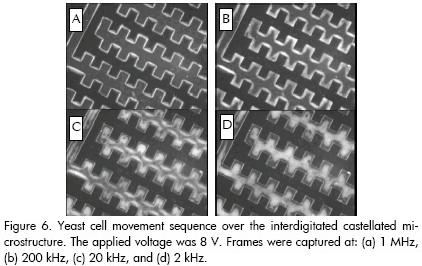
Such particle behaviour (i.e. particle collection at the centre of the electrode surfaces when working at relatively low frequencies in dielectrophoretic-based microdevices) has also been observed by other researchers when working on handling, characterising and separating microparticles (Green and Morgan, 1998; Green et al., 2000; 2000a).
Such pattern was tentatively attributed to the combination of an electrophoretic effect in aiding cells to cross electrode boundaries, coupled with a negative dielectrophoretic force in directing them to the centre of the electrode surface (Pethig et al., 1992). However, Ramos et al.s impressive review worked out an order-of-magnitude estimation of forces acting in c-DEP microelectrodes, concluding that the fluid motion moves particles away from the electrode edge for the low-frequency range and into well defined regions on top of the electrodes (Ramos et al., 1998).
The aforementioned review guided us to perform the numerical analysis of electrohydrodynamic (EHD) effects (i.e. fluid motion as a result of thermally-induced medium inhomogeneities, originated on DEP-based microdevices) (Fernández, 2000). This work demonstrated that the fluid motion around the interdigitated microelectrodes consists of a couple of whirlpools rotating in opposite directions at low frequencies (see Figure 7); whilst the left-hand one spins counter-clockwise, the other rotates clockwise. If there are particles in the solution and the applied voltage is high enough, those particles could show the tendency to concentrate at the centre of the electrodes.
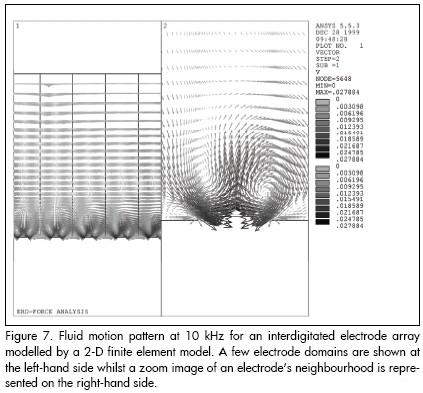
Figure 7 shows that the particles are drawn down by fluid motion onto the electrode edge and then into the centre, where they collect. This effect is frequency-dependent, as shown in our results with yeast cells. In view of the practical results, it is evident that other normally unconsidered effects (e.g. EHD and electro-osmotic effects), must be taken into account when working at low-frequency ranges in addition to the primarily studied dielectrophoretic force also influencing particle motion. Indeed, such motion actually results from the hydrodynamic viscous drag force affecting the particles which can be originated by EHD effects.
Conclusions
This work was conceived for exploring the possibilities of dielectrophoresis-based microsystems aimed at bioparticle handling. A microsystem was thus designed and fabricated by microfabrication techniques: platinum microelectrode lift-off patterned on a silicon substrate and bulk silicon micromachining techniques. Microcavity walls were moulded by means of a photopatternable resin. This process resulted in a micropool of known volume being formed, allowing the use of the same sample volume during all the experiments.
From the experimental point of view, the occurrence of negative dielectrophoresis was verified in yeast cells which were collected at the bay regions of interdigitated castellated microelectrodes. S. cerevisiae were also trapped at the electrode edges as a consequence of positive dielectrophoresis. Such bioparticle behaviour agreed with that expected for nonviable yeast cells and can be explained by the double-shell particle model. Furthermore, the bioparticle distribution agreed with that predicted by the simulated electric field profile.
This research was mainly dedicated to c-DEP effects and it was observed that electrohydrodynamic (EHD) phenomena may alter or even control particle behaviour in the presence of low-frequency electric fields. It must be remarked that despite the huge range of applications, DEP theoretical and numerical studies up to now have concentrated on electric field and dielectrophoretic force distributions. However, the lack of calculation taking into account the influence of other related phenomena such as EHD should be noted. Of course, the influence of EHD phenomena has to be considered when designing much more reliable and controllable c-DEP-based devices, especially when working with media having high conductivity and high applied voltages.
Numerical electric field calculations were validated, because yeast cells (influenced by n- or p-DEP) were observed to collect over the microelectrodes, according to distributions dictated by electric field profile. Moreover, particles were observed to agglomerate following patterns established by the aforesaid electric field distribution. In other words, particle distribution can be predicted by simulations of the electric field profile over the electrodes. This ability will be valuable when microparticles have to be assembled on electrode surfaces following a desired pattern.
To conclude, we would like to say that following the introduction of microfabrication technologies, dielectrophoresis has become a promising tool at the forefront of the selective handling and separation of cells, bacteria and other micro-organisms, as well as viruses and possibly biomacromolecules such as DNA and proteins. In view of this, dielectrophoresis as a subject for research in general, and microsystems aimed at bioparticle microhandling hinging upon dielectrophoretic phenomena in particular, have a brilliant future. Some reasons for this are that microsystems can be manufactured with suitable dimensions for those required by microparticle manipulation, and the dielectrophoretic force has magnitudes comparable to those of other forces available for performing these tasks. Furthermore, the method is non-invasive, does not require the use of antibodies or other labelling (although dielectric labelling to increase specificity could be an option) and can be employyed at either single-cell or multiple-cell level.
Acknowledgements
The authors are grateful to the National Microelectronics Center (CNM) in Barcelona, Spain, especially to Dr. Errachid Abdelhamid for manufacturing and processing the microchip tested here.
Bibliography
Abidin, Z. Z., Dowes, L. and Markx, G. H., Novel electrode structures for large scale dielectrophoretic separations based on textile technology., Journal Biotechnology, Vol. 130, 2007, pp. 183-187. [ Links ]
Dalton, C. and Kaler, K., An integrated PDMS microfluidic device for dielectrophoretic separation of malignant cells., Source: Proceedings of the 3rd International Conference on Microchannels and Minichannels, Vol. part B, 2005, pp. 411-418. [ Links ]
Fernández F.H. Design, assembly and testing of microsystems for dielectrophoresis-based bioparticle electrohandling, Doctoral thesis, Department of Electronics, University of Barcelona, Barcelona, Spain 2000. [ Links ]
Figeys, D. and Pinto, D. Lab-on-a-chip: A revolution in biological and medical sciences., Analytical Chemistry, Vol. 72, 2000, pp. 330a-335a. [ Links ]
Fuhr, G. and Shirley, S. G., Biological application of microstructures., Topics in current chemistry, Vol.194, 1998, pp. 83-116. [ Links ]
Green, N. G. and Morgan, H., Separation of submicrometre particles using a combination of dielectrophoretic and electrohydro-dynamic forces., Journal of Physics D: Applied Physics, Vol. 31, 1998, pp. L25–L30. [ Links ]
Green N. G., Ramos, A. and Morgan, H., AC electrokinetics: a survey of sub-micrometre particle dynamics., Journal of Physics D: Applied Physics, Vol. 33, 2000, pp. 632-641. [ Links ]
Green N. G., Ramos, A. and González, A., Electric field induced fluid flow motion on microelectrodes; the effect of illumination., Journal of Physics D: Applied Physics, Vol. 33, 2000a, pp. L13–L17. [ Links ]
Hoettges, K., Hughes, M., Cotton, A., Hopkins, N. and Mcdonell, M., Optimizing particle collection for enhanced surface-based biosensors., IEEE in Medicine and Biology Magazine, Vol. 22, 2003, pp. 68-74. [ Links ]
Huang, Y., Wang, X-B., Tame, J. A. and Pethig, R., Electrokinetic behaviour of colloidal particles in travelling electric fields: studies using yeast cells., Journal of Physics D: Applied Physics, Vol. 26, 1993, pp. 1528-1535. [ Links ]
Krommenhoek. E. E., Gardeniers, J. G. E., Bomer, J. G., Van Den Berg, A., LI, X., Ottens, M., Van Der Wielen, L. A. M., Van Dedem, G. W. K., Van Leeuwen, M., Van Gulik, W. M. and Heijnen, J. J., Monitoring of yeast cell concentration using a micromachined impedance senso., Sensors and Actuators B: Chemical, Vol. 115, 2005, pp. 384-389. [ Links ]
Müller T., Pfennig A., Klein, P., Gradl, G., Jäger, M. and Schnelle, T., The potential of dielectrophoresis for single-cell experiments., IEEE in Medicine and Biology Magazine, Vol. 22, 2003, pp. 51 - 61. [ Links ]
Paul, C. and Harrison, J., Transport, manipulation and reaction of biological cells on-chip using electrokinetic effects., Analytical Chemistry, Vol. 69, 1997, pp. 1564 – 1568. [ Links ]
Pethig, R., Huang, Y., Wang, X-B. and Burt, J. P. H., Positive and negative dielectrophoretic collection of colloidal particles using interdigitated castellated microelectrodes., Journal of Physics D: Applied Physics, Vol. 24, 1992, pp. 881-888. [ Links ]
Pohl, H. A. and Schwar, J. P., Factors affecting separations of sus-pensions in nonuniform electric fields. Journal of Applied Physics, 1959, Vol. 30, pp. 69-73. [ Links ]
Pohl, H. A. and Crane, J. S., Dielectrophoretic force, Journal of Theoretical Biology., Vol. 37, 1972, pp. 1-13. [ Links ]
Ramos, A., Morgan, H., Green, N. G. and Castellanos, A., AC electrokinetics: a review of forces in microelectrodes structures., Journal of Physics D: Applied Physics, Vol. 31, 1998, pp. 2338-2353. [ Links ]
Rosenthel, A. and Voldman, J., Dielectrophoretic traps for single-particle patterning., Biophysical Journal, Vol. 88, 2005, pp. 2193-2205. [ Links ]
Talary M., Burt, J. P. H., Tame, J. A. and Pethig, R., Electromanipulation and separation of cells using travelling electric fields., Journal of Physics D: Applied Physics, Vol. 29, 1996, pp. 2198-2203. [ Links ]
Talary, M. S., Burt, J. P., Tame, J. A. and Pethig, R., Future trends in diagnosis using laboratory-on-a-chip technologies., Parasitology, Vol. 117, 1998, pp. S191-S203. [ Links ]
Tang, J., Gao, B., Huaizhi, G., Velev, O., Qin, Lu-chang. and Otto. Z., Assembly of 1D Nanostructures into Sub-micrometer Diameter Fibrils with Controlled and Variable Length by Dielectrophoresis., Advanced Materials, Vol. 15, No. 16, 2003, pp. 1352-1355. [ Links ]
Velev, O. and Kaler, E., In situ assembly of colloidal particles into miniaturized biosensors., Langmuir, Vol. 15, 1999, pp. 3693–3698. [ Links ]
Wang X-B., Huang, Y., Burt, J. P., Markx, G. H. and Pethig, R., Selective dielectrophoretic confinement of bioparticles in potential energy wells., Journal of Physics D: Applied Physics, Vol. 26, 1993, pp. 1278-1285. [ Links ]
Wälti, C., Germishuizen, W., Tosch, P., Kaminski, C. and Davies, G. Electrokinetic manipulation of DNA. Journal of Physics D: Applied Physics, Vol. 40, 2007, pp. 114-118. [ Links ]
Zakhem, H., Lanoisellé, J., Lebovka, A., Nonus, M. and Vorobiev, E., Behavior of yeast cells in aqueous suspension affected by pulsed electric field., Journal of colloid and interfase science, Vol. 300, 2006, pp.553-563. [ Links ]














