Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Ingeniería e Investigación
versão impressa ISSN 0120-5609
Ing. Investig. v.31 n.1 Bogotá jan./abr. 2011
A study of low-cost adsorbent materials for removing Cr(VI) from aqueous waste effluent
C. Vargas-Nieto1, J. G. Carriazo2, E. Castillo3
1 Chemist. M.Sc. Candidate in Science - Chemistry, Universidad Nacional de Colombia. claudiavargasn@gmail.com
2 Ph.D. in Chemistry. Professor. Department of Chemitry. Universidad Nacional de Colombia. jcarriazog@unal.edu.co
3 Ph.D. in Chemistry, Universidad de Barcelona. Professor, Deparment of Chemistry, Universidad Nacional de Colombia. ecastillo@unal.edu.co
ABSTRACT
The present paper shows very high potential for two types of solid (a commercial alumina and material obtained by composting, i.e. matured compost) on Cr(VI) adsorption/ elimination in aqueous solution using a concentration range close to those previously detected in waste-water from Colombian industries. Both had important properties for eliminating Cr(VI), the compost being more important as it represents low-cost material. Optimal conditions for chromium adsorption on alumina and compost were established. Initial Cr(VI) alumina concentration was 10 mgL-1, with 100 mL/g volume of solution per adsorbent mass, pH=2.0, 1 hour equiliium time and 150 rpm stirring. For compost, initial Cr(VI) concentration was = 3 mg L-1, 50 mL/g volume of solution per adsorbent mass, pH=2.5, 3 hour equiliium time and 150 rpm stirring. The experiments showed that compost adsorption properties could be enhanced by adding small quantities of alumina. Compost could thus be chosen as a promising material for use in bioremediation chromium-containing waste water in a management programme for using solid waste in for minimising environmental impact.
Keywords: Chromium removal, chromium adsorption, waste water treatment, adsorption on compost, adsorption on alumina.
Received: September 7th 2009. Accepted: January 30th 2011
Introduction
The presence of heavy metals in aqueous media is a dangerous situation for human health, since many of them are toxic ions. The most important problem regarding these metals is the impossibility of biodegrading them; however, several methodologies are available for eliminating them or reducing their content in solution and some of these methods seek to convert heavy metal ions to less toxic species or immobilise these ions to avoid their biological and ecological diffusion. The main transformations are due to changes in oxidation which have a strong effect on metal ion mobility, in some cases showing higher metal solubility thereby facilitating their persistence in aqueous media; however, in other cases solubility can be decreased, yielding metal immobilisation. The usual methods for removing heavy metals from industrial effluent include precipitation, reductionoxidation and ionic exchange. However, such processes have drawbacks such as high cost, incomplete metal ion elimination or the production of residues requiring additional treatment. Adsorption has become an alternative method during the last decades for removing metals from waste water using a variety of solid materials, such as clays (Fritzen et al., 2006; Zhu et al., 2008), alumina, activated carbon (Mohana and Pittman, 2006; Mor et al., 2007; Park et al., 2006) and different types of domestic waste (Gibert et al., 2005; Wei, Y et al., 2005).
Applying strategies developed through new technologies based on the use of low-cost adsorbents have been widely studied regarding a policy of handling and using solid waste to increase their reuse and minimise their environmental impact. That has been confirmed by an increasing number of studies on metal removal using available waste material from different sources, such as vegetable oil extraction residues (Blázquez et al., 2009, Malcok and Dundar., 2006), clarified sludge, rice husk ash, fly ash and saw dust (Bhattacharya et al., 2008), rice processing byproducts (Tarley and Arruda., 2004), beer yeast waste (Han et al., 2006) and some seeds (Fiol et al., 2006). The literature has shown compost to be an appropriate material for adsorbing metal ions (Boni and Sbaffoni., 2009, Wei et al., 2005) due to this material´s characteristics such as important lignocellulose, hemicellulose and lignin content. These resources are readily and widely available in Colombia and compost can thus be prepared in large quantities at the site where treatment is required. Such metal removal may be significantly enhanced by the chemical stabilisation of metal species by inducing additives to the compost, such as organic ligands (Kumpiene et al., 2008). It can thus be inferred that composting can contribute towards reducing the availability of metals compared to other biostabilisation techniques because abundant scientific evidence proves so (Smith, 2008).
The objective of this work was to evaluate the potential of compost to adsorb chromium(VI) species in aqueous solutions. This metal commonly yields several chemical species having oxidation states (III) and (VI) in aqueous solution, the last state, Cr(VI), being the most toxic for human beings because of its high mobility in CrO42- and HCrO4- ion form due to carcinogenic and mutagenic effects, besides inducing dermatitis and lung disease (Cieslak-Golonka, M, 1995; Gómez and Callao, 2006; Levina and Lay, 2005). A widely-recognised material in some adsorption process (alumina) was also evaluated for eliminating Cr(VI)ions. Optimal conditions for obtaining the highest Cr(VI) adsorption levels in a batch reactor were established for both materials to estimate the viability of using compost combined with materials enhancing its removal ability for eliminating chromium from aqueous effluent.
Materials and methods
Adsorbent materials
Three adsorbents were used: commercial alumina (supplied by Carlo Erba) characterised by X-ray diffraction and two composts. The first compost (compost 1) was prepared on a farm located near Tenjo (Cundinamarca, Colombia) which had initially been destined for an organic vegetable crop. This compost was obtatained by mixing several waste materials (crop, pasture, cattle manure and soil residue) in a compost pile. The second compost (compost 2) was supplied by the Marengo Agricultural Centre (National University of Colombia) in Tibaitatá (Cundinamarca, Colombia). The compost piles were matured for 18 to 24 months in both cases. These composts´ physical features were being black, having a soil smell, dried soft texture and lacking non-decomposed particles.
Characterisation techniques
Philips PW 1820 equipment was used for X-ray diffraction (XRD) analysis with copper anticathode (Cu-Ka radiation: l=1.54056Å) and Bragg-Brentano configuration, using the forced powder technique, 0.05 step size and 2 second step time. An FEI QUANTA 200 microscope was used for scanning electron microscopy (SEM) analysis, taking three micrographs at different points for each sample. Samples were metallised by gold sputtering for all observations.
Adsorption study and chromium analysis
A 500 mg L-1 solution of Cr(VI) was prepared from K2Cr2O7 (Merck, 99%) in distilled/deionised water. The experiments were carried out in 125 mL conical flasks, with continuous stirring (150 rpm in shaker type stirrer) at room temperature (20°C), using a 25 mL solution volume and 5 to 50 mg L-1Cr(VI) concentrations. Various adsorbent quantities were used, and solution pH was adjusted with NaOH and HNO3 diluted solutions. Aqueous samples were centrifuged for 5 minutes before Cr(VI) determination.
Residual Cr(VI) concentration was determined by colorimetric method with 1,5 diphenylcarbazide (APHA, 1995), prepared in 5% v/v acetone for each analysis. A Perkin Elmer Lamda 2S spectrophotometer was used for taking absorbance measurements.
Results and discussion
Characterising adsorbents
Figure 1 shows the X-ray diffraction profile for the alumina used as adsorbent. The presence of several crystalline phases of alumina could be clearly observed, identified by comparing reflexion position and interplanar spacing (determined by Bragg equation (nλ = 2dsenθ ), with those values published in the pertinent literature. Major phases were -alumina (y- AL2O3) (2θ: 37.3°, 39.3°, 45.8°, 67.53°) and boehmite (-AlOOH) (2θ:: 28.15°, 38.25°, 49.17°, 55.24°). Other phases were also detected: bayerite (Al(OH)3) (2θ: 18.28°, 20.20°) and nordstrandite (2θ=14.43°).
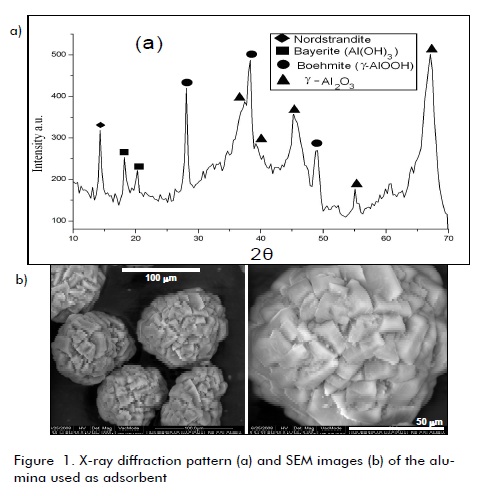
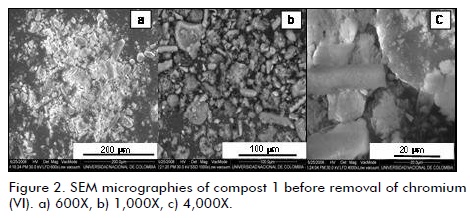
SEM images (Figure 1b) indicated that the alumina was constituted by semi-spherical particles formed by the agglomeration of small, rectangular crystals whereas Figure 2 shows SEM images for compost 1, revealing an irregular, varied morphology in all cases, confirming the compost´s high morphological heterogeneity (the same morphology was observed for compost 2). The compost samples were not characterised by XRD due to their amorphous features.
Studying Cr(VI) adsorption on alumina
Stirring suspension is a significant parameter for solute adsorption (in liquid medium) on a solid. This parameter was evaluated in all the experiments for optimising adsorption and the effect of stirring speed on the range being studied (50 to 200 rpm) was observed for Cr(VI) adsorption on both alumina and compost, indicating that maximum Cr(VI) adsorption level on solids was obtained with higher than 100 rpm speed. A 150 rpm speed was chosen for all tests.
Effect of contact time and pH
Figure3a shows that adsorption equiliium was quickly reached in a period of less than one hour, agreeing with other studies on this type of material for which equiliium time is achieved be fore two hours (Santos and Oliveira, 2003; Suman et al., 2007). This was explained by taking into account that a strong interaction between solute (HCrO4-) and adsorbent develops for low pH values due to the presence of (Al-OH2)+ groups having positive surface charge.
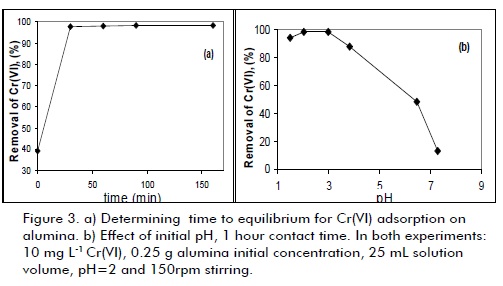
The pH effect was studied in 1 to 9 range; Cr(VI) adsorption decreased drastically when pH was increased (Figure 3b). Such strong dependence of pH was correlated with chromium species in solution and functional groups located on alumina surface. At acidic pH (≈2), in which higher level Cr(VI) elimination was observed, adsorbent surface was highly protonated, facilitating HCrO4- anion removal as a result of electrostatic interactionbased adsorption. However, surface protonation decreased when pH was increased. There was strong competition between CrO42- and OH- species present at high pH values. Thus, the number of positive sites on adsorbent surface became reduced at high pH, showing a decrease in Cr (VI) removal capacity.
Effect of adsorbent quantity and initial adsorbate concentration
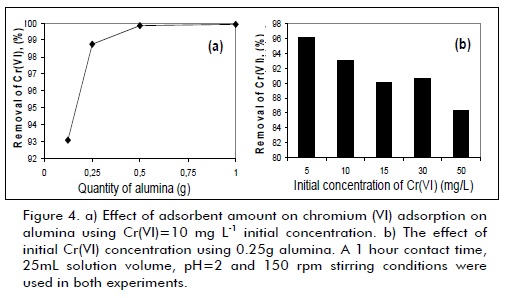
Figure 4a shows an increase in Cr(VI) elimination percentage as the amount of alumina was increased, attributed to the higher availability of active adsorption sites. In the conditions used here, 0.125 g alumina was sufficient for removing 93% of Cr(VI), revealing that this material was very effective in eliminating Cr(VI) from aqueous effluent. Initial Cr(VI) concentration was an important parameter because it partially determined the quantity of chromium to be adsorbed. Cr(VI) concentration effect is shown in Figure 4b, indicating that removal percentage decreased from 96% to 86% when chromium concentration was increased from 5 to 50 mg L-1. This effect could be explained by bearing in mind that all adsorbents have a finite number of active sites which become saturated at certain concentration values. This result confirmed commercial alumina´s high efficiency in removing chromium (VI), revealing that even with 50 mg L-1 Cr(VI) the solid achieved 86.3% removal.
Studying Cr(VI) adsorption on compost
A set of preliminary tests was carried out to select the compost having the best properties to establish each composting material´s adsorption capacity. Chromium concentration for the experiments was kept at similar levels (1.0 to 5.0 mg L-1) to those found in industrial waste-water analysed in the Universidad Nacional de Colombia´s Environmental Chemistry Laboratory (Chemistry Department) to approach how to treat such effluents. The results showed that compost 1 (Tenjo) had the best adsorption capacity (higher than 80% removal). Compost 2 (Marengo Agricultural Centre) was not effective as adsorbent due to the low chromium removal levels observed in the experiments (below 1% in all tests). Compost 1 was thus chosen as adsorbent material to be studied in depth and compared to commercial alumina. Figure 5a shows compost 1 response for Cr(VI) adsorption, changing pH and adsorbent material quantity. A higher Cr VI) elimination level (higher than 97%) was observed at pH 2.5, using 2.5 g compost for 100 mL solution. HCrO4- was the predominant species in chromium solution in these conditions and R-C-OH groups on compost surface were protonated. Increased chromium-(VI) elimination efficiency at acidic pH could be related to Cr(VI) reduction to Cr(III) by electron-donor chemical groups on compost surface, as has been suggested by several authors working with similar materials (Park et al., 2006; Park et al., 2008; Wei et al., 2005). Amine and carboxylic groups on material surface become protonated at acidic pH, making the surface more positive and increasing Cr(VI) anionic species removal levels (Park et al., 2006).
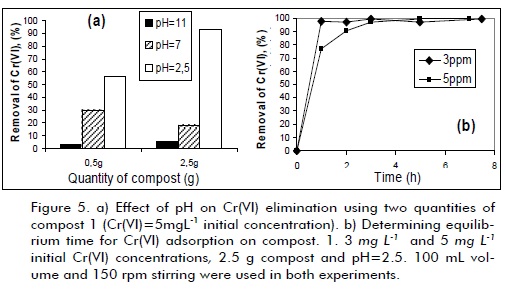
Determining equiliium time
A 3-hour equiliium time was determined for reaching maximum adsorption by using the best experimental conditions for chromium adsorption on compost 1, as indicated in Figure 5b (initial 5mg L-1 Cr(VI)-concentration). It could thus be concluded that equiliium time was dependent on initial Cr(VI) concentration: the higher the chromium species concentration, the longer time needed to achieve adsorption equiliium. The high Cr(VI) removal levels (more than 97%) revealed compost 1´s great potential for being used as adsorbent material in chromium elimination from aqueous media.
The effect of adsorbent quantity and initial adsorbate concentration
Increased Cr(VI) removal levels were observed with an increase in compost quantity (Figure 6a). This result was obviously coherent due to more adsorption sites being available with the greater quantity of compost . However, solid quantity variation from 1 to 10 g only increased removal levels by around 2%, probably due to an intensification of solid particle transport difficulties as a consequence of the greater amount of solid mass. Increasing initial Cr(VI) concentration showed a decrease of metal removal levels when using concentrations greater than 10 ppm (Figure 6b). Therefore, 100% Cr(VI) removal was only obtained at concentrations less than 10 ppm in the conditions used in this study, whereas 70% removal level was reached when 10 to 40 ppm metal concentrations were used.
Figure 6. a) The effect of compost 1 quantity on Cr(VI) removal (Cr(VI) =5 mg L-1 initial concentration). The effect of initial Cr(VI) concentration on its removal from aqueous solution using compost 1 (2.5 g compost). In both experiments: 100 mL solution, pH=2.5, 150 rpm stirring for 3h).
Adding alumina to compost
Many tests were carried out using compost and alumina mixtures at several ratios for ascertaining optimal conditions for removing higher quantities of Cr(VI) using alumina or compost (Table 1). The experiments assessed the possibility of using both materials for removing toxic metal ions by incorporating a higher adsorbent material content having low environmental impact and low cost (compost). These results indicated that small quantities of alumina (5%) led to improving removal levels (Figure 7), achieving 100% elimination after one hour.
Compost morphology after Cr(VI) adsorption
SEM micrographies of compost 1 after being used for chromium removal (not shown here), revealed particles having different morphology, but being similar in form and size to those of preliminary material (used before), indicating that this material had important mechanical strength properties in aqueous solution allowing it to be used in subsequent processes.
Figure 7. The effect of adding alumina to compost 1 for removing Cr(VI). Initial Cr(VI) concentration was 3.0 mgL-1, 2.5 g compost, 100 mL solu- tion, pH=2.5, stirring 150rpm.
Table 1. Optimal Cr(VI) adsorption parameters (higher that 97%) for compost or alumina
Conclusion
The present study showed the enormous potential of composting material for Cr(VI) species adsorption/elimination in aqueous media, metal concentrations corresponding to those found in waste water from Colombian industries. The compost had comparable adsorption properties (97% Cr(VI) removal) to those observed for commercial alumina. Compost adsorption properties could be enhanced (by up to 100%) by adding a small amount (5%) of alumina. Optimal adsorption conditions (higher than 97%) on alumina or compost were established. For alumina, these were Cr(VI)=10 mg L-1 initial concentration, 100 mL/g solution per mass of adsorbent volume, pH=2.0, 1 hour equiliium time and 150 rpm stirring and for compost these were initial Cr(VI) concentration=3 mg L-1, 50 mL/g solution per mass of adsorbent volume, pH=2.5, 3 hour equiliium time and 150 rpm stirring. This study let to choosing compost as a promising material for Cr(VI)-containing waste-water treatment forming part of a solid waste handling and reuse programme for minimising such residues´ environmental impact.
Acknowledgements
The authors would like to acknowledge the Universidad Nacional de Colombia (Bogotá) for financing the project (DIB 20201007535).
References
American Public Health Association (APHA)., Method 3500-Cr D, "Colorimetric Method", Standard Methods for the Examination of Water and Wastewater, 21st Ed., Washington, DC, Centennial Edition, 1995, pp. 3-59 -3-60. [ Links ]
Ahluwalia, S., Goyal, D., Microbial and plant derived biomass for removal of heavy metals from wastewater., Bioresource Technology, Vol. 98, 2007, pp. 2243-2257. [ Links ]
Blázquez, G., Hernáinz, F., Calero, M., Martín-Lara, M.A., Tenorio, G., The effect of pH on the biosorption of Cr (III) and Cr(VI) with olive stone., Chemical Engineering Journal, Vol 148,2009, pp. 473-479. [ Links ]
Bhattacharya, A.K., Naiya, T.K., Mandal, S.N., Dasa, S.K., Adsorption, kinetics and equilibrium studies on removal of Cr(VI) from aqueous solutions using different low-cost adsorbents., Chemical Engineering Journal, Vol. 137, 2008, pp. 529- 541. [ Links ]
Boni, M., Sbaffoni, S., The potential of compost-based biobarriers for Cr(VI) removal from contaminated groundwater: Column test., Journal of Hazardous Materials, 2009, pp. 1087-1095. [ Links ]
Castillo, E., Desarrollo de una metodología en continuo para reducir el contenido de metales pesados en lixiviados de Doña Juana., Convocatoria Nacional de Investigación 2006. Universidad Nacional de Colombia, Bogotá, 2006. [ Links ]
Cieslak-Golonka, M., Toxic and mutagenic effects of chromium (VI). A review., Polyhedron, Vol. 15, 1995, pp. 3667-3689. [ Links ]
Corporación Regional Autónoma (CAR)-Colombia., Resolución 3358,1990. [ Links ]
Fiol, N., Villaescusa, I., Martínez, M., Miralles, N., Poch, J., Serrarlos, J., Sorption of Pb(II), Ni(II), Cu(II) and Cd(II) from aqueous solution by olive stone waste., Separation and purification technology, Vol. 50 ,1,2006, pp. 132. [ Links ]
Fritzen, M., Souza, A., Souza, L., Nome, R., Fiedler, H., Nome, F., Distribution of hexavalent Cr species across the clay mineral surface-water interface., Journal of Colloid and Interface Science, Vol. 296, 2006, pp. 465-471. [ Links ]
Gibert, O., De Pablo, J., Cortina, J., Ayora C., Municipal compost-based mixture for acid mine drainage bioremediation: Metal retention mechanisms., Applied Geochemistry, Vol. 20, 2005, pp. 1648-1657. [ Links ]
Gómez, V., Callao, M.P., Chromium determination and speciation since 2000., Trends in Analytical Chemistry, Vol. 25, 2006, pp. 1006-1015. [ Links ]
Han, R., Li,H., Li,Y. Zhang, Y., Xiao, H., Shi, J., Biosorption of copper and lead ions by waste beer yeast., Jornal of Hazardous Materials, Vol 137 ,3, ,2006, pp.1569- 1576. [ Links ]
Kumpiene, J., Lagerkvist, A., Maurice, C., Stabilization of As, Cr, Cu, Pb and Zn in soil using mendments -A review., Waste Management, Vol. 28, 2008, pp. 215-225. [ Links ]
Levina, A., Lay, P., Mechanistic studies of relevance to the biological activities of chromium., Coordination Chemistry Reviews, Vol. 249, 2005. pp. 281-298. [ Links ]
Malcok, E., Y., Dundar, M., Adsorption of chromium (VI) on pomace-An olive oil industry waste: Batch and column studies., Journal of Hazardous Materials, B138, 2006, pp. 142-151. [ Links ]
Mohana, D., Pittman, C., Activated carbons and low cost adsorbents for remediation of tri- and hexavalent chromium from water., Journal of Hazardous Materials, B137, 2006, pp. 762-811. [ Links ]
Mor, S., Ravindra, K., Bishnoi, N. R., Adsorption of chromium from aqueous solution by activated alumina and activated charcoal., Bioresource Technology, Vol. 98, 2007, pp. 954- 957. [ Links ]
Park, D., Park, J., Yun, Y-S., Mechanisms of the removal of hexavalent chromium by biomaterials or biomaterial-based activated carbons., Journal of Hazardous Materials, B137, 2006., pp. 1254-1257. [ Links ]
Park, D., Yun, Y-S., Park, J., XAS and XPS studies on chromiumbinding groups of biomaterial during Cr(VI) biosorption., Journal of Colloid and Interface Science, Vol. 317, 2008, pp. 54-61. [ Links ]
Santos, M., De Oliveira, E., Heavy metals removal in industrial effluents by sequential adsorbent treatment., Advances in Environmental Research, Vol. 7, 2003, pp. 263-272. [ Links ]
Smith, S. A critical review of the bioavailability and impacts of heavy metals in municipal solid waste compost compared to sewage sludge., Enviroment International, 2009, Vol. 35, pp.142-156. [ Links ]
Suman, M., Khaiwal, R., Bishnoi, N., Adsorption of chromium from aqueous solution by activated alumina and activated charcoal., Bioresource Technology, Vol. 98, 2007, pp. 954- 957. [ Links ]
Tarley, C.R.T., Arruda., M.A.Z., Biosorption of heavy metals using rice milling byproducts characterisation and application for removal of metals from aqueous effluents., Chemosphere,Vol. 54 ,7,2004, pp. 987- 995. [ Links ]
Wei, Y., Lee, Y., Hsieh, H., XANES study of Cr sorbed by a kitchen waste compost from water., Chemosphere, Vol. 61, 2005, pp.1051-1060. [ Links ]
Zhu, S., Hou, H., Xué, Y., Kinetic and isothermal studies of lead ion adsorption onto bentonite., Applied Clay Science, Vol. 40, 2008, pp. 171-178. [ Links ]











 texto em
texto em 


