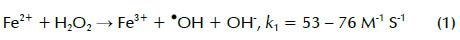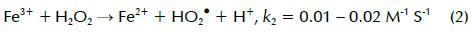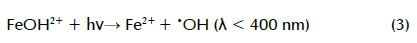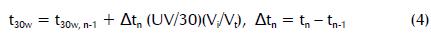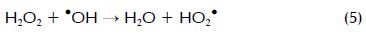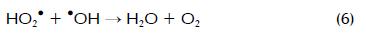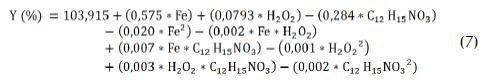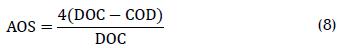Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Ingeniería e Investigación
versão impressa ISSN 0120-5609
Ing. Investig. v.32 n.1 Bogotá jan./abr. 2012
Solar photo-Fenton optimisation in treating carbofuran-contaminated water
Optimización del proceso foto-Fenton solar para el tratamiento de agua contaminada con Carbofurano
Manuel Alejandro Hernández-Shek1, Ana Cecilia Agudelo Henao2, Claudia Mendoza Marín3, Harlen Torres Castañeda4
1 Environmental Engineer, Universidad Nacional de Colombia, sede Palmira, Colombia. Grupo de Investigación Prospectiva Ambiental. E-mail: mahernan-dezsh@unal.edu.co.
2 PhD in Sciences - Physics, Universidad del Valle, Cali Colombia. Associated Professor, Departamento de Ciencias Básicas, Facultad de Ingeniería y Administración, Universidad Nacional de Colombia sede Palmira, Colombia. E-mail: acagude-loh@unal.edu.co.
3 PhD in Sciences - Chemistry, Universidad del Valle, Cali Colombia. Professor, Departamento de Química, Universidad ICESI, Cali, Colombia. E-mail: clame-rin@gmail.com.
4 MSc in Sciences - Chemistry. Universidad del Valle, Cali, Colombia. Associated Professor, Departamento de Ciencias Básicas, Facultad de Ingeniería y Administración, Universidad Nacional de Colombia sede Palmira. Colombia. E-mail: hgtorresc@unal.edu.co.
Received: April 20th 2011; Accepted: February 27th 2012
RESUMEN
Se desarrolló una metodología de superficie de respuesta, diseño Box-Benkhen, con el fin de optimizar el proceso foto-Fenton para la degradación de carbofurano (C12H15NO3) usando una planta piloto de colectores solares cilindro-parabólicos. El modelo Box-Benkhen incluyó cuatro variables: porcentaje de degradación de carbofurano, concentración inicial de carbofurano, concentración de peróxido de hidrógeno [H2O2] y concentración de hierro [Fe2+]. El proceso de degradación fue monitoreado a través de la concentración de carbono orgánico total y cromatografía líquida de alta resolución. Los resultados mostraron que una concentración de 93,2 mg l-1 de carbofurano se degradó completamente en un tiempo t30W = 15 min con 17,1 mg l-1 de Fe2+ y 121,6 mg l-1 de H2O2. El proceso foto-Fenton alcanzó 76,7% de mineralización. La biodegradabilidad fue evaluada usando la relación DBO5/DQO; este valor incrementó desde 0,04 hasta 0,52 en t30W = 20 min, mostrando la posibilidad de usar un tratamiento biológico a partir de ese momento.
Palabras clave: pesticida, carbofurano, foto-Fenton, biodegradabilidad, diseño Box-Benkhen.
ABSTRACT
Box-Benkhen design response-surface methodology was developed to optimise photo-Fenton degradation of carbofuran (C12H15NO3) by using a compound parabolic collector pilot plant. The four variables considered in Box-Benkhen design model included carbofuran degradation percentage, initial carbofuran concentration, hydrogen peroxide [H2O2] concentration and iron [Fe2+] concentration. Degradation was monitored by using total organic carbon concentration and high-performance liquid chromatography. A 93.2 mg l-1 carbofuran concentration was completely degraded in t30W = 15 min with 17.1 mg l;-1 Fe2+ and 121.6 mg l-1 H2O2. Photo-Fenton degradation led to 76.7% mineralisation. Biodegradability during optimisation was evaluated by using the BOD5/COD ratio; this value increased from 0.04 at the beginning of the process to 0.52 in t30W = 20 min, thereby showing the effectiveness of using biological treatments.
Keywords: Pesticide, carbofuran, photo-Fenton, biodegradability, Box-Benkhen design.
Introduction
Wastewater contaminated with pesticides is threatening human health and the environment (Mejía, 2001). Carbofuran (2,3-dihydro-2,2-dimethylbenzofuran-7-yl methyl carbamate C12H15NO3) is an insecticide and nematicide which is included in the general group of carbamate pesticides and is among the greatest number of pesticide poisonings and deaths around the world (Mahalakshmi, Banumathai et al., 2007; Li-An Lu, Ying- Shin et al., 2010; Katsumata, Matsuba et al., 2005). Carbofuran is the active compound in different commercial pesticides used to control pests and improve productivity of crops like soybeans, rice, sugarcane, tobacco, maize, potatoes and vegetables (Mejía, 2001; RAL-AL, 2008). Cleaning irrigation equipment and empty containers produces large volumes of water contaminated with high concentrations of carbofuran. This wastewater represents serious problems, given the lack of efficient technology treating it and because biological treatments are not able to degrade carbofuran due to its high toxicity and recalcitrance (Li-An, Ying-Shin et al., 2010; RAL-AL, 2008). Technologies must thus be designed to treat this wastewater, destroy recalcitrant substances and cause changes in its chemical structure.
Advanced oxidation processes (AOPs) have been extensively investigated for wastewater treatment. They could be applied as sole treatment or as a pretreatment to improve pesticide-containing wastewater biodegradability prior to biological treatment. AOPs mainly rely on creating highly oxidative non-selective free radicals, in most cases the hydroxyl radical (•OH) having 2.8V/SHE E° which is effective for persistent pollutant oxidation and mineralisation (Malato, Blanco et al., 2003; Ying-Shih, Chi-Fanga et al., 2010). The Fenton reaction combined with ultraviolet (UV) radiation is one of the most effective methods for degrading organic matter. Fenton reaction with H2O2 and Fe2+ is shown in equation 1; creating the less powerful hydroperoxyl radical (HO2, E° 1.42 V/SHE) is shown in equation 2 (Li-An Lu, Ying- Shin et al., 2010):
Fenton reaction rate (k1) is much faster than Fe2+ regeneration rate (k2); therefore, adding Fe2+ and H2O2 is required to keep the reaction going. The photo-Fenton reaction, a combination of H2O2 and UV irradiation below 400 nm with Fe3+ or Fe2+, is a promising treatment which can produce relatively more •OH compared to the Fenton treatment. This is mainly accomplished by photoreduction of Fe(OH)2+ (formed in Fenton reaction pH 2-3) to Fe2+, as shown in equation 3 (Li-An Lu, Ying- Shin et al., 2010). Regenerated Fe2+ could undergo further reaction with more H2O2 molecules, producing new •OH and forming a reaction cycle (Chiou, Chen et al., 2006). It has two advantages: facilitating the Fenton treatment without continuous addition of external Fe2+ and reducing ferric hydroxide sludge formation (Torrades, Saiz et al., 2008):
Solar photo-Fenton degradation has shown high efficiency in mineralising toxic pesticides and other organic pollutants (Malato, Blanco et al., 2003). The best reaction condition for mineralising target compound must be estimated to avoid wasting chemical reagents and improve process efficiency. Carbofuran degradation using photo-Fenton treatment was optimised in the present study by using a response surface (RS) Box-Benkhen design; the variables to be optimised were initial carbofuran concentration, Fe2+ concentration and H2O2 concentration. The experiments were performed on CPC pilot scale. Biodegradability parameters were measured during photo-Fenton degradation to identify when photo-treated water would become susceptible to being treated by biological methods.
Materials and Methods
Chemicals
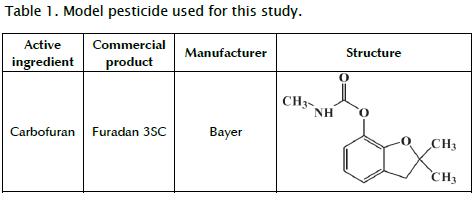
Analytical standard carbofuran (Sigma) was used for high-performance liquid chromatography (HPLC) quantification. Table 1 shows the commercial pesticide selected for the degradation experiments. HPLC grade methanol and acetonitrile were supplied by Merck. A Milli-Q ultra-pure water system (Millipore, Milford, MA, USA) was used throughout the study to obtain (HPLC) grade water used in the analysis. Fe2+, as iron sulfate heptahydrate (FeSO4.7H2O) (MOL LABS®), was used for the photo-Fenton degradation. Hydrogen peroxide (H2O2) reagent grade (30% w/v) from Riedel - De Haën was used. pH was adjusted (between 2.8 - 2.9) with a 2N sulphuric acid solution (H2SO4 96% purity from MOL LABS). A 0.1 M sodium bisulphate solution (NaHSO3) from Riedel-De Haën was used to remove residual hydrogen peroxide to stop the Fenton reaction and preserve the samples. Merckoquant tapes were used for periodic measurement of hydrogen peroxide. pH was stabilised (between 6 and 8) by drops of a sodium hydroxide solution (2M from MERCK) to precipitate the iron.
Analysis
Carbofuran concentration was measured via liquid chromatography (1 ml/min flow rate) in HPLC-UV (Agilent Technologies series 1100) on a C-18 column (4.6 mm, I.D, 250 mm, from Capital). Water-methanol-acetonitrile (45:35:20) was used as a mobile phase. Mineralisation was followed by measuring dissolved organic carbon (DOC) by direct injection of filtered samples in a Shima-dzu-5050A total organic carbon (TOC) analyser and calibrated with standard potassium phthalate solutions.
Biodegradability assays
The BOD5/COD ratio was measured after each photo degradation treatment. Biodegradability was reached when the BOD5/COD ratio was higher than the typical value for domestic wastewater (> 0.4) (Metcalf & Eddy, 1991).
Experimental setup for solar photochemical treatment
Photochemical experiments were performed in sunlight in a CPC pilot plant designed for solar photocatalytic applications (Fig. 1). This reactor was consisted of two modules involving five Pyrex glass tubes; total illuminated area was 1.2 m2 and volume 7.8 l.
22 l of water were recirculated in the photo reactor at the beginning of each photo-Fenton experiment and the appropriate amount of Furadan was added to reach the initial concentration of the pesticide to be degraded. The pH was then adjusted between 2.8 and 3.0 with concentrated sulphuric acid. Iron salt (FeSO4.7H2O) was added and it was necessary to wait 10 min for homogenisation. An initial hydrogen peroxide dose was added and samples were taken at different times to evaluate carbofuran degradation. The photo-Fenton reaction was verified through the presence of hydrogen peroxide every 5 min; its concentration could not drop below 5 mg/l. UV irradiation in the solar plant was recorded by a radiometer (Davis, Health EnviroMonitorTM). Incident irradiation could be evaluated regarding time by taking cloudiness and other environmental variations into account. Experiments could thus be compared by using a corrected t30W illumination time (Ballesteros, Casas et al., 2010; Mendoza-Marin, Osorio et al., 2010) by using equation 4:
where tn was the experimental time for each sample, UV was the average solar ultraviolet radiation measured during Δtn , and t30w was ''normalised illumination time''. Time refers to a constant 30Wm-2 (typical solar UV power on a perfectly sunny day around noon). Vt was the total volume of the water loaded in the pilot plant (22.0 l), Vi was total irradiated volume (7.8 l).
Experimental design
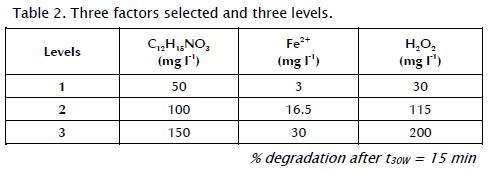
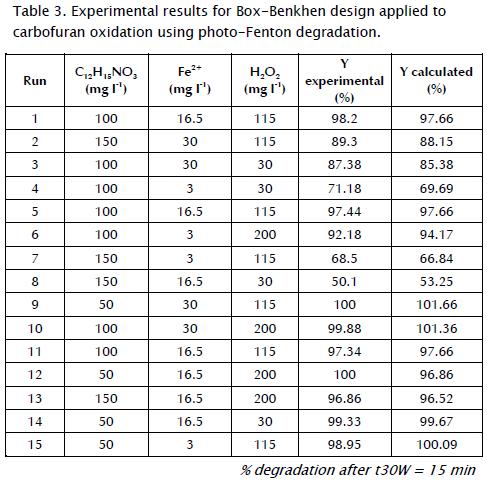
A three-factor, three-level Box-Benkhen design (BBD) was used to determine optimal chemical carbofuran degradation conditions. The method consisted of defining a minimum or low level (denoted as 1), a central or medium level (denoted as 2) and a high or maximum level (denoted as 3) for each experimental factor (Table 2).
Table 3 shows the matrix design, the conditions and results obtained for each experiment, and the response factor defined as degradation percentage of carbofuran after t30W = 15 min followed by HPLC. The data was statically analysed by using Statgraphics software. The experiments were performed randomly to avoid any systematic bias in the outcome (Ray, Lalman et al., 2009). The factors and the experimental levels for each factor were based on values found in the literature, available resources and preliminary experiments' results.
Results and Discussion
Effect of H2O2, Fe2+ concentrations on carbofuran degradation
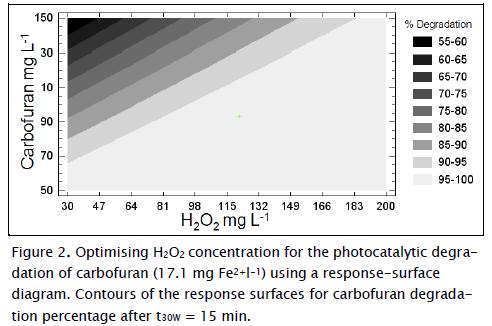
Amounts of H2O2 and Fe2+ have proven to be two of the most important variables during photo-Fenton degradation (Malato, Blanco et al., 2002; Pignatello, Oliveros et al., 2006). Figure 2 shows the response surface diagram for identifying the best H2O2 concentration required in photo-Fenton reaction to degrade a determined amount of carbofuran with 17.1 mg l-1 Fe2+ in t30w = 15 min. It can be noticed in the experimental outcomes that carbofuran degradation increased at higher H2O2 concentrations in the range studied here which was mainly due to greater •OH generation (Li-An Lu, Ying- Shin et al., 2010). However, several researchers have also reported the negative effect of H2O2-overdosed photo-Fenton system for the degrading a target compound (Malato, Blanco et al., 2002). At under-overdosed rate, H2O2 could react with •OH resulting in less powerful HO2• being formed, as shown in equation 5. Moreover, HO2• could further react with •OH and form water and oxygen, as shown in equation 6:
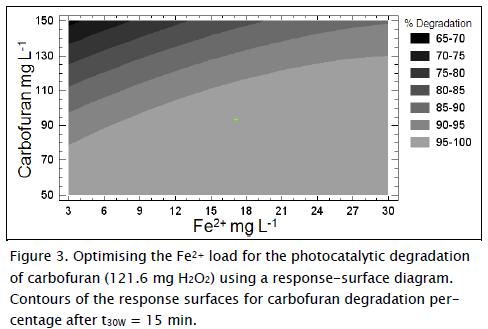
Figure 3 shows that carbofuran and DOC removal efficiency could be improved by increasing Fe2+ concentration. However, using high iron concentrations can be toxic to microorganisms in a biological reactor in coupled systems (Malato, Blanco et al., 2002); it produces ferric hydroxide sludge which can produce areas in the photo-reactor reducing solar light incidence and making it necessary to design another treatment for iron removal, thereby making the system more complex and expensive (Ballesteros, Casas et al., 2010; Mendoza-Marin, Osorio et al., 2010; García-Montaño, Torrades et al., Oliveros et al., 2006; Malato, Fernández et al., 2009).
Optimising photo-Fenton degradation applied to carbofuran degradation
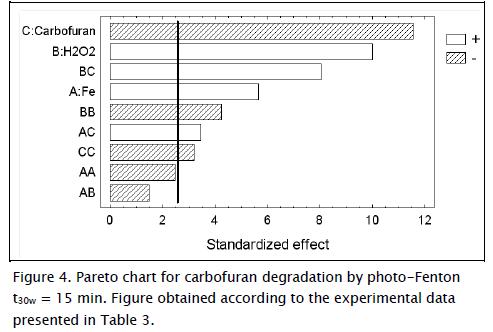
Figure 4 shows analysis of experimental results using a Pareto graph. The Pareto diagram was used to draw more significant conclusions regarding these variables and interactions. This graph shows the magnitude and importance of the effects (variables and interactions). It displays the absolute value of the effects on the ordinate and standard pseudo-error of the effects on the abscissa (95% confidence interval). The Pareto chart has a reference line (solid vertical line) and any effect surpassing this line was potentially important. The reference line was simultaneous margin of error. The signs + and - represented positive and negative effects. A positive effect indicated that carbofuran degradation increased in the presence of high levels of the respective variables within the range studied while a negative effect indicated that carbofuran degradation increased in the presence of low levels of these variables. Positive quadratic order polynomial coefficients indicated a synergistic effect, while negative coefficients indicated an antagonistic effect between or among the variables (Giraldo, Peñuela et al., 2010).
Experimental design methodology led to producing a reduced model directly relating response factors to influential variables. The reduced model's coefficients in polynomial expression were calculated by multiple regression analysis using Statgraphics Plus 5.1 software and representing the weighting for each variable. Optimal conditions could also be represented in polynomial expression (7), where Y (%) represented carbofuran degradation percentage after t30w = 15 min:
The negative quadratic factors for carbofuran, hydrogen peroxide and iron concentration in the polynomial expression corroborated the fact that these concentrations' load was an intermediate value in the range being tested. Higher carbofuran concentration values reduced its degradation as time elapsed whereas greater amounts of hydrogen peroxide and iron led to more successful carbofuran degradation.
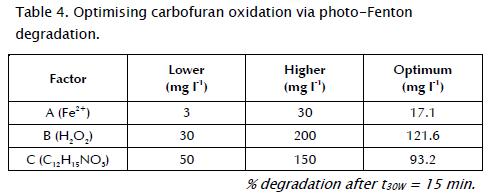
According to the response-surface diagrams (Fig. 2 - Fig. 3) and equation 7, the optimal combination of factors at t30W (15 min) for obtaining 100% carbofuran degradation is shown in Table 4.
To verify the validity of the equation model, Table 3 also indicates the estimated values for carbofuran degradation percentages calculated by equation 7. It should be noted that estimated values were close to those of the experimental data. Indeed, R2 and the reproducibility obtained by Statgraphics Plus 5.1 software were 98.7% and 96.3%, respectively. These results verified the model's validity.
Carbofuran degradation in optimised conditions
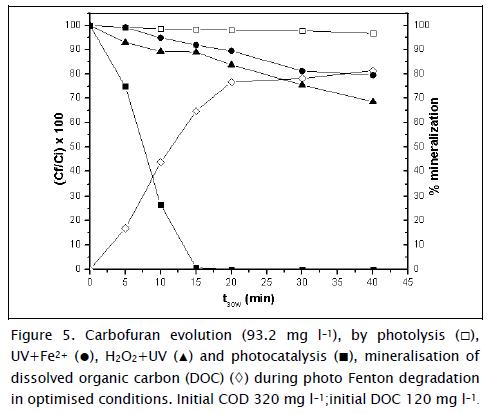
Figure 5 shows a new set of experiments carried out in optimised conditions: 93.2 mg l-1 C12H15NO3, 17.1 mg l-1 Fe2+, and 121.6 mg l-1 H2O2. The totality of carbofuran was eliminated after t30w = 15 min. Optimal carbofuran concentration was given different treatments (photolysis, optimal Fe2+ concentration + UV and optimal H2O2 concentration + UV) to analyse each factor's inference on carbofuran photo-treatment. The results indicated that initial carbofuran concentration was removed after t30w = 15 min; 65% mineralisation occurred after this time,. Carbofuran degradation was higher in the experimental H2O2 concentration + UV than in the experimental Fe2+ concentration + UV. This could be explained by using equation 3 in which one H2O2 molecule reacted with solar radiation below 310 nm to produce two •OH molecules (Malato, Blanco et al., 2003). Water has small amounts of iron which, together with hydrogen peroxide, promoted •OH formation (Malato, Fernández et al., 2009).
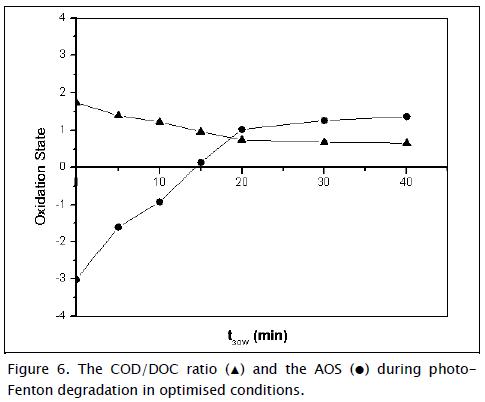
Photo-treated water's average oxidation state (AOS) (Eq. (8)) and COD/DOC ratio were determinant in photo-treated wastewater to evaluate photocatalytic effect on biodegradability and determine the minimum time necessary to increase wastewater biodegradability; both parameters were considered indirect measurements of probable biodegradability (Sitori, Zapata et al., 2009; Katitvichy-anukul and Suntronvipart, 2006):
The AOS ranged from +4 for CO2, representing the most oxidised state of carbon, to -4 for CH4. Figure 6 shows that AOS increased during the first 20 normalised minutes (t30w) until it reached a stable 1.3 value. This increase suggested oxidation reactions present during photo-Fenton degradation and intermediate products' chemical nature did not change significantly after 20 minutes. It was also observed that the COD/DOC ratio value decreased, confirming that the species were oxidised. The COD/DOC ratio was normalised at t30W=20 min, indicating that the species reached a point where oxidation and mineralisation became stabilised. According to carbofuran degradation pathways by photo-Fenton (Li-An Lu, Ying- Shin et al., 2010), mineralisation increased by up to 76.7% t30w=20 indicating that the furan ring or benzene ring was opened and, subsequently, mineralised to inorganic carbon dioxide and water via carbamatic acid and methyl amine. The sub-products formed during this stage were highly oxidised short-chain organic compounds; such compounds are difficult to oxidise but are usually biodegradable (Laperlot, Pulgarin et al., 2006).
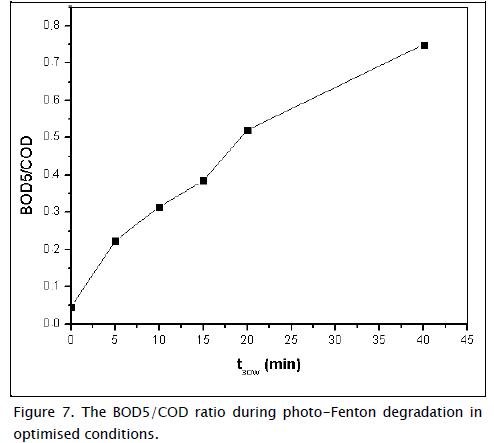
It has been shown that some more toxic and recalcitrant species than the initial compound can appear after treatment; these species are thus impossible to remove through common biological treatment (Mendoza-Marin, Osorio et al., 2010). Figure 7 shows the change in BOD5/COD ratio with exposure to sunlight. The initial BOD5/COD ratio was 0.04, indicating that the organic matter in the water was not biodegradable, and after t30W = 20 min its value reached 0.52, meaning that all carbofuran content had to be removed because even a low carbofuran concentration is very toxic for microorganisms.
Conclusions
It was found that the effluent obtained after photo-Fenton degradation was able to continue to complete decontamination via biological treatment.
Photocatalysis (t30W = 20 min) led to efficient mineralisation (76.7%) of organic-load in these types of discharge with low reagent consumption facilitating its industrial application.
It has been demonstrated that biological treatments cannot be applied while amounts of carbofuran remain due its high toxicity, even at low concentration. Photo-treatment must totally degrade carbofuran concentration. Biological systems should only be used to treat photo degradation products of carbofuran.
The Box-Benkhen experimental design allowed a constant carbo-furan value to be eliminated to obtain optimal iron and hydrogen peroxide values to avoid unnecessary reagent use and avoid additional processes for removing residual reagents.
Acknowledgments
The authors are grateful for the financial support received from the Universidad Nacional de Colombia (QUIPU 2010100812) and DIPAL (2020100626). They are also grateful to Norberto Benítez and Fabio Zuluaga from the Universidad del Valle's Chemistry Department.
References
Ballesteros, M., Casas, J., Oller, I., Malato, S., Sánchez, J., A comparative study of different tests for biodegradability enhancement determination during AOP treatment of recalcitrant toxic aqueous solutions., Ecotoxicology and Enviromental Safety, No. 73, 2010, pp. 1189-1195. [ Links ]
Chiou C., Chen Y., Chang-Tang C., Shie J., Li Y., Photochemical mineralization of di-N-butyl phthalate with H2O2/Fe3+, Journal of hazard materials. No. 135, 2006, pp. 344-349. [ Links ]
García-Montaño, J., Torrades, F., García-Hortal, J., Domenech, X., Peral, J., , Combining photo-Fenton process with aerobic sequencing batch reactor for commercial hetero-bireactive dye removal, Applied Catalysis B. Environmental, No. 67, 2006, pp. 86-92. [ Links ]
Giraldo, A., Peñuela, G., Torres, R., Pino, N., Palominos, R., Mansilla, H., Degradation of the antibiotic oxilinic acid by photo-catalysis with TiO2 in suspension, Water Research, No. 44, 2010, pp 5158-5167. [ Links ]
Katitvichyanukul, P., Suntronvipart, N., Evaluation of biodegradability and oxidation degree of hospital using photo-Fenton process as the pre-treatment method, Journal of Hazardous Materials, No 138, 2006, pp. 384-391. [ Links ]
Katsumata, H., Matsuba, K., Kaneco, S., Suski, T., Ohta, K., Yobico., Degradation of Carbofuran in aqueous solution by Fe (III) aquacomplex as effective photocatalysts., Journal of Photochem-istry and Photobiology, No. 170, 2005, pp. 239-245. [ Links ]
Laperlot, M., Pulgarin, C., Fernández, P., Maldonado, M., Pérez, L., Estrada, W., Oller, I., Gernjak S., Malato S., Enhancing bio-degradability of priority substances (pesticides) by solar photo-Fenton, Water Research, No. 40, 2006, pp. 1086-1094. [ Links ]
Li-An, L., Ying-Shin M., Kumar, M., Jih-Gaw, L., Photochemical degradation of Carbofuran and elucidation of removal mechanism. Chemical Engineering Journal, 2010, doi:10.1016/j.cej.2010.10.045. [ Links ]
Mahalakshmi, M., Banumathai, A., Palanichamy, M., Murungesan, V., Photocatalytic degradation of Carbofuran using semiconductor oxides., Journal of Hazardous Materials, No. 143, 2007, pp. 240-245. [ Links ]
Malato, S., Blanco, J., Vidal, A., Alarcon, D., Maldonado, M., Caceres, J., Gernjak, W., Applied studies in solar photocatalytic detoxification: an overview, Solar Energy, No. 75, 2003, pp. 329-336. [ Links ]
Malato, S., Blanco, J., Vidal, A., Richter, C., Photocatalysis with solar energy at a pilot-plant scale: An overview., Applied Catalysis B: Environmental, No. 37, 2002, pp. 1-15. [ Links ]
Malato, S., Fernández, P., Maldonado, I. Blanco, J., Gernjak, W., Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends, Catalysis Today, No. 147, 2009, pp. 1-59. [ Links ]
Mejía, M., Agricultura Ecológica. 1st edition. Terranova (ed). Bogota D.C, 2001., pp 55-51. [ Links ]
Mendoza-Marin, C., Osorio, P,. Benítez, N., Decontamination of industrial waste from sugarcane crops by combining solar photo-Fenton and biological treatments. Journal of Hazardous Materials, No. 177, 2010, pp. 851-855. [ Links ]
Metcalf & Eddy., Wastewater Engineering Treatment, disposal and reuse, McGraw-Hill (ed). New York, 1991. [ Links ]
Montgomery, D., Design and Analysis of experiments, 1st ed. John Willey & Sons (ed). New York, 2001. [ Links ]
Oller, I., Malato, S., Sanchez, J., Madonado, M., Gassó, R., Detoxification of wastewater containing five common pesticides by solar AOPs - biological coupled system., Catalysis Today, No. 129, 2007, pp. 69-78. [ Links ]
Red de acción en plaguicidas y sus alternativas en América Latina (RAP-AL). Septiembre 2008. http://www.rapal.org/articulos_files/Carbofurano.pdf/. [ Links ]
Ray S., Lalman J., Biswas N., Using the Box-Benkhen technique to statistically model phenol photocatalytic degradation by titanium dioxide nanoparticles, Chemical Engineering Journal, No 150, 2009, pp 15 - 24. [ Links ]
Pignatello, J., Oliveros, J., Advanced oxidation process for organic contaminant destruction based on Fenton reaction and related chemistry, Critical reviews in environmental science and technology, No 36, 2006, pp. 1-84. [ Links ]
Sitori, C., Zapata, A., Ollr, I., Gernjak, W., Agüera, A., Malato, S., , Decontamination industrial pharmaceutical wastewater by combining solar photo-Fenton and biological treatment, Water Research, No. 43, 2009, pp. 661-665. [ Links ]
Srimanta, R., Lalman, J. Biswas, N., Using the Box-Benkhen technique to statistically model phenol photocatalytic degradation by titanium dioxide nanoparticles., Chemical Engineering Journal, No. 150, 2009, pp. 15-24. [ Links ]
Torrades F., Saiz S., Garcia A., Montano J., Degradation of wheat straw black liquor by Fenton and photo-Fenton processes, Environmental Engineering Science. No. 25, 2008, pp. 92-98. [ Links ]
Ying-Shih, M., Chi-Fanga, S., Jih-Gaw, L., Degradation of Carbofuran in aqueous solution by ultrasound and Fenton process: Effect of system parameters and kinetic study. Journal of Hazardous Materials, No. 178, 2010, pp. 320-325. [ Links ]
Zapata, A., Velegraki, T., Sánchez, J., Mantzavinos, D., Maldonado, M., Malato, S., Solar photo-Fenton treatment of pesticides in water: Effect of iron concentration on degradation and assessment of ecotoxicity and biodegradability. Applied Catalysis B: Environmental, No. 88, 2009, pp. 448-454. [ Links ]
Zapata, A., Malato, S., Sánchez, J., Oller, I., Maldonado, M., Scale - up strategy for a combined solar photo-Fenton/Biological system for remediation of pesticide-contaminated water., Catalysis Today, No. 151, 2010, pp. 100-106.
[ Links ]