Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Ingeniería e Investigación
Print version ISSN 0120-5609
Ing. Investig. vol.31 no.1 Bogotá Jan./Apr. 2011
Synthesising unsaturated fatty alcohols from fatty methyl esters using catalysts based on ruthenium and tin supported on alumina
David Alexander Echeverri1, Luis Alberto Rios2, Juan Miguel Marín3
1 Chemical Engineering. M.Sc. in Chemistry. Ph.D. Candidate. Universidad de Antioquia, Colombia. edaez212@udea.edu.co.
2 Chemical Engineering. Ph.D. Natural Sciences., Universidad Técnica de Aachen, Germany. Professor, Universidad de Antioquia. larios@udea.edu.co.
3 Chemical Engineering. Ph.D. in Chemistry. Universidad de Antioquía, Colombia. Professor, Universidad de Antioquia. jmmarin@udea.edu.co.
ABSTRACT
Most promising catalysts for synthesising unsaturated fatty alcohols are based on group 8 metals with a promoter like tin, because the process can be carried out in moderate conditions, and these metals are less toxic than chromium. There have been no reports about the use of this catalyst to date using raw materials like methyl ester blends or evaluation reusing catalysts. This paper presents the hydrogenation of methyl esters from palm oil and commercial methyl oleate with Ru-Sn/Al2O3 catalysts prepared by impregnation, at moderate pressure and temperatur (5 MPa and 270°C). Greater selectivity to unsaturated alcohol and the less selectivity to methyl stearate was found for an optimal Sn:Ru=2 ratio. Hydrogenation of palm oil methyl esters with this catalyst produced a mixture of oleyl alcohol, saturated alcohols having 16-18 carbon atoms and heavy esters. Raw material had no great effect on catalyst activity. However, the catalyst showed deactivation through several uses due to decreased catalytic area.
Keywords: fatty alcohol, hydrogenation, methyl ester, palm oil, heterogeneous catalysis.
Received: October 06th 2009. Accepted: Feuary 07th 2011
Introduction
Fatty alcohols are valuable products due to their use as intermediates in manufacturing detergents and in direct applications as pharmaceuticals, cosmetics and textile auxiliaries (Monick, 1979). In particular, unsaturated fatty alcohols such as oleyl alcohol (cis-9-octadecan-1-ol) have advantages regarding saturated ones having the same chain length, such as lower melting point, higher solubility in water and the possibility of introducing functional groups into the C=C double bond (Egan, 1984). Unsaturated fatty alcohols are unique because they can only be obtained from natural raw materials. Industrial production is currently based on high-pressure hydrogenation (260-300°C and 25 -30 MPa) of methyl esters and fatty acids, obtained respectively by transesterification and hydrolysis of oil (Kreutzer, 1984; Voest, 1984). These processes use zinc chromite type catalysts promoting selective hydrogenation of the carbonyl group instead of the C=C double bond (Adkins and Sauer, 1937). The disadvantages of these processes lie in the severe reaction conditions and the leaching of hexavalent chromium known for its high toxicity in natural ecosystems.
At present, strong environmental regulations force the search for new more efficient and less polluting processes. Research during the last 20 years has been focused on Group 8 metal catalysts such as ruthenium with a second metal, such as tin, acting as promoter. These systems have resulted in methyl ester and fatty acid hydrogenation in less drastic conditions (8 MPa and 270°C). Besides, these metals are less toxic than chromium (Narasimhan, 1990; Cheah, 1992; Cheah, 1994; Tahara, 1997, Pouilloux, 1998; Pouilloux, 2001; Echeverri, 2009; Echeverri, 2010; Sanchez, 2011). As reported in these papers, there is an optimum molar ratio between promoter and active metal allowing maximum unsaturated alcohol selectivity.
All prior investigations have been carried out with pure raw materials such as methyl oleate. However, fatty acid methyl ester mixtures derived from oils such as sunflower, palm, palm kernel and coconut are used in the commercial synthesis of unsaturated fatty alcohol. Catalyst reuse is another very important aspect which has not been studied. Colombia is currently the world’s fifth producer of palm oil. A substantial percentage of oil is exported as crude oil and the rest is used in food applications (Jaimes, 2004; Ríos, 2006). Palm oil thus has great potential as feedstock for oleochemical applications having high added value.
This study evaluated the hydrogenation of commercial methyl oleate at 5 MPa and 270°C with monometallic and bimetallic ruthenium heterogeneous catalysts and tin supported by impregnation on g-alumina. The effect of the tin-ruthenium molar ratio on catalyst performance was also analysed. Based on these results, the catalyst showing the highest selectivity was studied in the hydrogenation of methyl esters derived from the olein fraction of palm oil at different times. Conversion and selectivity results for the catalyst are analysed for several types of reuse.
Experimental
Materials
.Methyl oleate (80% w/w), metal precursors (RuCl3.nH2O,SnCl2.2H2O,) and gamma alumina were obtained from Sigma -Aldrich. The palm oil olein fraction was obtained from Extractora Bajirá (Antioquia).
Oil palm fatty acid methyl ester (FAME) synthesis.
The RBD palm oil olein fraction was obtained by transesterification with methanol using 0.7% KOH by weight with respect to oil. The temperature was 65°C, maintained for 2 hours in a reflux system. These conditions had been previously optimized to obtain higher FAME yields. The oil/alcohol molar ratio was 1 to 6. Glycerol was separated from ester phase in a separating funnel. The latter was washed with hot water until neutral pH was obtained. The remaining methanol was removed by rotoevaporation in a water bath at 90°C.
Catalyst preparation.
Monometallic and bimetallic catalysts were synthesized by impregnation. There was 2% ruthenium on the support. Tin/ ruthenium molar ratios were 0.5, 1 and 2. The g-Al2O3 was previously degassed in nitrogen and the metal precursors were dissolved in water. The solid was then impregnated with this solution according to the method described by Narasimhan et al., (Narasimhan, 1990). The solids were dried under reduced pressure. Before use, the catalysts were calcined in air at 450°C and then reduced with hydrogen at 450°C and passivated in air at room temperature. Monometallic ruthenium and tin catalysts have been designated in this work as Sn/Al2O3 and Ru/Al2O3, respectively. Bimetallic catalysts have been designated as Ru-Sn/Al2O3-X, where X was the Sn:Ru molar ratio.
Characterising catalysts
.Surface area, pore size and pore volume were analysed using a Micromeritics Gemini V, using liquid nitrogen as adsorbate. The samples were degassed at 200°C in a nitrogen atmosphere for 1 hour before testing.
Hydrogenation reaction.
This was carried out in a batch reactor (27 mL) equipped with magnetic stirring The reactor was charged with methyl ester and the catalyst and then purged with hydrogen at 2MPa four times to remove air. The temperature was increased to 270°C at constant pressure (2 MPa) and subsequently increased to 5 MPa, keeping it stable during the reaction. Hydrogenation reaction progress was determined by taking samples at fixed times.
Product analysis
.Raw materials were physicochemically characterised including water content, saponification value, iodine value and acid value. Raw material and reaction product compositions were analysed by gas chromatography using a 50 m x 0.25 mm RTX-5 capillary column and a TCD detector. The carrier gas was hydrogen. The raw materials were derivatised to methyl esters before analysis. The analysis was performed by caliation curve. Complementary to the previous analysis, components were identified by gas chromatography with mass detector (quadrupole) using the column described above. Characterisation was also performed by Fourier transform infrared (FTIR) spectrometry with a Shimadzu IR Prestige-21, using KBr cells.
.Catalyst reuse
.The catalysts were recovered after the hydrogenation reaction. The reaction mixture was removed by microfiltration with a 0.45 mm Advantec MFS memane filter. Catalysts were then washed with hexane to remove most of the oil. The solid was dried in an oven at 60°C for one hour. The catalyst was used 4 times.
Results and discussion
1. Raw materials’ properties
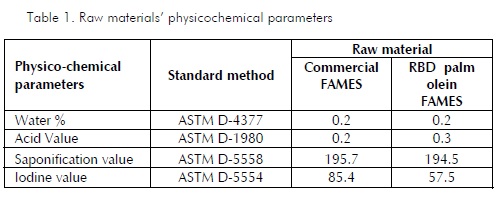
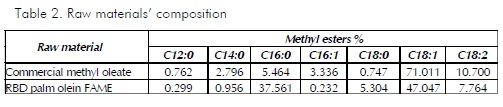
Tables 1 and 2 give the results of analysing the raw materials’ composition and physicochemical parameters. It should be noted that the main difference between the raw material from commercial sources and that derived from palm oil lay in the lowest unsaturated fatty acid content in the latter (oleic and linoleic). Palm oil had a higher percentage of saturated fatty acids (palmitic, stearic), reflected in its lower iodine content.
2. Characterising catalysts
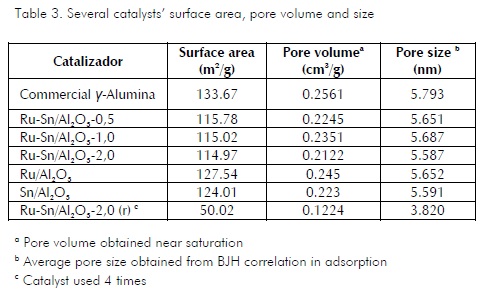
According to solid material classification, microporous ones are defined as those having smaller than 2 nm pore size, mesoporous materials have 2 to 50 nm pore size and macroporous ones have greater than 50 nm pore size. The solid catalysts obtained (Table 3) could be described as mesoporous. Regarding commercial alumina, catalysts impregnated on this support had less surface area and less pore size and pore volume, possibly due to some pores being covered by the metal support. Catalyst reuse (4 times) had a dramatic effect on its structural properties, especially on surface area which showed more than 50% reduction. Such decrease may have been due to agitator grinding effect and reaction product deposition in the pores of the catalyst.
3. Analysing reaction products
Changes occurring in functional groups participating in the reaction were identified according to raw material and hydrogenation product infrared spectra (Figure 1). The groups prone to reacting were the carbonyl group and the C=C double bond whose infrared bands mainly appeared at 1,742 and 1,642 cm-1 respectively. Decreased reaction product peak intensity corresponding to the carbonyl group was observed due to its reduction to alcohol (3,450 cm-1). It should be noted that the band at 1,642 remained, which may have been due to the non-saturation of the unreacted raw material and/or of oleyl alcohol formed.
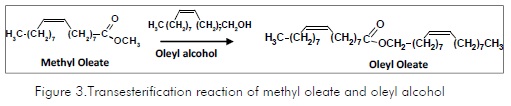
The main reaction products were identified as being oleyl alcohol, stearyl alcohol, palmitoyl alcohol and oleyl oleate (Figure 2). Oleyl alcohol was formed in the selective hydrogenation of methyl oleate in the carbonyl group; stearyl alcohol was formed by the hydrogenation of both the carbonyl group and the nonsaturation of methyl oleate or methyl stearate. In turn, palmitoyl alcohol was formed from methyl palmitate. Oleyl oleate was considered a heavy ester derived from the transesterification of methyl oleate with oleyl alcohol formed in an earlier stage (Figure 3) (Pouilloux, 2001).
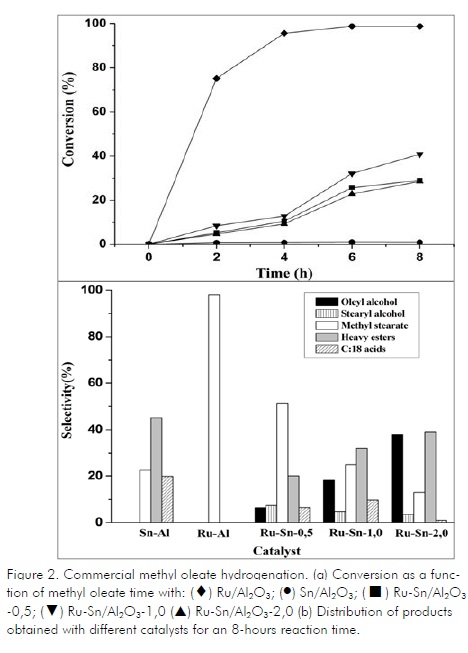
Methyl oleate conversion as a function of time is analysed in Figure 2a. Monometallic ruthenium catalysts were the most active, having almost total methyl ester conversion. Monometallic tin catalyst had the lowest methyl oleate conversion. Adding tin dramatically decreased monometallic ruthenium catalyst activity since, even for the lowest Sn/Ru ratio, conversion dropped to a maximum 20%. Tin can inhibit ruthenium activity when deposited on active sites or promote heavy ester formation blocking hydrogen access (Pouilloux, 2001).
The great effect of tin as a promoter of ruthenium properties was shown regarding the distribution of the products so obtained (Fig. 2b). Thus, monometallic ruthenium was highly active in the hydrogenation reaction but was not selective regarding unsaturated alcohol production and the main reaction was olefinic bond hydrogenation, as stated by other authors (Cheah, 1994). The main reaction product obtained depended on Sn:Ru ratio: methyl stearate (the saturated ester product of methyl oleate or heavy ester olefinic bond hydrogenation) (Figure 3). Increasing the Sn:Ru ratio decreased methyl stearate formation and increased heavy ester and oleyl alcohol formation. This indicated that adding tin had the effect of reducing catalyst selectivity to the olefinic bond and, at the same time, increased interaction with the carbonyl group. However, tin promoted the formation of heavy esters, as reported in other studies with these catalysts (Cheah, 1992; Cheah, 1994; Tahara, 1997).
The Ru-Sn/Al2O3-2,0 catalyst, which presented the highest selectivity in methyl oleate hydrogenation, was evaluated in RBD palm olein FAME hydrogenation for several hours (Figure 4a). Conversions were slightly lower using this substrate compared to commercial methyl oleate. This could have been attributed to catalyst deactivation by impurities in raw materials such as compounds containing chlorine, nitrogen, phosphorus, in addition to free fatty acids. Several studies have shown its negative influence on the performance of catalysts such as zinc chromite and copper (Voest, 1984; Poels, 1999). These impurities are mainly present in coconut oil and palm kernel oil.
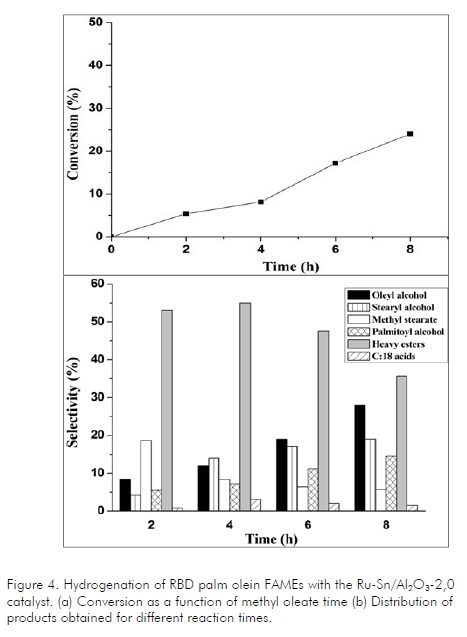
According to the distribution of products obtained from palm oil (Fig. 4b), it could be established that the main products were heavy esters, oleyl alcohol, methyl stearate, 18-carbon fatty acids, stearyl alcohol and palmitoyl alcohol. It is noteworthy that a considerably greater amount of saturated alcohol was obtained compared to commercial methyl oleate. This was due to higher saturated methyl ester content (methyl stearate) which was transformed into the corresponding alcohol. Likewise, the presence of methyl palmitate in the raw material produced palmityl alcohol. It was also noted that heavy esters decreased after 4 hours reaction while fatty alcohol formation increased due to the hydrogenation of the former. The product obtained using palm oil was semisolid at room temperature, unlike this one obtained from commercial methyl oleate which was liquid, though both raw materials are liquid at 25°C. This change could have been due to olefinic bond saturation during hydrogenation.
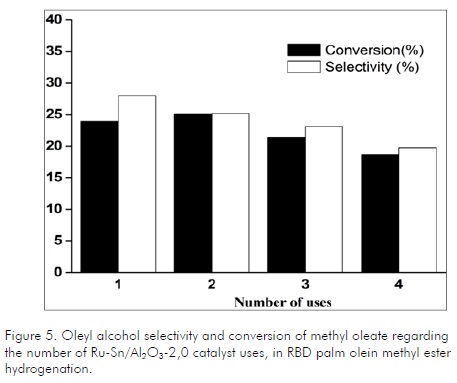
Regarding catalyst stability through several reuses (Fig.5), it was observed that conversion decreased by about 35% for the last reuse compared to the first one which could be considerable taking into account that it was used only 4 times. This loss of activity may have been caused by severe reduction in surface area, as shown by BET analysis. Selectivity to oleyl alcohol was also affected, possibly due to leaching of metals during the reaction mixture shown qualitatively during micro-filtration. It should be noted that the catalyst used was in the form of slurry. Therefore the catalyst was suspended in the liquid, undergoing attrition by mechanical agitation. Another type of reactor design that could improve catalyst stability would be fixed-bed; however, it would be necessary to ensure good mass transfer with the gas phase.
Conclusions
The bimetallic catalyst Ru-Sn/Al2O3-2,0 enabled selective methyl ester carbonyl group hydrogenation instead of that of the olefinic bond at moderate pressure. However, the main competitive reaction was heavy ester formation. Therefore, reaction time should be increased by more than 8 hours to hydrogenate these heavy esters and produce the respective fatty alcohols. The hydrogenation of palm oil methyl esters to respective alcohols was feasible with the catalyst mentioned, but saturated alcohols were also formed. Besides, catalyst activity became slightly decreased compared to that obtained using commercial methyl oleate as substrate. The catalyst can be reused up to 4 times; however, the most appropriate type of reactor must be evaluated to avoid loss of catalyst surface area and structural damage. Future studies will involve using different reactor design (for example fixed bed reactor) and assessing the effect of such configuration on catalyst selectivity and conversion.
Acknowledgements
The authors would like to thank the Universidad de Antioquia for providing financial support through its Group Sustainability Strategy.
References
Adkins, H., Sauer, J., The selective hydrogenation of unsaturated esters to unsaturated alcohols., Journal of the American Chemical Society, Vol. 59, No. 1, Ene.,1937, pp.1-3. [ Links ]
Cheah, K.Y., Tang, T.S., Mizukami, F., Niwa, S., Toba, M., Choo, Y.M., Selective hydrogenation of oleic acid to 9-octadecen-1-ol: catalysts preparation and optimum reaction conditions., Journal of the American Oil Chemists´ Society, Vol. 69, No. 5, May.,1992, pp.410-416. [ Links ]
Cheah, K.Y., Tang, T.S., Mizukami, F, Niwa, S, Toba, M., Hydrogenation of 9-octadecenoic acid by Ru-Sn-Al2O3 catalysts: effects of catalyst preparation method., Journal of the American Oil Chemists´ Society, Vol. 71 No. 5, May.,1994, pp. 501-506. [ Links ]
Echeverri, D.A., Marín, J. M., Restrepo, G. M., Rios, L.A., Characterization and carbonylic hydrogenation of methyl oleate over Ru-Sn/Al2O3: Effects of metal precursor and chlorine removal., Applied Catalysis A: General, Vol 366, No 2, Sept., 2009, pp. 342-347. [ Links ]
Echeverri, D.A., Marín, J. M., Rios, L.A., Hidrogenación de Metil Oleato con Catalizadores de Ru-Sn/Al2O3: Método Sol-gel vs. Impregnación., Información Tecnológica, Vol 21, No 3, May., 2010, pp. 77-86. [ Links ]
Egan, R., Earl, G.W., Ackerman, J., Properties and uses of some unsaturated fatty alcohols and their derivatives., Journal of the American Oil Chemists´ Society, Vol. 61, No.2, Feb., 1984; pp. 324-329. [ Links ]
Jaimes, D., Romero, C.A., Narváez, P.C., Principales tecnologías para la elaboración de oleoquímicos a partir de los aceites de palma y palmiste., Palmas, Vol. 25, No.1, Ene., 2004; pp. 47-64. [ Links ]
Kreutzer, U., Manufacture of Fatty Alcohols Based on Natural Fats and Oils., Journal of the American Oil Chemists´ Society, Vol. 61, No.2, Feb., 1984, pp.343-348. [ Links ]
Monick, J.A., Fatty alcohols, Journal of the American Oil Chemists´ Society., Vol. 56, No.2, Feb., 1979, pp. 853-860 A. [ Links ]
Narasimhan, C.S., Deshpande, V.M., Ramnarayan, K., Studies on ruthenium-tin boride catalysts I. Characterizaction., Journal of Catalysis, Vol.121 No.1, Ene., 1990, pp.165-173. [ Links ]
Narasimhan, C.S., Deshpande, V.M., Ramnarayan, K., Studies on ruthenium-tin boride catalysts II. Hydrogenation of fatty acid esters to fatty alcohols., Journal of Catalysis, Vol. 121, No.1, Ene., 1990, pp.174-182. [ Links ]
Poels, E.K., Sulfur deactivation of fatty esters hydrogenolysis catalysts., Journal of Catalysis, Vol. 186, No.1, Ago., 1999, pp.169-180. [ Links ]
Pouilloux, Y., Autin, F., Guimon, C., Barrault, J., Hydrogenation of fatty acids over ruthenium-tin catalysts; characterization and identification of active centers., Journal of Catalysis, Vol. 176, No.1, May.,1998, pp. 215-224. [ Links ]
Pouilloux, Y., Barrault, J., De Oliveira, K., Selective hydrogenation of methyl oleate into unsaturated alcohols in the presence of cobalt-tin supported over zinc oxide catalysts., Journal of Catalysis, Vol. 204, No.1, Dic., 2001, pp. 230-237. [ Links ]
Rios, L.A, Franco, A, Echeverri, D.A., Producción de alcoholes grasos a partir de aceites de palma y de palmiste utilizando procesos de hidrogenación catalítica. Una revisión Bibliográfica., Palmas, Vol. 27, No.1, Ene., 2006, pp. 65-76. [ Links ]
Sánchez, M.A., Mazzieri, V.A., Sad, M.R., Grau, R., Pieck, C. L., Influence of preparation method and boron addition on the metal function properties of RuSn catalysts for selective carbonyl hydrogenation., Journal of Chemical Technology & Biotechnology, Vol. 86, No 3, Oct., 2011, pp 447-453. [ Links ]
Tahara, K., Nagahara, E., Itoi, Y., Nishiyama, S., Tsuruya, S., Masai, M., Liquid-phase hydrogenation of carboxylic acid on supported Ru-Sn-alumina catalysts., Applied Catalysis A: General, Vol. 154, No.1, Jun., 1997, pp. 75-86. [ Links ]
Voeste, T., Buchold, H., Production of fatty alcohols from fatty acids., Journal of the American Oil Chemists´ Society, Vol. 61, No.2, Feb.,1984, pp. 350-352. [ Links ]











 text in
text in 


