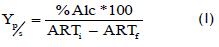Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Ingeniería e Investigación
versión impresa ISSN 0120-5609
Ing. Investig. vol.32 no.3 Bogotá dic. 2012
Obtaining superfine ethanol in a Cuban distillery
Obtención de alcohol extrafino en una destilería cubana
Y. Albernas1, M. González2, G. Corsano3, E. González4
1 Yailet Albernas Carvajal. Affiliation: Centro de Análisis de Procesos, Facultad de Química y Farmacia, Universidad Central Marta Abreu de Las Villas. M.Sc. in Chemical Industry Processes Analysis, Chemical Engineer, Universidad Central Marta Abreu de Las Villas. Santa Clara. Cuba. E-mail: yailetac@uclv.edu.cu
2 Meilyn González. Affiliation: Centro de Análisis de Procesos, Facultad de Química y Farmacia, Universidad Central Marta Abreu de Las Villas. Ph.D. in Technical Sciences, M.Sc. in Chemical Industry Processes Analysis, Chemical Engineer, Univer-sidad Central Marta Abreu de Las Villas. Santa Clara. Cuba. E-mail:mgonzalez@uclv.edu.cu
3 Gabriela Corsano. Affiliation: Instituto de Desarrollo y Diseño (CONICET-UTN), Argentina. Ph.D. in Engineering, M.Sc. in Computational Mechanics, Licentiate in Applied Mathematics, Universidad Nacional del Litoral, Santa Fe, Argentina. E-mail: gcorsano@santafe-conicet.gov.ar
4 Erenio González. Affiliation: Centro de Análisis de Procesos, Facultad de Química y Farmacia, Universidad Central Marta Abreu de Las Villas. Ph.D. in Technical Sciences, Ph.D. in Sciences, Chemical Engineer, Universidad Central Marta Abreu de Las Villas. Santa Clara. Cuba. E-mail: erenio@uclv.edu.cu
How to cite: Y. Albernas, M. González, G. Corsano, E. González. (2012). Obtain-ing superfine ethanol in a Cuban distillery. Ingeniería e Investigación. Vol. 32, No. 3. December 2012, pp. 47-52.
ABSTRACT
This paper describes obtaining superfine ethanol in a Cuban distillery from molasses as base raw material. The operational characteristics of the main stages for obtaining superfine alcohol have been described, emphasising alcohol fermentation due to its complexity in achieving process continuity; a Gantt chart led to determining a 31-hour process time and 5-hour cycle time. The influence of fermentation yield on process profitability was determined through mass and energy balances, demonstrating that a 4ºGL degree of alcohol was feasible. The main water-consuming elements were also determined (98% in molasses dilution) as well as steam con-sumption (91% during distillation). A preliminary analysis was made of the opportunities provided by material and energy integration, mainly for distillation, contributing towards a positive environmental impact.
Keywords: Superfine ethanol, molasses, profitability.
RESUMEN
En el presente trabajo se presenta un análisis global del proceso de alcohol extrafino en una destilería cubana, que parte de miel final como materia prima. Se realiza un análisis de las características operacionales de las principales etapas del proceso de obten-ción de alcohol extrafino y se hace énfasis en la fermentación alcohólica, dada su complejidad a la hora de mantener la continuidad del proceso que permite determinar, mediante el empleo del diagrama de Gantt, que el tiempo total del proceso es de 31 horas y el tiempo del ciclo es de 5 horas. Por medio de los balances de masa y energía se determina la influencia del rendimiento de la fermentación en la rentabilidad del proceso y se demuestra que es factible para un grado alcohólico por encima de 4 ºGL. Se determina, además, la incidencia de los principales consumidores de agua, demostrando que el 98% es consumido en dilución del mosto, así como los consumos de vapor dentro del proceso, en los que el 91% corresponde a la etapa de destilación. Se hace un análisis preliminar de las oportunidades de integración material y energética tanto existentes, sobre todo en la etapa de destila-ción, como aquellas que se pueden realizar con plantas aledañas, contribuyendo a un impacto ambiental positivo.
Palabras clave: Alcohol extrafino, miel, rentabilidad.
Received: February 24th 2011 Accepted: August 22th 2012
Introduction
The fossil fuel crisis has led to analysing existing biofuel re-sources during the last few years in an attempt to achieve great-er efficiency, emphasising material and energy integration and reducing the resulting environmental impact as addressed by Cavalett et al., (2011), Gonzalez et al., (2011), and Raei et al., (2010) regarding Pinch technology applied to heat exchange networks. Integration using residue from different processes has also been addressed by Abidin et al., (2011) considering CO2 recovery which is the main force in ethanol production.
There is higher steam consumption during the distillation stage in ethanol production where a desired degree of alcohol must be attained. Kansha et al. (2010) have reported a new methodology for integrating distillation columns using the columns' heat there-by reducing consumed steam whilst Gumienna et al., (2011) have addressed recycling stillage as a warming agent.
Albernas et al., (2010) have reported fermentation stage molas-ses processing time and used simulation to show the importance of obtaining high alcohol content in fermented broth, one of the main challenges being to maintain the temperature in the fer-menters, as explained by Nielsen et al., (2003) and shown in the example provided by Albernas et al., (2011), showing the effect of these factors' impact on optimal fermentation stage design.
Development
1. An overview of superfine ethanol production in a Cuban distillery
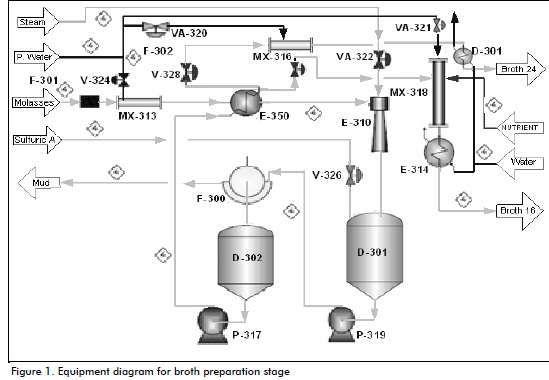
Broth preparation (Figure 1)
Molasses from general storage tanks are used in broth preparation, involving an initial pre-dilution which must reach around 40°Brix (°Bx) (Gallardo, 2005). Such pre-diluted molasses are filtered where solid impurities are removed from the molasses as they impair quality and therefore fermentation and final product quality. Sulphuric acid is supplied to this broth at 80°C to 90°C and 1.5-2.0 g/L acidity to obtain a desired pH during yeast growth in the main (mother) vats, ranging from 3.9-4.0 (Nielsen et al., 2003).
Some of the previously-diluted molasses go to an ejector which is fed with direct steam to sterilise them; they then go to a tank driven by heat exchanger plates and frames, keeping cooling water recirculation constant for cooling the broth at 35°C, using cold water from the cooling tower area as heat exchange medi-um. The broth at lower temperature then goes to a mixer which is fed with treated water and leaves it at 16°Bx. This Bx depends on the progress of fermentation fed into main vats (Nielsen et al., 2003). According to Villena (1999), the part of the broth which was not fed into the previous mixer goes to another mixer to ensure 24°Bx, because this is fed to subsidiary vats or fermenters (Albernas et al., 2011).
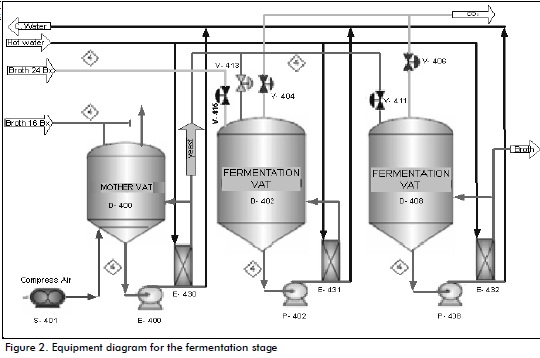
Fermentation stage (Figure 2)
The 16°Bx broth then enters the main vat; this is aerated with a blow-er during this process' maximum idle time which is also cooled by heat exchanger plates and frames to keep temperature near to 35ºC; the broth is then pumped to the fermenter.
24°Bx broth, regulated by a valve (20% input), has its density (being low due to fermentation) increased to 30%-40% where fermentation continues but with a new inlet; after a time (when Bx is half initial +1) the fermenter workload is completed (Villena, 1999). The 16ºBx broth is supplied to a fermenter during fer-mentation time, as above (ten fer-menters have been installed in the plant). According to Albernas et al., (2010), this process is semi-continuous during the fermentation stage, having a 30- to 40-hour fermentation cycle thereby ensuring that there will always be dead fer-menters waiting to be distilled to ensure distillation continuity, as recommended by Abidin et al., (2011) and also ensure CO2 production. The fermented broth is imme-diately pumped to the distillation stage. The fermenter dregs join the stillage stream which is sent to the yeast factory (adjacent to the ethanol plant), as discussed by Gumienna et al., (2011).
Distillation stage (Figure 3)
The raw material (known as wine), having 6% and 7% alcohol v/v, passes through wine heated at 35°C (increasing to 70°C) to decrease the amount of steam needed in the distillation column. This column (working under vacuum) provides ethanol at 60°GL and eliminates stillage passing to the torula yeast plant. It then goes to a pre-concentrator column to become concentrated to 75-80°GL (also working under vacuum) followed by a hydro-selector column where process alcohol is washed, decreasing alcohol content to 15-20ºGL (Gallardo, 2005; Seider et al., 2003). It then goes to a rectifier column, where it becomes concentrated to 96.3ºGL. Alcohol leaves two plates below the head and passes to a methyl elimination column, as indicated by Villena (1999) and Gallardo (2005).
2. Mass and energy balance during fermentation and distillation stages
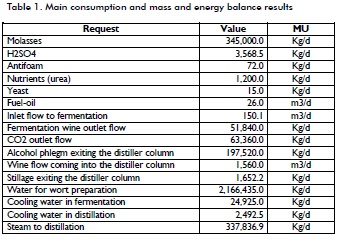
Analysing the process stages led to mass and energy balances for the main process points. It was taken that the plant operates at a continuous regimen (i.e. 900 HL/d (90 m3/d) extra fine alcohol production). The following Table gives the main consumption and mass and energy balance results.
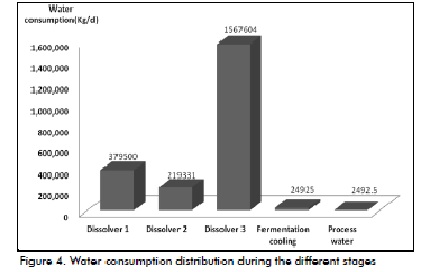
The data obtained during the fermentation stage agreed with Albernas et al., (2010). Figure 4 shows water consumption in preparing the broth, fermentation stages and cooling, including the distillation stage. It can be seen that the third dissolver consumed most water (71.45%) which was reasonable because all fermenters were diluted in this dissolver while the rest of the dissolvers involved using less °Bx and only the second wort was made in the mains vats (Villena, 1999).
Fermentation stage cooling requires low energy consumption compared to broth dilution (only 1.14% consumed water) but, as previously explained, this water is constantly circulating, thereby requiring no fresh feed water and ensuring efficiency in maintain-ing heat exchanger cleanliness. Distillation stage cooling includes process water and has a low value (0.11 %) because the plant integrates various streams (i.e. it is not necessary to use fresh water as the cooling medium), which will be discussed later on.
The hydro-selector column has high water consumption (6,185,520 Kg/d) (not discussed here because it is fresh water) but stillage slops from the pre-concentrator column and tail rectifier column occur as part of the integration between streams, allowing alcohol contained on these stillage to be recovered.
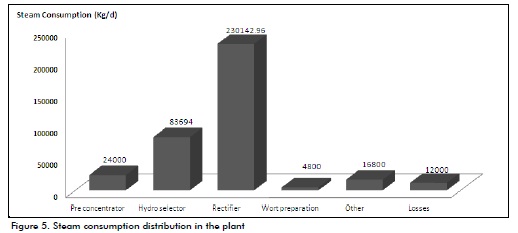
An analysis of the plant's steam consumption demonstrated that 90.95% of the total steam produced in the boiler (20,000 Kg/h) was consumed during the distillation stage (Figure 5), completely agreeing with Gallardo (2005). The rectifier column was the greatest consumer, requiring 62% of steam consumed; the high alcohol content required for extra fine alcohol is achieved in this column, according to Seider et al., (2003) and Villena (1999).
Figure 5 shows 3% steam loss (thereby threatening process economy) due to inefficient steam pipe insulation and leaks in some valves; this should be improved through pertinent investment. Fermenter sterilisation involved direct steam cleaning (4.5%) and broth preparation consumed 1.3% of the total for molasses sterilisation (Villena, 1999).
3. The alcohol fermentation stage's operational
Characteristics
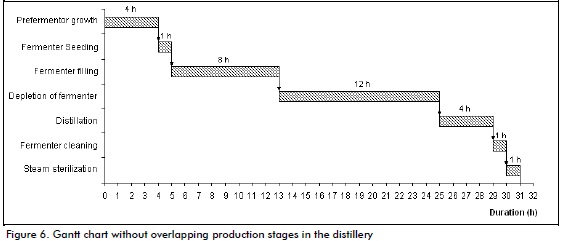
The ten fermenters in the plant have been located to guarantee continuity of the flow fed to the distillation stage, thereby achieving continuous production. Each shift lasts 8 hours in the plant, two fermenters being seeded for fermenting and another two for distilling (having already completed the cycle/dead population). Average process stage duration is shown below by Gantt chart (Figure 6) (Albernas et al., 2011).
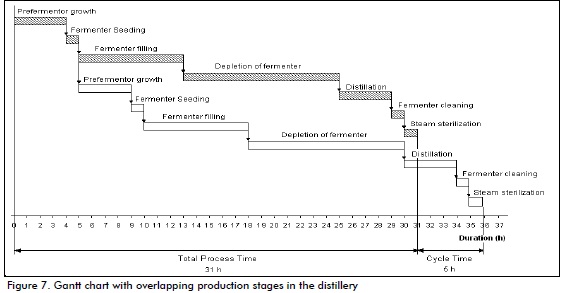
Total process time was 31 hours. A Gantt chart with overlapping stages showed that the cycle time for such a plant would be five hours (Figure 7). The five hour cycle time indicated that it would start again every five hours; this was primarily determined by the time needed by the main vat to regain population and one hour to seed the next fermenter.
It is very important that each operation takes place on time as delay means that process continuity cannot be achieved; also if an operation is finished before schedule this causes equipment to be underused. An operation may take longer and this delays the next stage and continuity becomes lost (Albernas et al., 2011).
4. Analysis of sensitivity to variation in fermentation yield
Product/substrate yield (Yp/s) was determined at 3ºGL minimum alcohol concentration and 6ºGL maximum concentration, following the equation described by Nielsen et al., (2003):
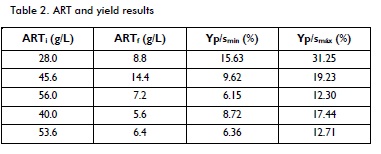
ARTi (initial) and ARTF (last) were obtained from the experimental data for the five fermentations, where the average value of both was considered, being 44.64 g/L and 8.48 g/L respectively.
Average minimum yield was 9.30% and average maximum yield 18.59%; such values were used for determining minimum and maximum actual degree of alcohol (by solving the previous equation), giving 3.36ºGL and 6.72ºGL respectively. They were evaluated in the distillation stage mass and energy balances analysed in section 2, varying these values within this range, in turn leading to different production values.
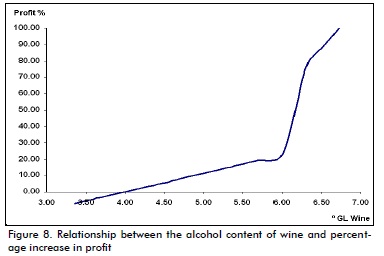
After obtaining ethanol production values for each alcohol fer-mentation value, total production cost and profit was determined by Microsoft Excel programming based on dynamic profitability indicators, taking into account investment in the plant, raw and manufactured material consumption, electricity, water, steam and fuel oil consumption and costs, according to Peters et al., (2003) and Seider et al., (2003), keeping production fixed to see how such economic indicators would be affected by alcohol variation, in turn affecting ethanol production and thereby the yield so obtained. Figure 8 shows that alcohol obtained during the fermentation stage is the crucial point because it determines production and thus process profitability. Analysing 3.36°GL to 3.70°GL alcohol variation demonstrated that the process was not profitable, as shown in the Figure below. The sensitivity analysis showed that an alcohol's minimum value to make the process profitable would have to be 4ºGL.
5. Opportunities regarding material and energy integration
The process involved some opportunities for material and energy integration and using calorie content and reduced steam consumption. They were:
- The wash-water hydro-selector column consisted of pre-concentrator stillage and rectifier tail; the alcohol which might have been contained in this stillage could be recovered and fresh water was not needed/used for this purpose, thereby agreeing with Gumienna et al., (2011);
- Concentrator column vapour was used as heating medium to heat wine in the distiller column;
- Vapour for condensing the rectifying column was used in heating the distiller column; and
- The hydro-selector column vapour was used for heating the methyl eliminator column.
The last three opportunities mentioned above agreed with those made by Kansha et al., (2010) and Gonzalez et al., (2011).
The opportunities arising from the distillery's proximity to a sugar factory were:
- Sugar factory steam supply surplus; this factory has a surplus of 480,000 Kg/d of bagasse during harvest time, representing 1,192,800 Kg/d of steam. The distillery only consumes a maximum of 384,000 Kg/d of steam; in a 90-day harvest period this would represent distillery savings of $ 485,688 by not having to consume fuel, as discussed by Cavalett et al., (2011);
- Energy integration was obtained in the distillation system by applying Pinch technology, as explained by Raei et al., (2010) reporting considerable energy-saving technologies which have been applied, as explained by Kansha et al., (2010), Gonzalez et al., (2011) and Cavalett et al., (2011);
- Stillage and fermentation residues were used in a torula yeast factory, leading to 18,000 Kg being obtained daily; and
- The CO2 produced during fermentation (63,360 Kg daily) was provided adjacent to a small plant purifying and compressing it, as recommended by Abidin et al. (2011), while the fusel oil (fusel alcohols) so obtained can also be used in producing diluents and varnish removers.
Everything discussed and analysed by Seider et al., (2003), Cava-lett et al., 2011, Gumienna et al., (2011) has suggested that this plant was working in line with the concept of material and energy integration, had a positive environmental impact and had added value as the sugar factory's sugar-cane bagasse provided the main raw material.
Conclusions
Overall analysis of obtaining extra fine alcohol determined that distillation was the most energy-consuming stage concerning steam consumption (91%) and that most water was consumed in the rectifier column during broth preparation (98%). The advantage of overlapping operations to reduce total cycle time and thus process time was thus confirmed. Alcohol fermentation (wine) values (over 4°GL) showed that the process was profita-ble, hence the importance of this stage. The plant was seen to have several opportunities regarding material and energy integration to increase production added value, some having been ex-ploited whilst others not so.
References
Abidin V., Bouallou C., Clodic D., Valorization of CO2 emissions into ethanol by an innovative process., Chemical Engineering Transactions, Vol. 25, 2011, pp.1-6. [ Links ]
Albernas Y, Verelst H., González E. and Pedraza J., Simulation of the batch fermentation stage in the process to obtain ethanol from final molasse., Chemical Engineering Transactions, Vol. 21, 2010, pp.931-936. [ Links ]
Albernas Y., González M., Pedraza J., González E., Visión global sobre la planificación de procesos discontinuos., Afinidad, Vol. LXVIII, No. 553, Mayo - Junio, 2011, pp. 203-209. [ Links ]
Cavalett O., Cunha M. P., Junqueira T. L., Dias M. O. S., Environmental and economic assessment of bioethanol, sugar and electricity production from sugarcane., Chemical Engineering Transactions, Vol. 25, 2011, pp.1007-1012. [ Links ]
Gallardo Aguilar I. Diseño y evaluación de equipos de destilación., Vías para el diseño de nuevas instalaciones de la industria de procesos químicos fermentativos y farmacéuticos, Editorial Científico - Técnica, 2005, capítulo 6, pp. 128-157. [ Links ]
González M., Verelst H., Espinosa R., González E., Simultaneous energy and water minimization applied to sugar process pro-duction., Chemical Engineering Transactions, Vol. 25, 2011, pp.177-182. [ Links ]
Gumienna M, Lasik M, Szambelan K, Czarnecki Z., Reduction of water consumption in bioethanol production from triticale by recycling the stillage liquid phase., Acta Scientiarum Polonorum. Technologic Alimentary. Vol. 10, No. 4, Oct-Dec, 2011, pp. 467-474. [ Links ]
Kansha Y., Kishimoto A., Tsutsumi A., A new design methodology for heat integrated distillation column based on self-heat recuperation., 19th International Congress of Chemical and Process Engineering CHISA 2010, 7th European Congress of Chemical Engineering ECCE 7, Prague, Czech Republic, 28 August - 1st September, 2010. [ Links ]
Nielsen J, Villadsen J., & Lidén, G., Bioreaction Engineering Principles, Second Edition, New York, Kluwer Academic/Plenum Publisher, 2003, chapter 9, pp. 339-420. [ Links ]
Peters, M. S., Timmerhaus K. D. and West R. E., Plant Design and Economics for Chemical Engineers, Fifth Edition, McGraw-Hill, 2003. [ Links ]
Raei B., Ghadi A., Bozorgian A. R., Heat Integration of heat exchangers network using pinch technology., 19th International Congress of Chemical and Process Engineering CHISA 2010, 7th European Congress of Chemical Engineering ECCE 7, Prague, Czech Republic, 28 August - 1st September, 2010. [ Links ]
Seider, W. D., Seader, J. D. & Lewin, D. R., Product and Process Design Principles. Synthesis, Analysis, and Evaluation, Second Edition, John Wiley and Sons, Inc., 2003, chapters 11, 12 and 17, pp. 367-381, 443-488, 563-609. [ Links ]
Villena M., Informe de proyecto de ampliación de destilería de alcohol rectificado de mieles de 500 a 900 HL/d, Alcoholes finos de caña. SA., Cienfuegos, Cuba, 1999, pp. 1-50. [ Links ]