Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Ingeniería e Investigación
Print version ISSN 0120-5609
Ing. Investig. vol.31 no.1 Bogotá Jan./Apr. 2011
Analysing a method for small and medium sized companies to rate oil quality during immersion frying
Efraín Hisnardo Rojas Uribe1 , Paulo César Narváez Rincón2
1 Chemical Engineering and Food Science and Technology Specialist, Univer-sidad Nacional de Colombia, Bogotá. ehrojasu@unal.edu.co
2M.Sc. Engineering. Ph.D. Engineering. Professor, Chemical and Environmen-tal Engineering Department, Chemical and Biochemical Processes Group, Universidad Nacional de Colombia, Bogotá. pcnarvaezr@unal.edu.co
ABSTRACT
This work studied the deterioration of commercial Frytol oil in controlled frying conditions using the anisidine index (AI) as standard evaluation method. The effect of tempera-ture and time was studied at 170°C for up to 10 hours and at 200°C for up to 6 hours in the presence of factors which speeded up oil deterioration: 1% and 8% water based on the mass of oil and non-lipid material, 1% and 6% based on the mass of oil. Air flow rate was kept con-stant at 25 L h-1. The relationship between AI, colour and viscosity was studied to evaluate the technical viability of measuring these variables as a function of time to be used as a method for rating oil deterioration by small and me-dium sized enterprises (SME). Oil deterioration was higher and faster at maximum study interval values for each ex-perimental factor, although only the effect of temperature was significant (p<0.05). Red and yellow on the Lovibond scale were weakly related (AI R2=0.558 and R2=0.526, respectively), colour depending on frying temperature. Viscosity measured at 30°C had the highest coefficient of determination (R2=0.809) and was independent of frying temperature. Viscosity is thus a variable which can be used for determining frying oil deterioration in SME as a conse-quence of a significant change from 10 cP to 45 cP which is closer to frying oil deterioration limit (AI 156). It can facilitate determination and lower associated costs, there-by improving product quality and customer confidence in them.
Keywords: immersed frying, viscosity, Lovibond colour, anisidine value, oil deterioration.
Received: October 05th 2009. Accepted: December 31th 2010
Introduction
Frying refers to cooking food by immersing it in hot oil or fat for a specified period, usually in the presence of air, where the oil acts as a medium for heat and mass transfer thereby producing fast and homogeneous heating of a product (Navas, 2005; Yagüe, 2003) and giving unique crispness, flavour and colour which is highly desired by consumers (Gupta, 2004; Choe, 2007). These characteristics are mainly due to different reactions occurring in the frying oil and the food itself which are produced by lipid oxidation compounds and Maillard reaction products (Navas, 2005).
Frying food is cooked during immersion by direct heat transfer from the hot oil to the cold food; oil temperature decreases when food is added to hot oil and moisture on food surface quickly changes from liquid phase to vapour phase, while water inside food diffuses to the surface, to finally reach vapour phase and travel through the frying oil to the air as shown by the presence of bubbles within the oil. Food starts to obtain its characteristic colour as frying advances a and oil is absorbed into the food, conferring a crunchy texture and its characteristic flavour (Gupta, 2004).
Factors such as high temperature and the presence of oxygen and water in the oil produce irreversible physical changes such as increased viscosity, colour and foaming, decreased smoke point and chemical reactions, including oxidation, hydrolysis and polymerisation, these being factors which promote oil deterioration (O'Brien, 2004; Saguy and Dana, 2003; Dobarganes, 2002, Sontag 1982). A high degree of oil deterioration can have negative effects on human health because some products having undesirable chemical reactions can act as enzyme inhibitors, vitamin destroyers, gastrointestinal irritants and/or mutagenic agents (Clark and Serbia, 1991, Zakrzewski, 1991; Keuneke, 1999).
Some studies have measured peroxide value, acid value, nonpolar material content, AI, polymer content, iodine value, thiobarbituric acid value or conjugated diene to determine the degree of oil deterioration (Pokorny et al., 2001; Navas, 2005, Gupta, 2004; Rosell, 2001; Shahidi, 2005); nonpolar material and AI are most commonly used for reliability and relationship to deterioration but they are difficult to implement in SME because of the highly specialised equipment needed, the technical background for personnel required for such analysis and higher associated costs (Benedito et al., 2007). AI measures the amount of α and β;unsaturated aldehydes present in oil; it measures secondary oxidation or oil’s history (O’Brien, 2004).
Some countries have established regulations to protect consumers from the risk of consuming food fried in deteriorated oils where the limit determining whether an oil cannot be used in food production corresponds to 25% polar material content (Firestone, 2004; Shahidi, 2005; Gertz, 2000, Sahin et al., 2008), i.e. equivalent to 156 AI (Boatella et al., 2000).
Currently there are no practical and objective systems for evaluating the deterioration of frying oil in SME because standardised methods for analysing frying oils use equipment and procedures involving cost, time and technologies beyond the reach of such companies (Navas, 2005; Benedito, 2007). The rapid test systems which are used in Europe and the USA have not become widespread in LatinAmerica because the necessary equipment is very expensive regarding SME budgets.
Accordingly, this research has studied a practical method for SMEs determining frying oil deterioration limit to facilitate monitoring frying oil quality in such companies which will help standardise processes and improve food's organoleptic characteristics, thereby promoting quality and consumer confidence in their products. Sociedad Industrial de Grasas Vegetales (C.I. SIGRA SA), which refines and produces cooking and frying oil, aims to increase the added value of their product Frytollíquido by providing technical support for its customers by developing this work.
The effect of temperature, time, initial moisture content and nonlipid material on Frytolliquid oil deterioration was studied during controlled frying at constant air flow rate and using AI for measuring deterioration. The relationship of AI with colour and viscosity was then established to determine its feasibility as a method for SME to rate frying oil deterioration. Selecting these two variables was based on the relationship between them and deterioration (Gupta, 2005), and the possibility of implementing easily applied procedures in SME where measurement would consist of comparison with predefined standards (i.e. Gardner colour and viscosity scales) used in industries such as resins, paints and coatings. This type of characterisation reduces the cost of equipment and analysis and the technical background required by the personnel in charge is low.
Materials and Methods
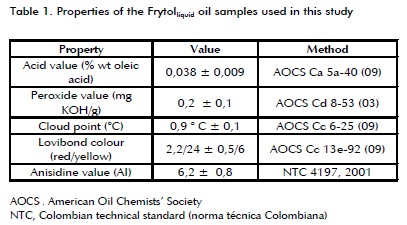
Materials. A sample of fresh Frytollquid oil (a mixture of soybean oil and palm olein) produced by C.I. SIGRA S.A. (Bogotá, Colombia) was used. The sample was a mixture of eleven random samples. The oil sample was characterised by acid value (AOCS Ca5a40 (09)), peroxide value (AOCS Cd853 (03)), cloud point (AOCS Cc625 (09)), red and yellow Lovibond colour with 5 "¼ cell (AOCS Cc13e92 (09)) and AI (NTC 4197, 2001). Table 1 shows average characterisation results where the value of each sample's properties was measured in triplicate (results in Table 1 are thus the average of 33 data items).
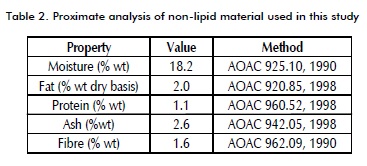
Nonlipid material was prepared from thirteen samples randomly taken from raw pie crust, obtained from Empanadas de la Cima Ltda (Bogotá, Colombia), homogenised by maceration and characterised by proximate analysis, as shown in Table 2.
4methoxyaniline (pAnisidine) and sodium sulphate anhydrous from Merck (Darmstadt, Germany) and 2,2,4trimethyl pentane from Mallinckrodt Baker Inc. (Phillipsburg, USA), were used for determining the AI. pAnisidine was purified following the procedure described in NTC 4197.
Methods. AI was measured according to the procedure described in NTC 4197, using a Perkin Elmer Model Lambda 3B visible light pectrophotometer (Perkin Elmer Inc., Massachusetts, USA) with 10 mm cell. The procedure established in AOCS Cc 13e92 was followed for measuring Lovibond colour using a Lovibond Colourimeter PFX 880 (The Tintometer Ltd, Wiltshire, UK) with 10 mm cell at 50°C.
Viscosity was measured according to the procedure described in ASTM D2196 in a Brookfield VISCO 20 concentric cylinder rotational viscometer (Malvern Instruments, Worcestershire, UK) using a RV / HA # 3 302S/ S33 needle at 30°C.
Experimental design. The effect of temperature, the presence of nonlipid material, time and humidity on Frytol lquid frying oil deterioration was studied by designing a multifactorial experiment for three variables at two levels and for one variable at three levels, as follows: 1% and 6% wt nonlipid material, 1% and 8% wt moisture, both based on the mass of the oil, 170°C and 200°C frying temperature, and time by sampling at 6, 8 and 10 hours for the 170°C test and at 4, 5 and 6 hours for the 200° C test. The higher the frying temperature, the higher the rate of reactions associated with deterioration; such pattern explained why test time was shorter at 200°C than 170°C. 24 tests were performed for studying these variables' effect (each conducted twice). Frying was controlled using RANCIMAT 679 (Metrohm AG, Switzerland) equipment, keeping system air flow rate constant at 25 L h1.
Tests were performed at 170°C for 9 hours and 200°C for 6 hours in controlled frying conditions in a RANCIMAT 679 (Metrohm AG, Switzerland) to determine the relationship between AI, viscosity and colour deterioration using 6% wt nonlipid material, 8% wt moisture and 25 L h1 air flow rate. The volume of oil used in each test was 70 ml. Samples were taken every hour and tests were conducted twice. Each sample was analysed for AI, dynamic viscosity and Lovibond colour.
The results were statistically analysed with STATGRAPHICS Centurion XV version 15.2.06 (StatPoint Inc. Virginia, USA).
Results and analysis
Evaluating frying oil deterioration by AI. Statistical analysis of the results from the experiment which evaluated the effect of time, temperature, moisture and nonlipid material on oil deterioration (ANOVA) showed that only the effects of time and temperature on AI were significantly different from zero at 95% confidence level (p<0.05) and an increase in these variables' value increased frying oil deterioration.
Figures 1 shows the effect of time and temperature on AI, while Figure 2 shows the effect of initial moisture and nonlipid material on AI. Two of the variables being studied were parameterised regarding the average value of the range studied in each Figure, even though the pattern was qualitatively similar throughout the range.
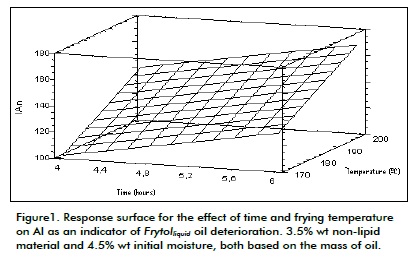
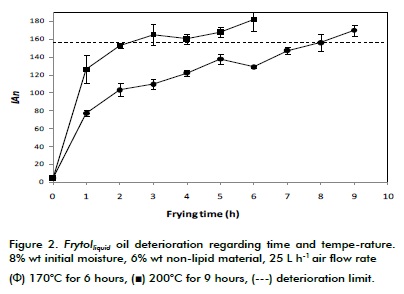
Figure 1 shows that increasing frying temperature led to increased deterioration of frying oil, confirming Gupta (2004) and Saguy's (2003) research in which they reported that if this variable were controlled then oil life would be extended. Deterioration increased with a rise in temperature because there was enough energy to eak the CC and CH covalent bonds in triglyceride, producing a wide variety of alkyl radicals acting as initiators for the chain reactions responsible for the deterioration of oil occurring through a free radicals mechanism (Schaich, 2005). This has an effect on reaction rate and this is why the oil had the same deterioration level in less time for tests at 200°C than 170°C, for the same initial moisture and nonlipid material conditions. For example, AI after 10 hours at 170°C was the same as less than 6 hours at 200°C in experiments with 1% wt initial moisture and 6% wt non lipid material.
Evaluating the relationship between AI, viscosity and colour. Figure 2 shows Frytollíquido oil deterioration regarding time and temperature. It confirms that AI increased by increasing time and temperature and that if, as has been reported by Boatella et al., based on a correlation between AI and nonpolar material content (Boatella et al., 2000), then the limiting value for establishing when an oil should be replaced would be 156 AI; a 30°C increase reduced the oil's lifetime by 6 hours in experimental design conditions.
Figure 3 shows the relationship between Lovibond red and AI during deterioration tests at 170°C and 200°C. Although there was a radical change in Lovibond red at 200°C from about 0.5 to 2.0 when AI exceeded deterioration limit value, such change was not observed at 170°C.
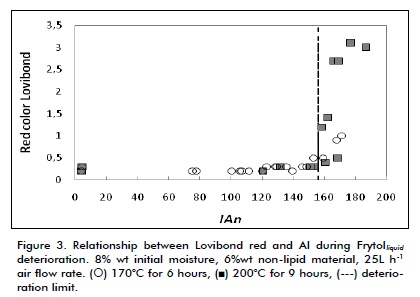
A similar pattern can be observed in Figure 4, but this time for Lovibond yellow, indicating that Lovibond colour was related to temperature. The coefficient of determination (R22) was calculated for Lovibond red and yellow, given the wide frying temperature range and the little control which SME can exert over this variable in quantifying the relationship between colour and oil deterioration. This included data regarding both temperatures tested (0.558 and 0.526, respectively) meaning that the relationship between colour and oil deterioration was low. However, statistical analysis showed that effect of temperature on colour was significantly different from zero at 95% confidence level (p<0.05), thereby confirming qualitative analysis.
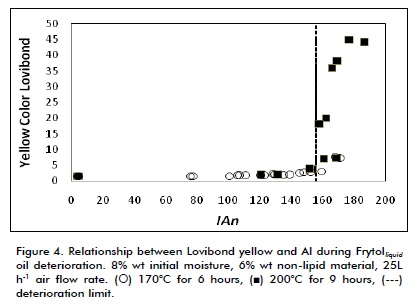
This study’s results were different to those obtained by Paul et al., (1996) who analysed the feasibility of determining oil deterioration through colour; they obtained acceptable results, although their research did not study the effect of frying temperature.
According to the results, SME following frying oil deterioration by using colour is not an appropriate technique.
Figure 5 shows viscosity regarding AI. It can be noticed in this Figure that there was a significant change in viscosity when deterioration limit value was reached (156 AI), despite temperature, which was reflected in a sigmoidal curve at 170°C and 200°C, inflection point being closer to deterioration limit. Frytolliquid oil viscosity at 30°C changed from less than 10 cP to around 45 cP and determination coefficient (R2) between viscosity and AI was 0.809. This coefficient's value was considered high enough to state that there was a statistical relationship between AI and viscosity. Statistical analysis showed that temperature had no significant effect on viscosity in the range being studied (p>0.05). Such pattern led to concluding that viscosity is an appropriate variable for SME to monitor the deterioration of oil.
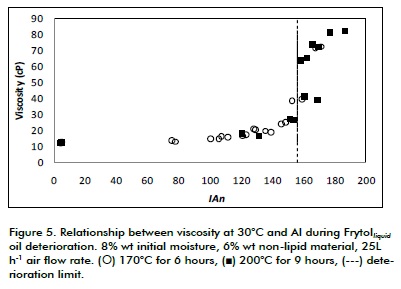
Behavior of viscosity as a function of frying temperature has been reported for different oils, as rapeseed oil, corn oil, sunflower oil and their mixtures, showing its feasibility as indicator variable of deterioration of oil (Santos, 2005).
Figure 6 shows FRYTOLliquid oil deterioration related to viscosity at 30ºC regarding time and temperature. Deterioration limit corresponded to 45 cP viscosity. Figure 6 confirms that when frying time was increased there was a clear change in viscosity which was more noticeable at 170°C than at 200°C and that deterioration limit was reached first at the higher temperature (about 3 hours at 200°C and 8 hours at 170°C).
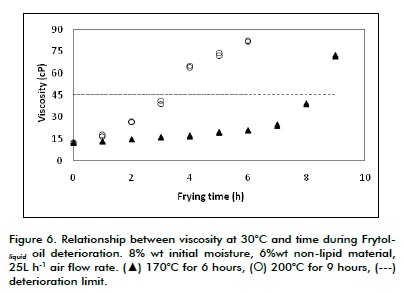
The change in viscosity was enough to establish the deterioration of oil through viscosity if a comparisonbased technique having a set of standards were to be implemented, similar to the viscosity measurement method used in the Gardner bubble viscometer range (ASTM D154507). If the Gardner Holdt scale were to be used, then FRYTOLliquid oil viscosity (closer to oil's deterioration limit value) would change from A3 to A or B.
Conclusions
Temperature and time had an effect on frying oil deterioration significantly different from zero (95% confidence level) in the range studied here. A 30°C increase in frying temperature reduced oil life by up to 6 hours in the conditions used in this research. There was a correlation between the deterioration of oil, measured by AI, and viscosity so that SME could use this physical property to control oil deterioration. Based on thes results, it is proposed that SME should adopt a comparative technique (similar to that described in ASTM D 154507) for ascertaining viscosity using a bubble viscometer whose cost and ease of use would be suitable for SME. Standardising the method and its economic assessment form the second stage for this research.
Referencias
AOAC 925.10, 1990., Solids/Total Solids, Moisture, Official Methods of Analysis of the Association of Official Agricultural Chemists (AOAC), 17th Edition, USA, 2000. [ Links ]
AOAC 920.85, 1998., Fat/Crude Fat, Fat/Ether Extract, Official Methods of Analysis of the Association of Official Agricultural Chemists (AOAC), 17th Edition, USA, 2000. [ Links ]
AOAC 960.52, 1998., Elemental Analysis / Nitrogen, Official Methods of Analysis of the Association of Official Agricultural Chemists (AOAC), 17th Edition, USA, 2000. [ Links ]
AOAC 942.05, 1998., Ash, Official Methods of Analysis of AOAC International, Official Methods of Analysis of the Association of Official Agricultural Chemists (AOAC), 17th Edition, USA, 2000. [ Links ]
AOAC 962.09, 1990., Fiber / Crude Fiber, Official Methods of Analysis of the Association of Official Agricultural Chemists (AOAC) [ Links ]
AOCS Ca 5a40 (09)., Free Fatty Acids, Official Methods and Recommended Practices of the American Oil Chemists´ Society, 6th Edition, USA, 2009. [ Links ]
AOCS Cd 853 (03)., Peroxide Value, Acetic AcidChloroform Method, Official Methods and Recommended Practices of the American Oil Chemists´ Society, 6th Edition, USA, 2009. [ Links ]
AOCS Cc 625 (09)., Cloud Point Test, Official Methods and Recommended Practices of the American Oil Chemists´ Society, 6th Edition, USA, 2009. [ Links ]
AOCS Cc 13e92 (09)., Lovibond (per ISO Standard), Official Methods and Recommended Practices of the American Oil Chemists´ Society, 6th Edition, USA, 2009. [ Links ]
ASTM D154507., Standard Test Method for Viscosity of Transparent Liquids by Bubble Time Method., American Standards for Testing Materials, USA, 2007. [ Links ]
Benedito, J., García, J. V., Dobarganes, M., Mulet, A., Rapid Evaluation of Frying Oil Degradation Using Ultrasonic Technology., Food Research International, Vol. 40, 2007, pp. 406-414. [ Links ]
Boatella, J., Codony, R., Rafecas, M., Guardiola, F., Recycled cooking oils: Assessment of risks for public health en European Parliament STOA Programme., Chambers, G., (ed.), European Parlament, Directorate General for Research. Luxembourgg, Septiembre, 2000., pp. 64. [ Links ]
Choe, E., Min, D. B., Chemistry of DeepFat Frying Oils., Journal of food science., Vol. 72, No. 5, 2007, pp. R77-R86. [ Links ]
Clark, W. L., Serbia, G. W., Safety Aspects of Frying Fats and Oils., Institute of Food Technologists., Vol. 45, No. 2, 1991, pp. 84-89. [ Links ]
Dobarganes, M. C., Velasco, J., Márquez, R. G., La calidad de los Aceites y Grasas de Fritura., Alimentación, Nutrición y Salud., Vol. 9, No. 4, 2002, pp. 109-118. [ Links ]
Firestone, D., Regulatory Requirements for the Frying Industry., En: Gupta, M. K., Warner, White P. J., (ed.), Frying technology and practices., AOCS Press., Champaign., 2004, pp. 200-216. [ Links ]
Gertz, C., Chemical and Physical Parameters as a Quality Indicator of Used Frying Fats., The European Journal Lipid Science Technology., Vol. 102, No. 8, 2000, pp. 566-572. [ Links ]
Gupta, M. K., The Effect of Oil Processing on Frying Oil Stability., En: Gupta, M. K., Warner, White P. J., (ed.), Frying technology and practices., AOCS Press., Champaign., 2004. [ Links ]
Gupta, M. K., Frying Oils, in Bailey´s Industrial Oil and Fats Products, Edible Oil and Fat Products: Products and Applications, Fereidoon Shahidi (ed.), 6th Ed., John Wiley and Sons., New York., USA., Vol. 4, 2005. [ Links ]
Keuneke, R., Health Destructive Effects of Frying., Total Health, Vol. 21, No.3, 1999, pp. 26-28. [ Links ]
Navas, J.A., Optimización y Control de la Calidad y Estabilidad de Aceites y Productos de Fritura., Tesis presentada a la Universidad de Barcelona España, Facultad de Farmacia, para optar al grado de Doctor en Medicamentos, Alimentación y Salud, 2005. [ Links ]
ICONTEC, NTC 4197, Grasas y Aceites Animales y Vegetales., Determinación del Índice de Anisidina., Equivalente a la ISO 6885:1998., Instituto Colombiano de Normas Técnicas y Certificación ICONTEC. I.C.S.: 67.200.20., Bogotá, Colombia, 2001. [ Links ]
O´Brien, R.D., Fats and Oils: Formulating and Processing for Applications., CRC Press., 2nd ed., Boca Ratón., USA., 2004. [ Links ]
Pokorny, J., (ed.), Yanishlieva, N., Gordon, M., Antioxidants in Food: Practical Applications., CRC Press., Woodhead Publishing Limited., Cambridge., 2001. [ Links ]
Rosell, J. B., Frying. Improving Quality., J. B. Rosell (ed.), Cambridge, England., Woodhead Publishing Limited., 2001. [ Links ]
Saguy, I.S., Dana, D., Integrated Approach to Deep Fat Frying: Engineering, Nutrition, Health and Consumer Aspects., Journal of Food Engineering., Vol. 56, 2003, pp. 143-152. [ Links ]
Sahin, S., Gülum, S. S., Advances in Deep Fat Frying., Contemporary Food Engineering Series., Sun, D., (ed.), CRC press, 2008. [ Links ]
Schaich, K., Lipid Oxidation: Theoretical Aspects, in Bailey´s Industrial Oil and Fats Products, Edible Oil and Fat Products: Chemistry, Properties and Health Effects, Fereidoon Shahidi (ed.), 6th ed., John Wiley and Sons., New York., USA., Vol. 1, 2005. [ Links ]
Shahidi, F., (ed.), Edible oil and Fat Products: Chemistry, Properties and Health Effects., En: Bailey´s Industrial Oil and Fat Products., Vol. 1, 6th ed., Wiley Interscience, New Jersey., 2005. [ Links ]
Santos, J., Santos, I., Souza, A., Effect of Heating and Cooling on Rheological Parameters of Edible Vegetable Oils., Journal of Food Engineering., Vol. 67, 2005, pp. 401-405. [ Links ]
Warner, K., Chemical and Physical Reactions in Oil During Frying. En: Gupta, M. K., Warner, White P. J., (ed.), Frying technology and practices., AOCS Press., Champaign., 2004. [ Links ]
Yagüe, M. A., Estudio de la Utilización de Aceites para Fritura en Establecimientos Alimentarios de Comidas Preparadas., Observatorio de la Seguridad Alimentaria., Septiembre, 2003., En internet: http://magno.uab.es/epsi/alimentaria/mangelesaylon.pdf. Visitada el 26 Agosto de 2008. [ Links ]
Zakrzewski, S., Principles of Envirommental Toxicology., American Chemical Society Professional Reference Book., Washington D.C., United States of America, 1991, pp 4455. [ Links ]











 text in
text in 


