Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Ingeniería e Investigación
Print version ISSN 0120-5609
Ing. Investig. vol.31 no.1 Bogotá Jan./Apr. 2011
A comparative study of the combustion properties of normal biogas-air mixture and oxygenenriched biogas-air
Karen Cacua1, Andrés Amell2, Luis Olmos3
1 Chemical Engineer, Master in Engineering, Universidad de Antioquia, Colombia. Member of the Grupo de Ciencia y Tecnología del Gas y Uso Racional de la Energía. karen.cacua@udea.edu.co
2 Master in Economics of Energy and Natural Resources. Teaching Faculty of Engineering, Universidad de Antioquia, Coordinator of the Grupo de Ciencia y Tecnología del Gas y Uso Racional de la Energía. National Director of the Programa de Investigación en Energía y Minería de Colciencias. anamell@udea.edu.co
3 Mechanical Engineer, Master in Engineering, Universidad de Antioquia. Member of the Grupo de Ciencia y Tecnología del Gas y Uso Racional de la Energía . luis.olmos@udea.edu.co
ABSTRACT
Research into renewable energy as energy alternatives and decreasing greenhouse gases from organic waste decomposition make biogas a promising alternative for fossil fuel substitution and an energy source from recovery of organic waste in urban, rural and agroindustrial areas.
This paper presents a sensitivity analysis of oxygen-enriched biogas combustion properties ranging from 22% to 35%. Results showed that properties such as deflagration speed, adiabatic flame temperature, dew temperature and CO2 and H2O percentage increased when oxygen percentage in air was increased. On the other hand, properties such as minimum ignition energy, stoichiometric air volume and dry fume volume decreased when the O2 percentage in air was higher than 21%.
Keywords: biogas, oxygen enrichment, greenhouse gas, combustion property.
Received: March 5th 2010. Accepted: November 24th 2010
Introduction
Current limitations concerning fossil fuel availability and climate change due to increased greenhouse gases have promoted research into alternative fuels during the last few years, especially those which are CO2-neutral. Biogas is an attractive alternative fuel because it is available as a decentralised energy source and can be produced wherever organic waste exists (Forsich et al., 2004).
Biogas is produced during anaerobic fermentation of organic waste and it primarily consists of CH4 (50-70%), CO2 (25-50%), H2 (1-1.5%), N2 (0.3-3%) and other minor species such as H2S. Biogas deflagration speed is lower than that of other fuels such as liquefied petroleum gas and natural gas. Also, biogas has a higher autoignition temperature and narrower inflammability interval. These characteristics are due to the presence of CO2 in biogas; CO2 produces thermal and kinetics effects affecting biogas combustion properties related to methane properties. These is a limitation on the use of biogas in different technologies (Forsich et al., 2004; Porpatham et al., 2008; Walsh et al., 1988).
However, biogas has significant energy potential that can be used in different applications such as cooking, heating and generating electric by internal combustion engines and gas turbines. This implies looking for alternatives to improve biogas combustion properties.
Enriching air with oxygen is a promising technique for improving low energy density and low deflagration speed fuels' combustion properties (Qiu and Hayden, 2009). Nitrogen dilutes reactive oxygen during combustion and absorbs part of the energy in exhaust gases due to its high calorific capacity; this decreases combustion efficiency. When oxygen is increased in the air, nitrogen is reduced and combustion efficiency is increased, so fuel consumption decreases.
Researchers have used oxygen-enriched air to study the combustion of several fuels. They have found beneficial effects such as increased energy transfer efficiency, adiabatic flame temperature, inflammability interval and deflagration speed. A decrease in minimum ignition energy has also been found (Baukal, 1998; Coombe and Nieh, 2007; Qiu and Hayden, 2009).
Little research has been found in relation to biogas combustion with oxygen-enriched air. Dahiya et al., (Dahiya et al., 1986) carried out research in 1986 about electricity generation using exhaust gas from biogas combustion with oxygen-enriched air using magneto-hydro-dynamics (MHD) technology. They found that temperature and exhaust gas electric conductivity increased by increasing oxygen in air. The state of the art has shown that no research has been done into biogas combustion with oxygenenriched air. Also, air separation technologies such as cryogenic distillation, memane and pressure swing absorption (PSA) have been lowering production cost and they are available in large-, medium- and small-scales. Research into kinetic, thermal, diffusive and fluid-dynamic phenomena regarding biogas combustion with oxygen-enriched air is thus relevant today.
The present research makes comparative analysis of biogas combustion properties which are affected when air is enriched with oxygen. Biogas combustion properties were determined using normal air (21%O2) and oxygen-enriched air for the comparative analysis. Sensitivity analysis was used to quantify variation.
Combustion properties
Combustion properties of fuel-air mixing are determined to ascertain a specific fuel's energy availability, the conditions for achieving combustion, the amount of air required to completely oxidise the fuel and the amount and composition of exhaust gases (Amell, 2002). These properties are iefly described below:
Stoichiometric air volume (Va)
Stoichiometric air volume is the normal or standard air required for stoichiometrically oxidising 1 m3 normal or standard gaseous fuel.
Wet fume volume (Vwf)
Wet fume volume is the total normal or standard wet fume volume produced by the combustion of 1 m3 normal or standard gaseous fuel. The unit of wet fume volume is normal or standard m3 of wet fumes per normal or standard m3 of gaseous fuel.
Dry fume volume (Vdf)
Dry fume volume is the total normal or standard dry fume volume produced by the combustion of 1 m3 normal or standard gaseous fuel. The unit of dry fume volume is normal or standard m3 of dry fumes per normal or standard m3 of gaseous fuel.
Maximum CO2 percentage
Maximum CO2 percentage is the relationship between CO2 volume and dry fume volume in percentage. This value is maximum when combustion is stoichiometric.
Dew point temperature (TD)
Hydrocarbon (CxHy) and hydrogen combustion produces water vapour in exhaust gases. Dew point temperature is the temperature where steam in the exhaust gases starts to condense. This temperature is important in studying water vaporisation enthalpy recovery from exhaust gases.
Adiabatic flame temperature (TAD)
Adiabatic flame temperature is the maximum temperature reached by exhaust gases when stoichiometric and adiabatic conditions are present during combustion and dissociation reactions are not present.
Laminar deflagration speed (SL)
Laminar deflagration speed is also known as flame speed. It is the lineal speed of a flame which is propagated in static air-fuel mixture. It is also known as the speed of burned area advancing to unburned area or the speed of energy being released from a fuel. This parameter depends on gas fuel type and the quantity of air in the air-fuel mixture (Amell, 1998).
Minimum energy ignition (EMI)
Minimum energy ignition (EMI) is one of the most important combustion properties to be considered when studying combustion propagation. It is defined as the amount of energy needed to supply an air-fuel mixture to start, keep and propagate oxidation. Oxidising composition and air-fuel pressure are important variables in determining minimum energy ignition (Amell, 2002; Lefebvre, 1999).
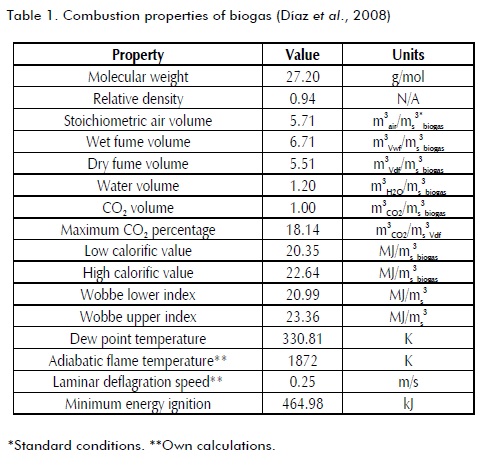
Combustion properties of biogas with normal air (21% O2)
Combustion properties of biogas with normal air (21%O2) are the basis for comparative analysis aimed at determining the effect of oxygen-enriched air. Table 1 shows the main combustion properties of biogas with normal air when the air-fuel mixture has stoichiometric proportions. These properties change when the oxygen content in air changes.
Methodology
Air composition was changed by increasing oxygen from 21% to 35% O2 in this study. Biogas composition was considered to be 40% CO2 and 60% CH4, This is typical composition for biogas produced from anaerobic fermentation of organic waste. The follow statements were considered to calculate the combustion properties of biogas having different enrichment levels (Amell, 2002):
-Air-fuel mixture combustion was stoichiometric. There were no dissociation reactions;
-The oxygen came from atmospheric air and was dry;
-Both fuel and air were ideal gases; and
-The turbulence effect was neglected and the flame was plane and laminar without being stretched (Law, 2006).
Biogas with normal air and with oxygen-enriched air stoichiometric reactions were as follows:
Normal air (21%O2)
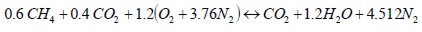 | (1) |
Oxygen-enriched air. Where r was the nitrogen/air ratio
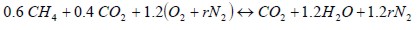 | (2) |
Considering reactions 1 and 2 and r values when oxygen composition was changed from 21% to 35%, combustion properties such as stoichiometric air volume, wet and dry fume volumes, maximum CO2 percentage and steam in wet fumes were determined using the methodology proposed by Amell et al., (2002).
Laminar deflagration speed and adiabatic flame temperature were determined using air-fuel mixture simulations in CHEMKIN software (Kee et al., 2004) and the GRIMECH 3.0 reaction mechanism (Smith et al., 1999). The air-fuel mixture temperature and pressure were 298 K and 1 atmosphere, respectively. A plane laminar premixed flame without stretching was considered for performing the simulations, i.e. temporal fluctuations in flame front surface area were not considered. This phenomenon has an effect on instantaneous deflagration speed.
Minimum ignition energy was determined considering a spherical flame and heat lost from minimum flame surface in the gaseous fuel combustion in a time interval relating flame front and deflagration speed (Eq. 3) (Kondo et al., 2003).
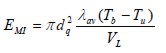 | (3) |
where, dq was critical quenching diameter, ρbb was exhaust gas combustion density, Cav and λav were calorific capacity and thermal conductivity, respectively. These variables were calculated at average temperature between adiabatic flame temperature (Tb) and gas temperature before combustion (Tu). Critical quenching diameter was calculated with the following equation (Eq. 4) (Kondo et al., 2003):
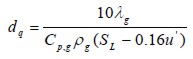 | (4) |
where, λg was gas thermal conductivity, Cp,g was gas calorific capacity, ρb b was gas density and u’ was turbulence intensity.
Turbulence was not considered in this study (u’=1) due to a plane flame model being used. Flow regime was considered laminar.
Sensitivity analysis was used for determining proportional variation of combustion properties when air composition was changed. An oxygen-enriched air factor (OEAF) was introduced. Sensitivity was defined according to the following equation (Eq. 5):
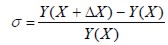 | (5) |
where σ was a measure of change in Y regarding its initial value due to perturbation ΔX in X. In this case, the inlet variable was OEAF and output variable was the combustion properties being analysed. OEAF is defined in Eq.6:
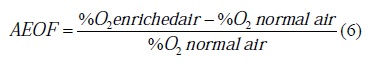 | (6) |
Results and discussion
Table 2 shows that combustion property variations were affected by oxygen-enriched air.
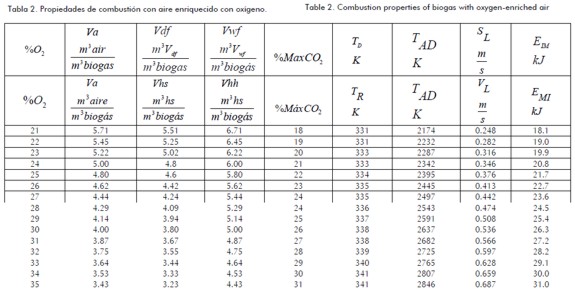
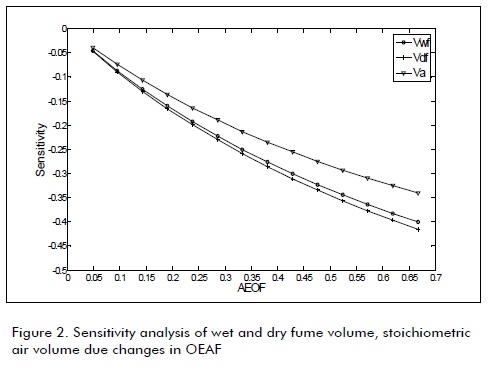
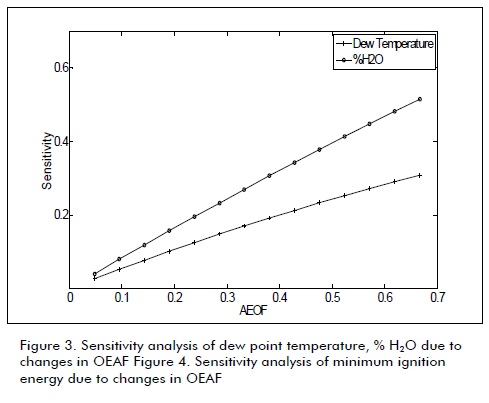
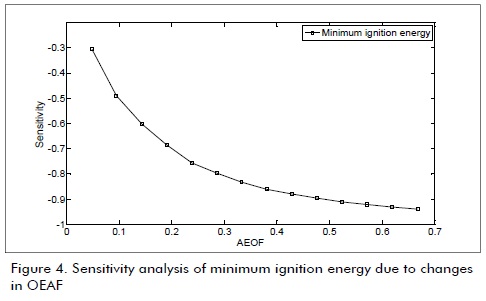
The next figures were obtained from the data in Table 1 Table 2, and Equation 3. These figures show the sensitivity of combustion properties such as Va, Vdf, Vwf, dew point temperature, adiabatic flame temperature, deflagration speed and minimum igni-Figures 1, 2, 3 and 4 show biogas combustion property sensitivity due to changes in OEAF. Figure 1 shows positive sensitivity of laminar deflagration speed increasing its value with increased OEAF.
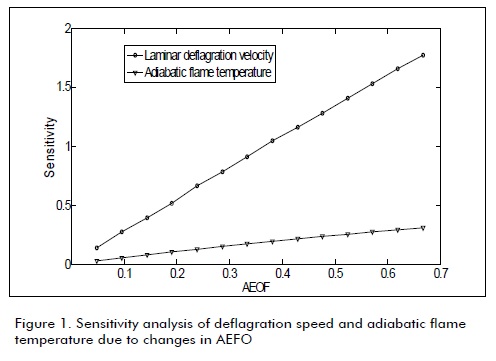
Combustion transmission from burned area to unburned area was greater due to increased air-biogas reactivity, decreasing the adverse effect of CO2 presence on laminar deflagration speed.
Adiabatic flame temperature was increased when OEAF was increased, as shown in Fig.1. Such increase is used in cogeneration and heat recovery systems. Fig.2 shows a decrease in stoichiometric air volume for biogas combustion when OEAF was increased; this was equally due to decreased nitrogen in air when fume volumes were decreased.
Dew point temperature increased due to increases in partial pressure accompanied by decreases in wet fume volume. This produces a corrosion risk in evacuation system due to increased water condensation.
Fig.4 shows the effects of oxygen-enriched air on minimum ignition energy. Decreases in minimum ignition energy were observed when OEAF was increased due to increases in airbiogas reactivity requiring less energy in the same conditions.
Conclusions
The oxygen-enriched air present in biogas combustion improved some combustion properties, such as thermal conductivity and increased biogas oxidation reaction kinetics, decreasing the effects of CO2 presence.
Laminar burning speed and minimum ignition energy were the most sensitive properties to a change of oxygen in air in biogas combustion.
The presence of oxygen-enriched air in biogas combustion decreased stoichiometric air combustion and fume volumes. It also increased adiabatic flame, dew point temperature and maximum CO2 percentage.
Laminar deflagration speed showed high sensitivity to changes in air composition. This effect could compensate for negative effects due to CO2 presence in biogas.
Minimum ignition energy was significantly affected and decreased when OEAF was increased due to increases in air-biogas reactivity and deflagration speed,
The marked effect on minimum ignition energy with changes in air composition was important in this study due to its effects on ignition times and air-biogas mixture flammability intervals.
Oxygen-enriched air has potential use in renewable fuels, such as biogas, and its possible use should be investigated in available natural gas technologies.
Acknowledgements: The authors gratefully acknowledge financial support by COLCIENCIAS, the Universidad de Antioquia´s A1 and A group sustainability strategy 2009/2010 and the project entitled "Optimi-sing dual diesel-biogas motors for functioning in Colombian air pressure."
References
Amell, A. A., Combustión del gas y quemadores., Ediciones CESET, Universidad de Antioquia., 1998, p. 88. [ Links ]
Amell, A. A., Estimación de las propiedades de combustión de combustible gaseosos., Ediciones CESET, Universidad de Antioquia, 2002, p. 73. [ Links ]
Barnett, H. C.,Hibbard, R. R., Basic considerations in the combustion of hydrocarbon fuels with air., Cleveland, Ohio, 1957. [ Links ]
Baukal, C. E., Oxygen-Enhanced Combustion, Air products and chemicals., CRC Press., 1998, p. 369. [ Links ]
Coombe, H. S., Nieh, S., Polymer membrane air separation performance for portable oxygen enriched combustion applications., Energy Conversion and Management, Vol. 48, No. 5, 2007, pp. 1499-1505. [ Links ]
Dahiya, R. P., Ami, C., Sharma, S. C., Dayal, M., Investigations of seeded combustion products of biogas/air-O2 systems., Energy Conversion and Management, Vol. 26, No. 2, 1986, pp. 253-258. [ Links ]
Díaz, C. A., Amell, A. A., Suárez, J. L., Comparison of combustion properties of simulated biogas and methane., Ciencia Tecnología y Futuro, Vol. 3, 2008, pp. 227-238. [ Links ]
Forsich, C., Lackner, M., Winter, F., Kopecek, H., Winther, E., Characterization of laser-induced ignition of biogas-air mixtures., Biomass and Bioenergy, Vol. 27, No. 3, 2004, pp. 299-312. [ Links ]
Kee, R. J., Rupley, F. M., Miller, J. A., Coltin, M. E., Grcar, J. F., Meeks, E, Moffat, H. K., Lutz, A. E., CHEMKINTM Software Release 4.0, San Diego, CA., Reaction Design, Inc., 2004. [ Links ]
Kondo, S., Takahashi, A., Tokuhashi, K., Calculation of minimum ignition energy of premixed gases., Journal of Hazardous Materials, Vol. 103 No. 1-2, 2003, pp. 11-23. [ Links ]
Law, C., Combustion physics., United States of America, Cambridge University Press., 2006, p. 241- 244. [ Links ]
Lefebvre, A., Gas Turbine Combustion., United States of America, Tylor & Francis, 1999, p. 50- 52. [ Links ]
Porpatham, E., Ramesh, B., Nagalingam, B., Investigation on the effect of concentration of methane in biogas when used as a fuel for a spark ignition engine., Fuel, Vol. 87 No. 8-9, 2008, pp. 1651-1659. [ Links ]
Qiu, K., Hayden, A. C. S., Increasing the efficiency of radiant burners by using polymer membranes., Applied Energy, Vol. 86 No. 3, 2009, pp. 349-354. [ Links ]
Smith, G., Golden, D., Frenklach, M., Moriarty, N., Eiteneer, B., Goldenberg, M., Bowmas, T., Hanson, R., Song, S., Gardiner, W., Lissianski, V., and Qin, Z., http://www.me.berkeley.edu/gri_mech/, Access Date: 12-12-2009 [ Links ]
Walsh, J., Ross, C., Smith, M., Harper, S., and Wilkins, A., Handbook on biogas utilization., United States of America, U.S. Department of Energy., 1988, p. 131. [ Links ]











 text in
text in 


