Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Ingeniería e Investigación
Print version ISSN 0120-5609
Ing. Investig. vol.31 no.2 Bogotá May/Aug. 2011
The past, present and near future of materials for use in biodegradable orthopaedic implants
Clara Eugenia Plazas Bonilla1, Jairo Ernesto Perilla2
1 Pharmaceutical Chemistry, DEA Food Science and Technology, Doctoral Student of Materials Science and Technology. Assistant Professor, Universidad Nacional de Colombia, Colombia. ceplazasb@unal.edu.co
2 Chemical Engineering, Masters in Chemical Engineering, Ph.D. Engineering Polymers. Associate Professor, Universidad Nacional de Colombia. jeperillap@unal.edu.co,
ABSTRACT
The aim of bone replacement or fracture treatment methodologies is to induce tissue regeneration respecting anatomy and try to recover functionality. This goal was initially achieved in the 17th century by using animal or human grafts and several medical devices made of natural and synthetic materials are currently used having a whole range of chemical and physical properties. Research in this field continues to seek a solution to the disadvantages usually found when using grafts: immunological reactions, the risk of microbiological contamination, the absence of donors, the need for several surgical interventions and the risk of disease transmission. Basic and applied research must thus be carried out not only in the development of biology and studies about embryonic stem cells but also in the field of new material development. This tendency may be clearly detected by looking at the vast numbers of studies related to using metallic, polymer and ceramic materials and, at present, compound or hybrid materials having potential use in orthopaedic implants. Most of them fulfil conditions regarding biocompatibility and non-toxicity and could be considered when designing biodegradable materials thereby making it feasible to identify a range of research subjects on biomaterials. This paper starts by identifying material development periods and then establishes the advantages and disadvantages of groups which have been considered for bone regeneration and identifies some guidelines which should be taken into account in the field of biodegradable materials in the near future. There is still a long way to go in this subject, especially regarding the field of materials science and technology.
Keywords: biomaterial, orthopaedic implant, biodegradability, bone regeneration.
Received: March 10th 2010
Accepted: June 17th 2011
Introduction
Diseases related to articulations noticeably affect the world’s population; chronic articulation diseases are the main cause of disability in older adults and osteoporosis affects 50% of the female population and 25% of the male population aged over 50. Given that it is expected that the world population over 50 years old will increase by 100% in 2020 (United Nations, 2004; Carvajal, 2007) and that osteoporosis-related fractures have almost doubled in recent years, then the next decade will require a significant advance in the field of skeletal muscle sciences (The Bone and Joint Decade, 2009).
The evolution of biomaterial research can be described as follows. The first generation dated from the 1960s to 1970s where steel and polyethylene were the classic materials, aimed at achieving very similar physical properties to the tissue being replaced and where the aim was to obtain a material which did not react with organic tissue (Navarro, 2005). The second generation was linked to the development of the aeronautics industry and materials such as titanium and its alloys whose notable features are inertia and passivity. However, there are drawbacks to adapting material to an organism and the high probability of rejection. The third generation (in the 1990s) was based on the principle that the body does not have to adapt itself to the material, but that such material has to do so (Jacota, 2008). The term active biomaterial started to be used for identifying materials presenting similarities in composition or surface to that of a biological system, typical examples being calcium phosphates, hydroxyapatite and bio-glass (Jacota, 2008; Zárate and Reyes, 2006). The fourth generation was characterised by the fact that its object was to obtain materials interacting in-depth with biological systems by regulating a particular biological process, becoming integrated into the body and being degradable and which were able to fight against infection. They were designated smart materials due to their properties which were adapted to external stimuli, or in which functions and interaction with biological systems could be controlled by sensors (Jacota, 2008).
Designing and studying materials to be used for implants must consider that introducing a foreign element inside a alive organism is an event that leads to an interface between implant material and tissue where an interaction and a sequence of effects take place due to surface tension, free surface energy, electrical properties, material hydrophilicity and the presence of ion groups. Such interaction also concerns susceptibility to microbiological contamination of the material due to its surface (Barcellos et al., 1998). An important segment in orthopaedic material research has thus been addressed for evaluating biocompatibility aspects or the lack of some event regarding an adverse biological reaction (Alcaide et al., 2009).
Biodegradability can be considered as a process which must happen at a speed allowing tissue healing, so it is appropriate to consider that an appropriate material must allow progressive load transfer to the bone being healed simultaneously with the formation of new tissue, meaning that it has to be able to perform having properties related to osteoinductive, osteoregenerator and osteoconductive material (Pariente et al., 2006). Physical properties are another essential parameter when designing these materials since they must have good mechanical resistance, adequate fatigue strength and appropriate density and weight. Likewise, they have to be antiseptic or sterilisable and easily repro ducible for large-scale processing at the lowest possible cost (Navarro, 2005). Applying the material usually determines the physical properties to be controlled, as in the case of ceramic and injectable polymeric materials used in non-invasive surgical techniques in osteoarticular tissue involving rheological characterisation (Fatimi, 2009). This issue leads to considering the rheological behaviour characteristic of biological tissues, where it must be recognised that modelling these properties in live tissue results from the cells' microscopic properties, such study being difficult to carry out (C. Verdier, 2003).
Likewise, regardless of the material's chemical nature, implant structure and shape have a marked influence (Kong et al., 2007; Likibi et al., 2005), making porosity and topography decisive factors in facilitating joining bone (Davies, 2007; Kong et al., 2007; Likibi et al., 2005; Navarro, 2005).
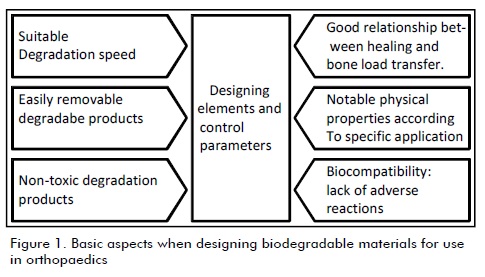
Bioabsorbable materials were developed to avoid the need for a second surgical intervention for removing an implant. This is why they have to have adequate rigidity for bone to start healing, keeping its mechanical properties while forming bone callus, followed by their degradation (Ambard et al., 2005; Bordenave and Baquey, 2004; Navarro, 2005). Figure 1 summarises the main aspects considered in biodegradable material design for orthopaedic applications.
Degradable biomaterials
Natural and synthetic polymers, ceramics and biological glass and some metals and alloys have been most used in bone regeneration (Navarro, 2005; Tharanathan, 2003). Some notable aspects are introduced below for each of them (excluding metallic materials because they are not considered as biodegradable).
Natural and synthetic polymers
Concerns about sustainable development have led to boosting the study of biopolymer-based materials obtained by chemical synthesis or as a product of live organism metabolism (Bordes et al.,2009) which is why biopolymers are used in designing biomedical devices (Martins et al., 2008; Pashkuleva et al., 2007, Pavithra and Doble, 2008; Rojas et al., 2008). Although synthetic polymers offer advantages over natural derivatives since they can be designed according to the required properties (Armelin, 2002; Middleton and Tipton, 2000; Thombre and Sarwade, 2005), natural polymers cover an important segment of research due to advantages such as biocompatibility, being easily obtained, their low cost and the feasibility of chemically modifying them (Martins et al., 2008; Vaz et al., 2003). It is worthwhile mentioning the number of studies regarding incorporating natural polymers into synthetic polymers for improving their degradability and biocompatibility (Armelin, 2002). Some natural and synthetic biopolymers having potential application in implants are mentioned below.
Polysaccharides: Starches, lignocellulosic products (wood), pectins and gums come within this category. Starch, for instance is a low-cost, highly hydrophilic polymer which is easily available and can be used as a biodegradable additive (Martins et al., 2008; Pashkuleva, et al., 2007). Cellulose is the most naturally occurring polymer which is why it has been the subject of wide research as a potential biodegradable material when modifications are made to change its structure (Metabolix, 2009).
Lipids and proteins: Casein, collagen and serum are obtained from animal sources and gluten, soy and zein from vegetable sources.
Microbial polyesters or polyhydroxyalkanoates (PHA): These are produced by some microorganisms, having 30% to 80% of their dry cell weight. Polyhydroxybutyrate (PHB) is this family’s main homopolymer; it is highly crystalline and presents a wide processing window. Poly hydroxybutyrate-co-hydroxyvalerate (PHBV), poly hydroxybutyrate-co-hydroxyhexanoate (PHBHx), poly hydroxybutyrate-co-hydroxyoctanoate (PHBO) and poly hydroxybutyrate-co-hydroxyoctadecanoate (PHBOd) are widely used in medicine due to their biocompatibility (Maia et al., 2004). Copolymer properties vary according to their hydroxyvalerate (HV) content; when this is higher, there is increased resistance to impact and a drop in melting and glass transition temperatures occurs (Bordes et al., 2009).
Lactic acid polyesters: Lactic acid is a quiral molecule which has two stereoisomeric forms: L and D. It is obtained by chemical synthesis from several raw materials or through biological processes, L stereoisomer being the most abundant biological monomer (Pan and Inoue, 2009; Anderson and Shive, 1997; Ambrose and Clanton, 2004). Polymerisation of lactic acid by esterification leads to low molecular weight polymers which have several applications in the pharmaceutical field; however, they are not used in implants due to their poor mechanical properties. To obtain high molecular weight PLA typically it is used the intermediate product called lactide. Lactide becomes polymerised by a ring-opening mechanism generating high molecular weight polymers. PLA mechanical properties and biodegradation can be manipulated by D and L unit content in the polymer (Bordes et al., 2009). The homopolymer derived from l-lactic acid is useful in orthopaedic applications due to its high crystallinity, high tensile strength, low elongation and high modulus; it is frequently used for cell culture, tissue regeneration and orthopaedic implants and in tissue engineering due to its non-toxic properties and good compatibility (Santos and Rodríguez, 2009).
PLLA has also been used as copolymer with other synthetic and natural polymers (Shum et al., 2005; Mano et al., 2008). Polymer units D and L, being amorphous, have low tensile strength, high elongation and high degradation rate (Armelin, 2002). In studies involving fasteners such as rods, wires and screws made of lactic acid-derived polymers for treating fractures it has been reported that a high percentage of cases had no complications and that these are advantageous because a second surgery is not necessary (Rokkanen et al., 2000).
Polycaprolactone (PCL): This is obtained by polymerising ε-caprolactone in the presence of metal alkoxides, it is biodegradable and semi-crystalline (Bordes et al., 2009; Peña et al., 2006). PCL is widely used in biodegradable sutures; for increasing its rate of degradation, it is combined with D and L-lactic acid copolymers (Metabolix, 2009). It is not frequently used due to its low vitreous transition and low melting point but its advantage lies in its easy degradation by enzymatic mechanism.
Other synthetic polymers having biodegradation properties would include aliphatic polyesters, aromatic copolyesters and polyesteramides (Armelin, 2002).
Ceramic materials
There is great interest in using these materials for bone regeneration ( Martínez et al., 2008) due to their potential bioactivity based on their structural similarity with bone mineral phase and suitable biochemical reaction produced by interphase ceramicbone. The apatites, and more specifically hydroxyapatite, though being highly brittle, have good physical properties such as good temperature resistance, corrosion resistance, wear resistance and high hardness. Aluminium oxide and zirconia, called bio-inert ceramics (Jacota, 2008), have broad application in orthopaedics because of their high wear resistance; they are used in bone articulation implants, bone cement for filling small defects, reabsorbable materials for stimulating bone regeneration and metallic covered implants (Navarro, 2005). Biodegradable ceramics can be classified into calcium ceramics and bioglass.
Calcium phosphate ceramics (hydroxyapatites). These are good materials for use in bone system implants because of their goodm histological and in vivo performance (Pereda, 2005), associated with some factors such as calcium/phosphorus ratio (Ca/P), crystallographic structure and porosity. Regarding their application, several phases can be used; stability is highly influenced by temperature and water content. It should be pointed out that shape and particle size are important characteristics regarding the biological reaction of hydroxyapatite implants and that their tension, compression strength and wear strength depend on a particular material's porosity. They are used in granules for small implants which do not have to withstand great stress, as in the case of medium ear implants, porous material for stimulating bone growth or in cement that can be implanted in a viscous state and which settles in vivo (Fernández, 2005; Laquerriere et al., 2005).
Bioglass: These are amorphous materials whose vitreous structure is produced by linking several tetrahedrons from a specific ionic group forming a non-reticular crystal structure, meaning that they do not have periodicity inside their crystal structure. Among the four oxides producing classic glasses proposed by Zachariesen (SiO2, GeO2, B2O3 and P2O5), the most known oxides able to form a crystal structure are silicon oxide (SiO2) and phosphorus pentoxide (P2O5). This leads to SiO2-based and P2O5-based glasses. They are characterised by being haemocompatible by their ability to become adapted to bone’s mineral phase composition and by possible modifications which may be made to their degradation speed by changing their chemical composition. Their clinical applications include their use in releasing antibacterial ions (copper and silver) and in dentistry for fluor release (Navarro, 2005).
Hybrid or composite materials
A broad spectrum of chemical structures can be obtained by polymer chemical handling and the use of technical processing (i.e. electrospinning). Applying bioactive material surface treatments or nanoparticles can lead to obtaining nanocomposites having improved mechanical properties making the material more suitable for the body and thus replacing materials used at the present time (Fundación OPTI, 2008, Chen et al., 2010). Because biodegradable polyesters approved for human use by the Food and Drug Administration (FDA) degrade by autocatalysis and easily lose their mechanical properties, then materials have been proposed having a slower rate of degradation, polymer choice and developing organic-inorganic hybrid nanomaterials involving sol-gel synthesised ceramics or bioactive glasses being a key aspect in providing the material with excellent biological properties (Baino and Vitale-Brovarone, 2011; Valliant and Jones, 2011).
Inorganic / clay reinforced polymer nanocomposites
Polymers can be reinforced by dispersing in them inorganic compounds, as a way of combining advantages related to the polymer matrix process and advantages related to fillers, for example high modulus, good resistance to high temperatures and oxidation resistance (Wu and Mather, 2009). Clays normally used for manufacturing polymer nanocomposites can be classified according to their morphology; the most used are montmorillonite and saponite. Clay particle size and particle size ratio modify polymers' properties producing nanobiocomposites, these being materials having good biodegradability (Li et al., 2008). A broad variety of materials can thus be obtained, including organomodified layered silicate (OMLS) based on different biopolymers, different clays and using organocompounds via several synthesis methods, this being a way for improving some individual materials' properties (Bordes et al., 2009).
Surface treatment
Surface coatings represent an important tool in the biomaterial field for implants used for modifying bone surface (Goto, 2005) so that topography becomes changed, such as polymer composition and its ability to absorb water and ability to bind to osteoblast is promoted by mineral matrix formation (Advincula et al., 2006; Chai and Ben-Nissan, 1999; Pavithra and Doble, 2008). The low temperature required, high coating adherence (Lu et al., 2007) and thermal spraying methods (Gaona, 2007) justify solââ¬âgel use. Because not all types of treatment are suitable for bone implant biomaterial, the most useful treatments are presented below.
Physicochemical treatment: These offer surface protection or activation. Such methods would include physical vapour deposition (PVD), chemical vapour deposition (CVD), ion implantation, sputtering, pulsed deposition, laser ablation and pulverised ion exchange.
Chemical treatment: These modify the initial material’s surface chemical composition; acid / basic treatment and electrochemical treatment are included in this group. The sol-gel procedure used for ceramic layer coating synthesis for covering biomaterials’ surface (i.e. titanium or modified titanium) fit into this group. Precursor selection and gel formation variations lead to controlling composition, structure and homogeneity of biologically interesting coatings such as titanium oxide, bioglass and calcium phosphate (Chai and Ben-Nissan, 1999; Jacota, 2008; Uhlmann et al., 1997). It is a good procedure for improving structures’ biocompatibility and avoiding metallic implant corrosion. Organic modified silanes (ORMOSILS) obtained by these techniques produce bioactive materials for bone replacement, a field where research has been focused on trying to improve substrate-coating layer integration (Gupta and Kumar, 2008; Schottner, 2001; Zarzycki, 1997).
Biological treatment: A material’s surface can be functionalised to bind to active molecules which are able to control cellular adhesion and at the same time induce a cellular response by immobilising peptides, proteins or growth factors.
Materials having biological reaction
Biomolecules have been incorporated into several materials’ scaffolds for optimising chemical, physical and biological properties for biomedical applications or for biologically activating these surfaces (Akkouch, 2008). Two trends may be identified in such development of bioactive, bioabsorbable materials, some stimulating molecular and cellular responses in a controlled way, others acting as temporal supports regarding bone defects in bone renewal (Yoshida et al., 2006). The former consists of developing tridimensional scaffolds without cells which could act as support for and house cells once implanted in vivo and the latter initially colonise scaffolds through progenitor cell in vitro conditions for being implanted and replacing damaged tissue (Navarro, 2005).
Conclusions
Regenerative medicine demands a wide range of approaches for changing the way of confronting illness by treatment, i.e. those related to the skeletal-muscle system. Although studies in this field of medicine explore potential regeneration of adult stem cells, another objective involving nanotechnology has focused on offering tissue engineering-related therapies involving the use of absorbable polymer materials and materials emulating different types of extra-cellular matrix present in tissues and the use of scaffolds that (once implanted) can be reabsorbed and renew injured tissue by new tissue having new vascularisation and nerve connections.
Due to the required materials' properties being defined regarding their specific application and the nature of interactions between an implant and the adjacent tissue, disregarding such interaction could develop adverse body reactions leading to rejection of the implant, which is why developing, designing and characterising such materials must involve physical and chemical characterisation, such biological evaluation leading to approaching their bioabsorbability, bioactivity and biocompatibility properties.
Using composites is a promising option in producing biodegradable materials for bone regeneration, involving selecting the polymer and incorporating active components at a molecular level where porous structure allows cell development and nutrient transmission. There is still a long way to go because no scaffolds have been reported to date having a good relationship between structural and mechanical properties that could simulate trabecular or cortical bone. Natural and synthetic biodegradables polymers are thus an interesting option due to their biocompatibility and the possibility of being metabolised once bone tissue has been regenerated; combined with ceramic materials, such as bioglass and hydroxyapatite, they present properties reinforcing bone regeneration. These properties have led to studying hybrids or composite materials because the involved materials’ individual properties can be improved, either through modifications or applying chemistry and physics to materials’ engineering.
Considerations to be taken into account in the evolution of new materials for biodegradable implants can be summarises as follows: the inevitable control of the cell/cell, cell/protein and protein/ surface interactions and a combination of specific properties of individual components contributing towards the development of new materials leading to the development of individual components’ optimised behaviour and the optimisation of their end behaviour.
References
Advincula, M., Rahemtulla, F., Advincula R., Ada E., Lemons J., Bellis S., Osteoblast adhesion and matrix mineralization on sol-gel-derived titanium oxide., Biomaterials, Vol. 27, No 10, 2006, pp. 2201-2212. [ Links ]
Akkouch, A., Incorporation de Fibronectine et d'albumine de sérum bovin à un biopolyère composé de Polypyrrole et de Poly (l-acide lactique) pour promouvoir la régénération tissulaire., tesis presentada a la Universidad de Laval, para optar el grado de Maestro en Ciencias en Medicina Experimental, 2008. [ Links ]
Alcaide, M., Serrano, M., Pagani, R., Sanchez, S., Vallet, M., Portoles, M., Biocompatibility markers for the study of interactions between osteoblasts and composite biomaterials., Biomaterials, Vol. 30, No 1, 2009, pp. 45-51. [ Links ]
Ambard, D., Le-Lez, S., Pedrono, A., Swider, P., Un modèle de prévision de cicatrisation de linterface os-implant., ITBMRBM, Vol. 26, No 1, 2005, pp. 117-126. [ Links ]
Ambrose, C., Clanton, T., Bioabsorbable Implants: Review of Clinical Experience in Orthopedic Surgery Department of Orthopedic Surgery., Annals of Biomedical Engineering, Vol. 32, No 1, 2004, pp. 171-177. [ Links ]
Anderson, J., Shive, M., Biodegradation and biocompatibility of PLA and PLGA microspheres., Advanced Drug Delivery Reviews, Vol. 28, No 1, 1997, pp. 5-24. [ Links ]
Armelin, E., Síntesis y Caracterización de nuevas poliesteramidas: Estudio de sus propiedades., tesis presentada a la Universi- Universidad Politécnica de Cataluña, para optar el grado de Doctor en Ciencias, 2002. [ Links ]
Baino, F., Vitale-Brovarone, C., Three-dimensional glass derived scaffolds for bone tissue engineering: Currents trends and forecast for the future., Journal of Biomedical Materials Research A, Vol. 97A, No 4, 2011. [ Links ]
Barcellos, I., Carobrez, S., Pires, A., Alvarez, M., In vivo and in vitro responses to poly (ethylene terephthalate-co-diethylene glycol terephthalate) and polyethylene oxide blends., Biomaterials, Vol. 19, No 22, 1998, pp. 2075-2082. [ Links ]
Bordenave, L., Baquey, C., Médecine nucléaire et prothèses., Médecine Nucléaire - Imagerie fonctionnelle et métabolique, Vol. 28, No 12, 2004, pp 623-630. [ Links ]
Bordes, P., Pollet, E., Avérous, L., Nano-biocomposites: Biodegradable polyester/nanoclay systems., Progress in Polymer Science, Vol. 34, 2009, pp. 125-155. [ Links ]
Carvajal, M., Las caídas y fracturas de cadera en el adulto mayor., Revista Medica de Costa Rica y Centroamérica, Vol. LXIV, No 581, 2007, pp. 199-202. [ Links ]
Chai, C., Ben-Nissan, B., Bioactive nanocrystalline sol-gel hydroxyapatite coatings., Journal of Materials Science: Materials in Medicine, Vol. 10, No 8, 1999, pp. 465-469. [ Links ]
Chen, S., Osaka, A., Hayakawa, S., Shirosaki, Y., Tsuru, K., Morphology and structure of organosilica hybrid particles derived from tetramethoxysilane and viniltrimetoxisilane via catalyst-free sol-gel route., Journal of Materials Chemistry, Vol. 20, 2010, pp. 7337-7339. [ Links ]
Davies J., Bone bonding at natural and biomaterial surfaces., Biomaterials, Vol. 28, No 34, 2007, pp. 5058-5067. [ Links ]
Fatimi, A., Comportement rhéologique de biomatériaux pour lingénierie ostéoarticulaire et dentaire: matrices extracellulaires synthétiques et suspensions phosphocalciques., IRBM, Vol. 30, No 3, 2009, pp. 139 -140. [ Links ]
Fernández, E., Obtención y caracterización de nuevos cementos óseos de fosfatos de calcio en el sistema CaHPO4-Ca3 (PO4)2., tesis presentada a la Universidad Politécnica de Cataluña, para optar el grado de Doctor en Ciencias de los Materiales, 2005. [ Links ]
Fundación OPTI., Ministerio de Industria, Turismo y Comercio, Gobierno de España, Observatorio de Prospectiva Tecnológica Industrial, Aplicaciones Industriales de las Nanotecnologías en España en el Horizonte 2020., España, 2008. On line at http://www.navarrainnova.com/pdf/2009/ nanoindustrial2020OPTI.pdf, Consultado Julio 2011. [ Links ]
Gaona, M., Recubrimientos biocompatibles obtenidos por Proyección Térmica y estudio in vitro de la función osteoblástica., tesis presentada a la Universidad de Barcelona para optar el grado de Doctor en Ciencias Químicas, 2007. [ Links ]
Goto, T., Surface coating technology for biomaterials- morphology and nanostructure., Control International Congress Series, Vol. 1284, 2005, pp 248-256. [ Links ]
Gupta, R., Kumar, A., Bioactive materials for biomedical applications using sol-gel technology., Biomedical Materials, Vol. 3, No. 3, 2008, pp1-15. [ Links ]
Jacota, S., Films Minces de Dioxyde de Titane Déposés sur Titane par MOCVD: Microstructure et Biocompatibilité., tesis presentada a la Universidad de Toulouse, para optar el grado de Doctor en Ciencia e Ingeniería de Materiales, 2008. [ Links ]
Kong, L., Ao, Q., Wang, A., Gong, K., Wang, X., Lu, G., Gong, Y., Zhao, N., Zhang, X., Preparation and Characterization of a Multilayer Biomimetic Scaffold for Bone Tissue Engineering., Journal of Biomaterials Applications, Vol. 22, No. 3,2007, pp 223-239. [ Links ]
Laquerriere, P., Grandjean-Laquerriere, A., Jallot, E., Nardin, M., Frayssinet, P., Nedelec, J.M., Laurent-maquin, D., Influence des propriétés physico-chimiques d'hydroxyapatites sur le comportement cellulaire., Revue Européenne de Technologie Biomédicale, Vol. 26, No 3, 2005, pp. 200-205. [ Links ]
Li, H., Zhai, W., Chang, J., Effects of Wollastonite on Proliferation and Differentiation of Human Bone Marrow-derived Stromal Cells in PHBV/Wollastonite Composite Scaffolds., Journal of Biomaterials Applications, Vol. 00, 2008, pp. 1- 16. [ Links ]
Likibi, F., Assad, M., Coillard, C., Chabot, G., Rivard, C., Intégration et apposition osseuses des biomatériaux orthopédiques métalliques poreux et non poreux., Annales de chirurgie, Vol. 130, No 4, 2005, pp. 235-241. [ Links ]
Lu, X., Wang, Y., Liu, Y., Wang J., Qu S., Feng, B., Weng J., Preparation of HA/chitosan composite coatings on alkali treated titanium surfaces through sol-gel techniques, Materials Letters, Vol. 61, No. 18, 2007, pp. 3970-3973. [ Links ]
Maia, J., Santana, M., Re, M., The effect of some processing conditions on the characteristics of biodegradable microspheres obtained by an emulsion solvent evaporation process, Braz., Journal of Chemical and Engineering, Vol. 21, No. 1, 2004, pp. 1-12. [ Links ]
Mano, J. F., Hungerford G., Gomez, J.L., Bioactive Poly (L-Lactic Acid)-Chitosan Hybrid Scaffolds., Materials Science and Engineering C, Vol. 28, No 8, 2008, pp. 1356-1365. [ Links ]
Martínez, A., Esparza, H., Carvajal, G., Ortiz, J., Caracterización estructural y morfológica de hidroxiapatita nanoestructurada: estudio comparativo de diferentes métodos de síntesis., Superficies y Vacío, Vol. 21, No 4, 2008, pp. 18-21. [ Links ]
Natural origin scaffolds with in situ pore forming capability for bone tissue engineering applications., Acta Biomaterialia, Vol. 4, No 6, 2008, pp. 1637-1645. [ Links ]
Metabolix Bioindustries Evolution., Bioplásticos. On line at http://www.metabolix.com, Consultado Nov. 2009. [ Links ]
Middleton, J., Tipton, A., Synthetic biodegradable polymers as orthopedic devices., Biomaterials, Vol. 21, 2000, pp. 2335- 2346. [ Links ]
Navarro, M., Desarrollo y caracterización de materiales biodegradables para regeneración ósea., tesis presentada a la Universidad Politécnica de Cataluña para optar el grado de Doctor en Ciencias, 2005. [ Links ]
Pan, P., Inoue, Y., Polymorphism and isomorphism in biodegradable polyesters., Progress in Polymer Science, Vol. 34, No 7, 2009, pp. 605-640. [ Links ]
Pariente, J., Bordenave, L., Villars, F., Renard, M., Delmond, S., ricain, J., Baquey, C., Centre d'innovations technologiques biomatériaux du CHU de Bordeaux., ITBM-RBM, Vol. 27, No 4, 2006, pp. 150-155. [ Links ]
Pashkuleva, I., Azevedo, H., Reis, R., Surface Structural Investigation of Starch-Based, Macromol. Biosci., Vol. 8, No. 2, 2007, pp. 210-219. [ Links ]
Pavithra, D., Doble, M., Biofilm formation, bacterial adhesion and host response on polymeric implants-issues and prevention., Biomed. Mater. Vol. 3, No 3, 2008, pp. 1-13. [ Links ]
Peña, J., Corrales, T., Izquierdo, I., Doadrio, A., Vallet, M., Long term degradation of poly(3-caprolactone) films in biologically related fluids., Polymer Degradation and Stability, Vol. 91, No 7, 2006, pp. 1424 -1432. [ Links ]
Pereda, O., Metodología de empleo de la hidroxiapatita coralina HAP-200 en Ortopedia y Traumatología., Rev. Cubana Ortop. Traumatol., Vol 19, No 1, 2005, pp. 35-40. [ Links ]
Rodriguez Santos Jr, A., Analysis of the Growth Pattern of Vero Cells Cultured on Dense and Porous Poly (L-Lactic Acid) Scaffolds., Materials Research, Vol. 12, No 3, 2009 pp. 257-263. [ Links ]
Rojas, M., Vallejo, B., Perilla, J. E., Los biopolímeros como materiales para el desarrollo de productos en aplicaciones farmacéuticas y de uso biomédico, Ingeniería e Investigación, Vol. 28, No 1, 2008, pp. 57-71. [ Links ]
Rokkanen, P., Böstman, O., Hirvensalo, E., Makela, E., Partio, E., Patiala, H., Vainionpaa, S., Vihtonen, K., Tormala, P., Bioabsorbable fixation in orthopaedic surgery and traumatology., Biomaterials, Vol. 21, No 24, 2000, pp.2607-2613. [ Links ]
Schottner, G., Hybrid Sol-Gel-Derived Polymers: Applications of Multifunctional Materials., Chemistry of Materials, Vol. 13, No 10, 2001, pp. 3422-3435. [ Links ]
Shum, A. W. T., Li, J., Mak A. F. T., Fabrication and Structural Characterization of Porous Biodegradable Poly(DL-Lactic-Co-Glycolic Acid) Scaffolds with Controlled Range of Pore Sizes., Polymer Degradation and Stability, Vol. 87, No. 3, 2005, pp.487-493. [ Links ]
Tharanathan, R., Biodegradable films and composite coatings: past, present and future., Trends in Food Science & Technology, Vol. 14, No 3, 2003, pp. 71-78. [ Links ]
The Bone and Joint Decade 2000-2010., Report 2009. On line at http://www.boneandjointdecade.org/HTML/musconline/images/BJD%20annual%20report%202009.pdf, Consultado Julio 2011. [ Links ]
Thombre, S., Sarwade, B., Synthesis and Biodegradability of Polyaspartic Acid: A Critical Review, Journal of Macromolecular Science., Part A: Pure and Applied Chemistry, Vol. 42, No. 9, 2005, pp. 1299-1315. [ Links ]
Uhlmann, D., Teowee, G., Boulton, J., The Future of Sol-Gel Science and Technology., Journal of Sol-Gel Science and Technology, Vol. 8, No 1-3, 1997, pp. 1083-1091. [ Links ]
United Nations, Department of Economic and Social Affairs, World Population Prospects., Revision: Analytical Report, 2004. On line at http://www.un.org/esa/population/publications/WPP2004/2004Highlights_finalrevised.pdf, Consultado Julio 2011. [ Links ]
Valliant, E., Jones, J., Softening bioactive glass for bone regenerative sol-gel hybrid materials., Soft Matter, Vol. 7, 2011, pp. 5083-5095. [ Links ]
Vaz, C., de Graaf, L., Reis, R., Cunha, A., In vitro degradation behavior of biodegradable soy plastics: effects of crosslinking with glyoxal and thermal treatment., Polymer Degradation and Stability, Vol. 81, 2003, pp. 65-74. [ Links ]
Verdier, C., Rheological Properties of Living Materials. From Cells to Tissues., Journal of Theoretical Medicine, Vol. 5, No 2, 2003, pp. 67-91. [ Links ]
Wu, J., Mather, P., POSS Polymers: Physical Properties and Biomaterials Applications., Polymer Reviews, Vol. 49, No 1, 2009, pp. 25-63. [ Links ]
Yoshida, M., Langer, R., Lendlein, A., Lahann, J., From Advanced Biomedical Coatings to Multi-Functionalized Biomaterials., Polymer Reviews, Vol. 46, No 4, 2006, pp. 347- 375. [ Links ]
Zárate, B., Reyes, A., Injertos óseos en cirugía ortopédica., Cirugía y Cirujanos, Vol. 74, No 3, 2006, pp. 217- 222. [ Links ]
Zarzycki, J., Past and Present of Sol-Gel Science and Technology., Journal of Sol-Gel Science and Technology, Vol. 8, No. 1-3, 1997, pp. 17-22. [ Links ]











 text in
text in 


