Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Ingeniería e Investigación
Print version ISSN 0120-5609
Ing. Investig. vol.33 no.1 Bogotá Jan./Apr. 2013
F. Forero L.1, C. A. Vélez P.2, A. P. Sandoval A.3
1 Freddy Forero Longas. Ingeniero agroindustrial, Universidad del Valle, Cali, Colombia. Affiliation: Ph. D. (c) Ingeniería de Alimentos, Universidad del Valle, Cali, Colombia. Email: freddy.forero@correounivalle.edu.co
2 Carlos Antonio Vélez Pasos. PhD Food engineering, Universidad de Campinas, Brasil. Affiliation: Professor, Universidad del Valle, Cali, Colombia. Email: carlos.velez@correounivalle.edu.co
3 Angelica Sandoval Aldana. PhD Ingeniería de Alimentos, Universidad del Valle, Colombia. Affiliation: Professor, Universidad del Tolima, Ibague, Colombia. Email: angelsan78@gmail.com
How to cite: F. Forero L., C. A. Vélez P., A. P. Sandoval A., Ultrafiltration and osmotic evaporation applied to the concentration of cholupa (Passiflora maliformis) juice., Ingeniería e Investigación. Vol. 33, No. 1. April 2013, pp. 35 - 44.
ABSTRACT
Cholupa it's an exotic fruit that has excellent organoleptic characteristics but highly sensitive to thermal treatments. Fruit was harvested in commercial fields and for each process stage, the following physicochemical parameters were analyzed: soluble solids, pH, total solids, viscosity, and turbidity. Was used a Pellicon2® ultrafiltration system (10 kDa) with flat membranes and filtration area 0.5 m2, osmotic evaporator had a polypropylene hydrophobic hollow fiber module MD020CP2N® with area 0.104 m2. Prior to ultrafiltration, in order to obtain a higher permeate flow, juice was subjected to enzymatic treatment with commercial mixtures, found that doses of Maxoliva® (50 µl/100g) and Rapidase® (40 µl/100g) are best treatment, which had a significant effect (P<0.05) reducing the viscosity and increase soluble solids; in clarification process by ultrafiltration, permeate flux rate has a behavior that fulfills Darcy law, with 19.4 and 6.0 l m-2 h-1, as maximum and minimum, respectively. Finally, osmotic evaporation process produce a concentrate of 65.15±0.36o Brix with homogeneous appearance, dark yellow color, which when it's reconstituted dissolves easily in water. The results show that ultrafiltration and osmotic evaporation proved to be effective and synergistic technologies to get a good quality concentrate, that preserve much original characteristics of juice. This is the first work reported about cholupa juice processing.
Keywords: Membranes, Evaporation, Ultrafiltration, Juice, Diffusion.
RESUMEN
La cholupa es una fruta exótica que tiene excelentes características organolépticas, pero muy sensibles a los tratamientos térmicos. La fruta fue cosechada en cultivos comerciales y para cada etapa del proceso, los parámetros fisicoquímicos analizados fueron: sólidos solubles, pH, sólidos totales, turbidez, viscosidad. Se utilizó un sistema de ultrafiltración Pellicon2® (10 kDa) con membranas planas y área de filtración 0.5 m2, el evaporador osmótico fue un módulo de fibras huecas (MD020CP2N®) con área 0.104 m2. Antes de la ultrafiltración el jugo se sometió a tratamiento enzimático, encontrando que las dosis de Maxoliva® (50 μl/100g) y Rapidase® (40 μl/100g) son el mejor tratamiento (P<0.05) para reducir la viscosidad y aumentar los sólidos solubles; en el proceso de clarificación por ultrafiltración, el flux de permeado tiene un comportamiento que cumple la ley de Darcy, con 19.4 y el 6.0 l m-2h-1, como máximo y mínimo, respectivamente. Por último, en el proceso de evaporación osmótica se obtiene un concentrado de 65.15±0.36 oBrix, el cual tiene un aspecto homogéneo, color amarillo oscuro, el cual al ser reconstituido se disuelve fácilmente en agua. Los resultados evidencian que la ultrafiltración y evaporación osmótica demostraron ser tecnologías eficaces y sinérgicas para obtener un concentrado de buena calidad, que conserva las características originales del jugo. Este es el primer trabajo reportado sobre el procesamiento del jugo de cholupa.
Palabras clave: Membranas, Evaporación, Ultrafiltración, Jugo, Difusión.
Received: May 22th 2012 Accepted: March 8th 2013
Introduction
Membrane processes have many advantages over other techniques of conservation, being the main, that product quality is usually maintained, since it operates at low temperatures, have low energy requirements and great flexibility operation (Jiao et al., 2004); (Alkhudhiri et al., 2012). Ultrafiltration has been successfully tested in processing a wide variety of fruits juices such as cactus (Cassano et al., 2010), tangerine (Anderson-Cook et al., 2009), kiwi (Tasselli et al., 2007), apple (Onsekizoglu et al., 2010), which has led to products with very good physicochemical properties and good acceptance in market, more common limitation in ultrafiltration applications it's the membrane fouling that reduces permeate flux during process (Saha et al., 2006); (Cassano et al., 2007b); (Yazdanshenas et al., 2010).
Within this range of operations with membranes, osmotic evaporation (OE) has emerged as a potential technique, applicable to food, chemical, cosmetic and pharmaceutical industry, this operation has aroused considerable interest, in liquid food processing, as fruit juices concentration (Shaw et al., 2001); (Valdés et al., 2009) milk, tea and other heat sensitive products, because they can work at atmospheric pressure, room temperature and nearly isothermal conditions (Nii et al., 2002) eliminating non-enzymatic browning reactions, color degradation, flavor and aroma loss, coupled with low energy consumption.
The porous membrane in the OE system is in intimate contact with circulating liquids, feed temperature is low and close to that brine, due to polymer hydrophobicity, membrane cannot be wetted by liquid creating an vapor - liquid interface at pores entrance (Forero et al., 2011); difference in water activity between aqueous solution and brine, translates into a vapor pressures difference, becoming driving force for water vapor transport (Romero, 2003b). The vapor pressure differential across membrane, is usually obtained with salts solutions such as NaCl, CaCl2, MgCl2, MgSO4 (Bandini et al., 2002); (Bui et al., 2003) and some organic liquids as glycerol and polyglycol (Celere et al., 2004); (Celere et al., 2005) which generally have high solubility, low water activity and high surface tension.
As to osmotic evaporation applications, for orange case, concentrate showed a similar composition of organic acids and sugars at initial juice, but with a 20% loss in vitamin C, possibly due to oxidation phenomena (Cisse et al., 2005); in melon study, glucose, fructose, sucrose and vitamin C were retained, without color degradation, but with a 30% reduction in phenolic compounds (Vaillant et al., 2005). Passion fruit concentrate by this technique, has superior organoleptic characteristics, when compared to traditional one obtained by thermal evaporation, especially in vitamin C content, osmotic evaporation produces a slight aromatic compounds loss (Vaillant et al., 2001a); Pineapple juice not reported significant physicochemical changes, color is highly preserved and without aromas loss (Hongvaleerat et al., 2008) this behavior was very similar to kiwi (Cassano et al., 2007a) and camu - camu (Rodrigues et al., 2004), where the main quality factors were vitamin C loss and color change. The aim of this study was to evaluate membrane technologies, ultrafiltration and osmotic evaporation to laboratory scale, as nonthermal processing alternatives to concentrate cholupa juice (Passiflora maliformis), which is a fruit of excellent physicochemical characteristics and with a planted acreage increasing in Colombia.
Materials and methods
Chemical reagents
Calcium chloride (CaCl2) used was commercial grade (94% purity), solutions were prepared with distilled water 0.5 µS/cm (Distiller 82100, Schott, Germany), enzyme mixtures Maxoliva® (65000 AVJP/g) and Rapidase® (95000 AVJP/g) (DSM Food, USA) (AVJP/g = pectinase activities per gram).
Fruit and juice characterization
Cholupa (Passiflora maliformis) fruits were collected in commercial crops located in Rivera city (02o49'01.8"N, 075o30'20.6"W, 608 masl, Colombia), fruits was removed with a disinfected stainless steel scissors, packed in paper bag, marked with date and accession number. In laboratory were examined and separated those showing decay, insect attack or fungi; plant material was disinfected for three minutes by immersion in sodium hypochlorite solution 1% (v/v), removing excess with distilled water and paper towels. A 30 fruits sample were evaluated for: length and width with a digital caliper (500-197-20B ± 0.02 mm, Mitutoyo, USA), total weight, pulp, peel and seed (310M ± 0.1g, Precisa Instruments, Switzerland) to estimate yields, juice was analyzed for soluble solids (Brix ± 0.1% - 20 ± 0.01 oC) digital refractometer (Rx7000, Atago, Japan), pH potentiometer (Handylab ± 0.01 pH, Schott, Germany) was previously standardized with buffer solutions pH 7 and 4 (20 ± 0.1 oC), total solids content (920.151 AOAC method), viscosity to 25 ± 0.5 oC (Dv III Ultra ± 0.1 cP, Brookfield, USA), turbidity (2100N ± 0.2 NTU, Hach, USA).
Enzymatic treatment
Was applied to reduce fouling in ultrafiltration and concentration membranes, was established an experimental design type response surface central composite with 13 treatments, having as factors: type enzyme (Maxoliva®, Rapidase®) and amount applied (minimum: 10 µl/100g , maximum 60 µl/100g), each treatment consisting of 100 g juice, was placed in a water bath (37±1 oC), with constant magnetic stirring (80 RPM) for one hour, after this period a thermal shock was performed (80±2 oC - 30 s) to inactivate enzymes; response variables (triplicate) were total solid, soluble solids, pH, viscosity, turbidity. The results were analyzed using Design Expert 8 trial® software (Stat-Ease, USA), analysis of variance (ANOVA) was conducted with F test and P <0.05.
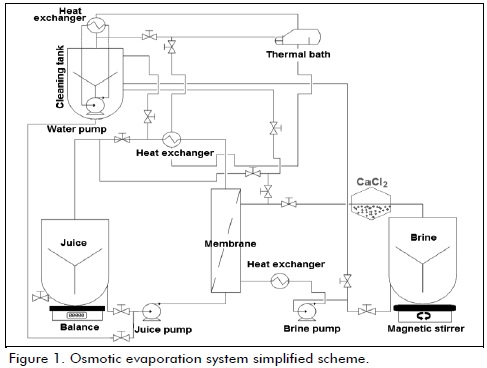
Ultrafiltration
After analyzing experiment results for enzymatic maceration, treatment that most decreased viscosity and increased soluble solids was applied to juice (10 l), is pre filtered with a standard mesh (200 µm), and then passed across Pellicon2® (Millipore, USA) system, contains a cassette Biomax® type flat membrane, made of modified polyethersulfone arranged in parallel and separated by polypropylene screens, membrane has a nominal molecular weight limit (10 kDa) and filtration area (0.5 m2), process was carried out with total recycle of retentate, using a peristaltic pump (4 l/min), to room temperature (28 oC), permeate and retentate was analyzed in triplicate for soluble solids, pH, viscosity, total solids and turbidity.
Osmotic evaporation
Ultrafiltered juice was concentrated using a MD020CP2N® (Microdyn, Germany) module, provided with a polypropylene hydrophobic membrane, unit has 40 hollow fibers with internal and external diameter 2.8 mm and 1.8 mm, respectively, average pore size is 0.2 µm, membrane area 0.104 m2, peristaltic pumps were used with flow rate 1 m/s (brine) and 0.08 m/s (juice), brine had 45% w/v concentration (CaCl2), 0.33 ± 0.02 water activity (Aqualab 4TE ± 0.003 aw, Decagon, USA), the feed : brine ratio was 1:7 (l), saline concentration was kept constant by saturation with fresh chloride, working temperature was 28 oC, evaporation flux was calculated as weight loss every 30 minutes, final concentrate was analyzed in a similar way that ultrafiltrate.
Results and discussion
Fruit characterization
The fruits of cholupa (Passiflora malliformis) showed a length/width ratio 1.21 ± 0.12, was observed that are oval with a long slightly higher, similar to passion fruit, this type of shape difficult cholupa packing and handle bulk is preferred, average weight was 47.28 ± 5.64 g. The yields were, juice (16.2 ± 1.3 g/100 g), peel (42.8 ± 3.8 g/100 g), seed (41.0 ± 2.6 g/100 g), this low percentage of juice compared to passion fruit (33.0 ± 0.15 g/100 g) (Kulkarni et al., 2010), it is one factors that has limited industrial use of this fruit, excellent physicochemical and sensory juice, make it a potential raw material for the beverage sector.
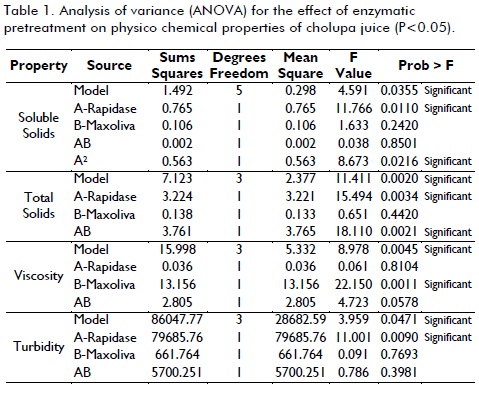
Enzymatic treatment
The implementation of enzymatic maceration at all levels were effective in reducing viscosity and increasing soluble solids (figure 2), found that doses of Maxoliva® (50 µl/100g) y Rapidase® (40 µl/100g) are best treatment (P<0.05), resulting in a juice viscosity 8.26 ± 0.38 (cP) and soluble solids 16.98 ± 0.20 (oBrix); Maxoliva® was statistically significant (P<0.05) to reduce viscosity (>50%), explained by its composition based on pectinases, which act directly on primary cell tissue in pulp, producing a pectin solubilization (Florez et al., 2003) , compound largely responsible for initial viscosity (16.48 ± 0.51 cP), pectin degradation produces a reduction in water holding capacity and therefore, water is released into system to further reduce viscosity (Lee et al., 2006), behavior consistent with that reported for passion fruit (Vaillant et al., 2001b), fruit very similar to cholupa.
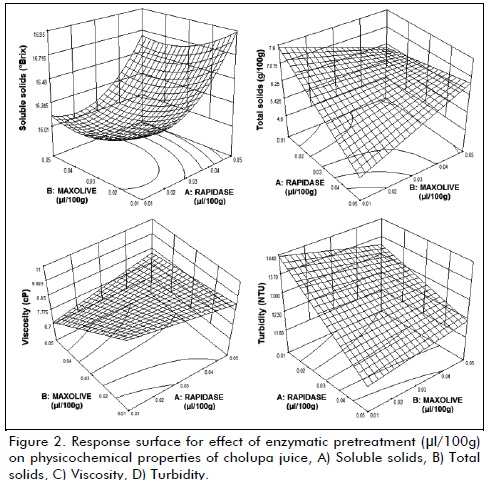
Rapidase® was statistically significant (P<0.05) to increase soluble solids content while decreased total solids and turbidity, this mixture is formed by enzymes of system cellulose (hemicelluloses, endoglucanases, exoglucanase, cellobiase) (Ortega et al., 2004) endoglucanases probably acted on long chains in amorphous regions of cellulose fibers producing oligosaccharides and increasing availability of non-reducing ends in molecules on which they exert their action; exoglucanases causing release of cellobiose units, being attacked by cellobiase forming glucose monomers, simultaneously hemicelluloses that are closely associated with cellulose fibers are also converted into simple sugars, through various mechanisms (Lemos et al., 2003); (Ribeiro et al., 2010).
Ultrafiltration
The prefiltering process was very useful to prevent fouling almost immediate in ultrafiltration membrane, at remove larger particles that are predominantly cell walls that were not broken by enzymatic treatment, likewise, use of enzymes facilitated clarification, due to large viscosity reduction, resulting in a better permeate flux, this benefit has been reported previously by other authors (Yu et al., 2004); (Vladisavljevic et al., 2003).
As for the physicochemical characteristics of ultrafiltered juice (Table 1), pH values ??are not altered by this operation, otherwise total solids, that as expected, significantly increased in retentate and down in permeate, viscosity was also greatly reduced in final filtered juice, coming very close to water, being good indication effectiveness of ultrafiltration to remove insoluble particulate matter, turbidity was reduced significantly, showing a clear yellow juice, translucent, without suspended particles, behavior of these characteristics is similar to that reported for ultrafiltrate pineapple juice (De Barros et al., 2003) and kiwi (Tasselli et al., 2007).
In the ultrafiltration process of cholupa juice, it was evident that membrane behavior changes significantly over time, mainly due to surface fouling and concentration polarization (Yazdanshenas et al., 2010); (De Bruijn et al., 2006), these two phenomena are aspects of same problem, which is the components accumulation retained in filter layer, both of which introduce additional resistance to membrane side in contact with juice, being responsible for gradual reduction in permeate flux and selectivity of process, these phenomena can occur simultaneously or intermittently (Rai et al., 2007).
Figure 3 shows evolution of permeate flux versus time, which presents a behavior that follows Darcy's law, in which the permeate flux is a function of transmembrane pressure and a total resistance, three characteristics zones are appreciated for this type of process; zone I corresponds to a rapid initial decrease in permeate flux from 19.4 to 10.7 (l/m2h) due to sudden fouling of membrane pores by solute molecules, causing pore blockage and reducing the effective size (Cassano et al., 2007b). Zone II represents a gradual decline (9.9 a 6.6 l/m2h) that continues over time significantly longer than zone I, decline is explained by formation of gel layer on membrane surface, this is formed with increasing amount of retained solute molecules (De Bruijn et al., 2006) pore blocking occurs on membrane surface, preventing fouling of interior structure.
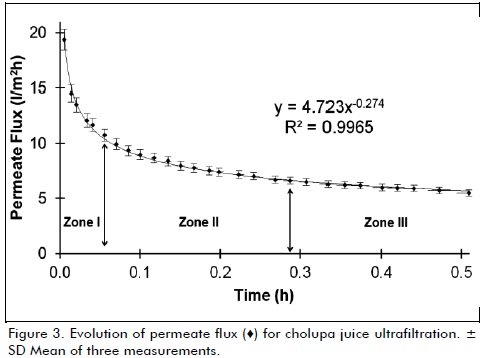
Finally, solutes form a cake, which controls the final stage of filtration, zone III corresponds to stationary permeate flux (6.0 l/m2h), when gel layer thickness reaches its maximum, which is determined by parameters of operation, in very dilute solutions steady flux takes too long to be achieved (Rai et al., 2006), this phenomenon is known as "concentration polarization" where accumulation of particles retained on membrane is controlled by two opposing mechanisms of mass transfer: convection of particles to membrane surface carried by the permeate flow and back diffusion of particles from membrane surface to inside of suspension, at steady state both transport mechanisms are balanced (Yazdanshenas et al., 2010).
Osmotic evaporation
Was reached a concentration of 65.15 ± 0.36 oBrix, after 8 hours evaporation flux is greatly reduced (figure 4), making it technically unfeasible to continue the process beyond these concentrations, for working conditions established; analyzing physicochemical parameters of concentrate juice (table 1), shows that final pH decreased about 12% compared to initial, explained this by increase in the proportion of organic acids, behavior similar to the viscosity and turbidity, all mainly due to strong water removal, color change to dark yellow hues, can be related to final turbidity that exceeded 10000 NTU, which is the limit of equipment used to measure this characteristic, behavior is expected and is mainly due to the large amount of sugars and pigments, finally it was observed that concentrate is completely dissolved in water, presents a homogeneous appearance and pleasant flavor similar to fresh juice (Bailey et al., 2000).
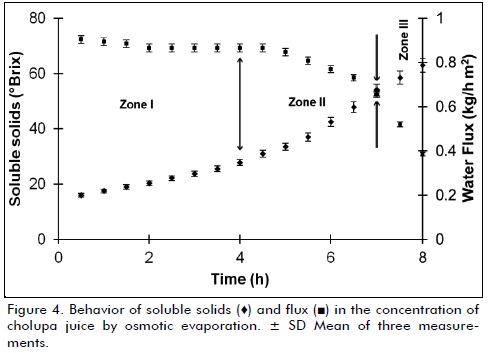
It was observed the presence of three clearly defined areas in osmotic evaporation process (figures 4 and 5), in zone I, evaporation flux remained almost constant (0.87 ± 0.015 kg/m2h) for a period of 5 hours, explained that for two main reasons, first, low fouling caused by juice on membrane because it is ultrafiltered, be almost free of insoluble particles and second is due to low initial viscosity, that allows to achieve higher transfer coefficients, resistance by viscous effects are negligible for this stage (Bui et al., 2006); zone II can be considered a transitional stage, where flux starts a gradual decline period from 0.84 to 0.76 (kg/m2h) over a period of 1.5 hours (Bui et al., 2005).
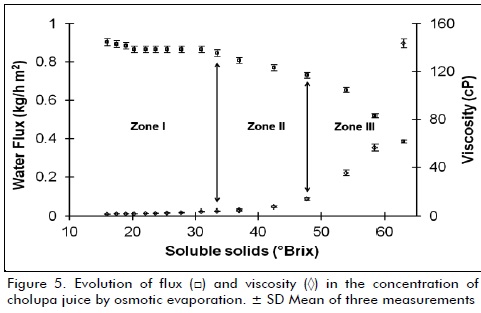
Zone III shows a rapid drop in flux of water removed from 0.73 to 0.38 (kg/m2h) in just 1.5 hours, mainly due to large change in viscosity (13.5 to 143.5 cP) by increased soluble solids, what triggers formation of a boundary layer with high resistance to mass transfer (Martínez-Díez et al., 2000), another effect that occurs simultaneously is decrease in water activity and therefore vapor pressure, generated by saturation of soluble solids, affecting the pressure differential generated between brine and juice, thereby altering equilibrium liquid - vapor in hydrophobic membrane (Romero, 2003a); all these changes, reduce pressure differential, which is driving force of process, as mentioned above.
Fruits are essential in a balanced diet as carriers mainly of carbohydrates, minerals and vitamins; however, the product perishability and hassle of preparation, among others, represent an obstacle for fresh consumption. These problems coupled with lack of time in today's society make fruit juices processed without heat, meet requirements for use in the daily diet, being more comfortable to use, maintain initial organoleptic characteristics, transport cost, long life. The juice concentrate obtained, meets the above requirements, becoming a great alternative raw material for food industries, looking to innovate with exotic flavors and aromas, in areas such as drinks: soda, energizing, children, functional.
Conclusions
Ultrafiltration and osmotic evaporation were shown to be effective and synergistic technologies to obtain a concentrated juice with good physico chemical and sensory quality. The application of enzymatic pretreatment favored the development of ultrafiltration processes, without adversely affecting juice properties; membrane fouling is a very complex phenomenon difficult to describe theoretically, depends on physical and chemical parameters, this produces high energy consumption, increased frequency of cleaning and reduced membrane life time. Cholupa juice could be concentrated, at room temperature (28 oC), by osmotic evaporation to as much as 65.15 ± 0.36 oBrix, with average evaporation flux of 0.38 to 0.87 kg/m2h. A comparative analysis from economic and energy viewpoint is suggested for future work in this area, in order to assess osmotic evaporation competitiveness, because although the proven advantages of this technique, low yields and long process times are the main disadvantaged. These techniques are a great alternative for exotic fruit processing and in which the traditional processes of concentration, significantly degrade physical chemical and organoleptic properties.
References
Alkhudhiri, A., Darwish, N., Hilal, N., Membrane distillation: A comprehensive review., Desalination, Vol. 287, No. 2012, pp. 2-18. [ Links ]
Anderson-Cook, C. M., Borror, C. M., Montgomery, D. C., Response surface design evaluation and comparison., Journal of Statistical Planning and Inference, Vol. 139, No. 2, 2009, pp. 629-641. [ Links ]
Bailey, A. F. G., Barbe, A. M., Hogan, P. A., Johnson, R. A., Sheng, J., The effect of ultrafiltration on the subsequent concentration of grape juice by osmotic distillation., Journal of Membrane Science, Vol. 164, No. 1-2, 2000, pp. 195-204. [ Links ]
Bandini, S., Sarti, G. C., Concentration of must through vacuum membrane distillation., Desalination, Vol. 149, No. 1-3, 2002, pp. 253-259. [ Links ]
Bui, A. V., Nguyen, H. M., Joachim, M., Prediction of water activity of glucose and calcium chloride solutions., Journal of Food Engineering, Vol. 57, No. 3, 2003, pp. 243-248. [ Links ]
Bui, A. V., Nguyen, H. M., Joachim, M., Characterisation of the polarisations in osmotic distillation of glucose solutions in hollow fibre module., Journal of Food Engineering, Vol. 68, No. 3, 2005, pp. 391-402. [ Links ]
Bui, V. A., Nguyen, M. H., The role of operating conditions in osmotic distillation and direct contact membrane distillation - a comparative study., International Journal of Food Engineering, Vol. 2, No. 5, 2006, pp. 1-12. [ Links ]
Cassano, A., Conidi, C., Drioli, E., Physico-chemical parameters of cactus pear (opuntia ficus-indica) juice clarified by microfiltration and ultrafiltration processes., Desalination, Vol. 250, No. 3, 2010, pp. 1101-1104. [ Links ]
Cassano, A., Drioli, E., Concentration of clarified kiwifruit juice by osmotic distillation., Journal of Food Engineering, Vol. 79, No. 4, 2007a, pp. 1397-1404. [ Links ]
Cassano, A., Marchio, M., Drioli, E., Clarification of blood orange juice by ultrafiltration: Analyses of operating parameters, membrane fouling and juice quality., Desalination, Vol. 212, No. 1-3, 2007b, pp. 15-27. [ Links ]
Celere, M., Gostoli, C., Osmotic distillation with propylene glycol, glycerol and glycerol-salt mixtures., Journal of Membrane Science, Vol. 229, No. 1-2, 2004, pp. 159-170. [ Links ]
Celere, M., Gostoli, C., Heat and mass transfer in osmotic distillation with brines, glycerol and glycerol-salt mixtures., Journal of Membrane Science, Vol. 257, No. 1-2, 2005, pp. 99-110. [ Links ]
Cisse, M., Vaillant, F., Perez, A., Dornier, M., Reynes, M., The quality of orange juice processed by coupling crossflow microfiltration and osmotic evaporation., International Journal of Food Science & Technology, Vol. 40, No. 1, 2005, pp. 105-116. [ Links ]
De Barros, S. T. D., Andrade, C. M. G., Mendes, E. S., Peres, L., Study of fouling mechanism in pineapple juice clarification by ultrafiltration., Journal of Membrane Science, Vol. 215, No. 1-2, 2003, pp. 213-224. [ Links ]
De Bruijn, J., Bórquez, R., Analysis of the fouling mechanisms during cross-flow ultrafiltration of apple juice., LWT - Food Science and Technology, Vol. 39, No. 8, 2006, pp. 861-871. [ Links ]
Florez, L. M., Vaillant, F., Hollander, H., Ariza-Nieto, M., Passion fruit juice sacs: Biochemical characterization and enzymatic treatment., Tropical Science, Vol. 43, No. 1, 2003, pp. 28-34. [ Links ]
Forero, F., Velez, C. A., Analysing transfer phenomena in osmotic evaporation., Ingenieria e Investigacion, Vol. 31, No. 3, 2011, pp. 40-49. [ Links ]
Hongvaleerat, C., Cabral, L. M. C., Dornier, M., Reynes, M., Ningsanond, S., Concentration of pineapple juice by osmotic evaporation., Journal of Food Engineering, Vol. 88, No. 4, 2008, pp. 548-552. [ Links ]
Jiao, B., Cassano, A., Drioli, E., Recent advances on membrane processes for the concentration of fruit juices: A review., Journal of Food Engineering, Vol. 63, No. 3, 2004, pp. 303-324. [ Links ]
Kulkarni, S. G., Vijayanand, P., Effect of extraction conditions on the quality characteristics of pectin from passion fruit peel (passiflora edulis f. Flavicarpa l.)., LWT - Food Science and Technology, Vol. 43, No. 7, 2010, pp. 1026-1031. [ Links ]
Lee, W. C., Yusof, S., Hamid, N. S. A., Baharin, B. S., Optimizing conditions for enzymatic clarification of banana juice using response surface methodology (rsm)., Journal of Food Engineering, Vol. 73, No. 1, 2006, pp. 55-63. [ Links ]
Lemos, M. A., Teixeira, J. A., Domingues, M. R. M., Mota, M., Gama, F. M., The enhancement of the cellulolytic activity of cellobiohydrolase i and endoglucanase by the addition of cellulose binding domains derived from trichoderma reesei., Enzyme and Microbial Technology, Vol. 32, No. 1, 2003, pp. 35-40. [ Links ]
Martínez-Díez, L., Florido-Díaz, F. J., Vázquez-González, M. I., Study of polarization phenomena in membrane distillation of aqueous salt solutions., Separation Science and Technology, Vol. 35, No. 10, 2000, pp. 1485 - 1501. [ Links ]
Nii, S., Jebson, R. S., Cussler, E. L., Membrane evaporators., Journal of Membrane Science, Vol. 201, No. 1-2, 2002, pp. 149-159. [ Links ]
Onsekizoglu, P., Bahceci, K. S., Acar, M. J., Clarification and the concentration of apple juice using membrane processes: A comparative quality assessment., Journal of Membrane Science, Vol. 352, No. 1-2, 2010, pp. 160-165. [ Links ]
Ortega, N., de Diego, S., Perez-Mateos, M., Busto, M. D., Kinetic properties and thermal behaviour of polygalacturonase used in fruit juice clarification., Food Chemistry, Vol. 88, No. 2, 2004, pp. 209-217. [ Links ]
Rai, P., Majumdar, G. C., Das Gupta, S., De, S., Effect of various pretreatment methods on permeate flux and quality during ultrafiltration of mosambi juice., Journal of Food Engineering, Vol. 78, No. 2, 2007, pp. 561-568. [ Links ]
Rai, P., Rai, C., Majumdar, G. C., DasGupta, S., De, S., Resistance in series model for ultrafiltration of mosambi (citrus sinensis (l.) osbeck) juice in a stirred continuous mode., Journal of Membrane Science, Vol. 283, No. 1-2, 2006, pp. 116-122. [ Links ]
Ribeiro, D. S., Henrique, S. M. B., Oliveira, L. S., Macedo, G. A., Fleuri, L. F., Enzymes in juice processing: A review., International Journal of Food Science & Technology, Vol. 45, No. 4, 2010, pp. 635-641. [ Links ]
Rodrigues, R. B., Menezes, H. C., Cabral, L. M. C., Dornier, M., Rios, G. M., Reynes, M., Evaluation of reverse osmosis and osmotic evaporation to concentrate camu-camu juice (myrciaria dubia)., Journal of Food Engineering, Vol. 63, No. 1, 2004, pp. 97-102. [ Links ]
Romero, J., Analysis of boundary layer and solute transport in osmotic evaporation., AIChE Journal, Vol. 49, No. 11, 2003a, pp. 2783-2792. [ Links ]
Romero, J., Modeling heat and mass transfer in osmotic evaporation process., AIChE Journal, Vol. 49, No. 2, 2003b, pp. 300-308. [ Links ]
Saha, N. K., Balakrishnan, M., Ulbricht, M., Polymeric membrane fouling in sugarcane juice ultrafiltration: Role of juice polysaccharides., Desalination, Vol. 189, No. 1-3, 2006, pp. 59-70. [ Links ]
Shaw, P. E., Lebrun, M., Dornier, M., Ducamp, M. N., Courel, M., Reynes, M., Evaluation of concentrated orange and passionfruit juices prepared by osmotic evaporation., Lebensmittel-Wissenschaft und-Technologie, Vol. 34, No. 2, 2001, pp. 60-65. [ Links ]
Tasselli, F., Cassano, A., Drioli, E., Ultrafiltration of kiwifruit juice using modified poly(ether ether ketone) hollow fibre membranes., Separation and Purification Technology, Vol. 57, No. 1, 2007, pp. 94-102. [ Links ]
Vaillant, F., Cisse, M., Chaverri, M., Perez, A., Dornier, M., Viquez, F., Dhuique-Mayer, C., Clarification and concentration of melon juice using membrane processes., Innovative Food Science & Emerging Technologies, Vol. 6, No. 2, 2005, pp. 213-220. [ Links ]
Vaillant, F., Jeanton, E., Dornier, M., O'Brien, G. M., Reynes, M., Decloux, M., Concentration of passion fruit juice on an industrial pilot scale using osmotic evaporation., Journal of Food Engineering, Vol. 47, No. 3, 2001a, pp. 195-202. [ Links ]
Vaillant, F., Millan, A., Dornier, M., Decloux, M., Reynes, M., Strategy for economical optimisation of the clarification of pulpy fruit juices using crossflow microfiltration., Journal of Food Engineering, Vol. 48, No. 1, 2001b, pp. 83-90. [ Links ]
Valdés, H., Romero, J., Saavedra, A., Plaza, A., Bubnovich, V., Concentration of noni juice by means of osmotic distillation., Journal of Membrane Science, Vol. 330, No. 1-2, 2009, pp. 205-213. [ Links ]
Vladisavljevic, G. T., Vukosavljevic, P., Bukvic, B., Permeate flux and fouling resistance in ultrafiltration of depectinized apple juice using ceramic membranes., Journal of Food Engineering, Vol. 60, No. 3, 2003, pp. 241-247. [ Links ]
Yazdanshenas, M., Tabatabaee-Nezhad, S. A. R., Soltanieh, M., Roostaazad, R., Khoshfetrat, A. B., Contribution of fouling and gel polarization during ultrafiltration of raw apple juice at industrial scale., Desalination, Vol. 258, No. 1-3, 2010, pp. 194-200. [ Links ]
Yu, J., Lencki, R. W., Effect of enzyme treatments on the fouling behavior of apple juice during microfiltration., Journal of Food Engineering, Vol. 63, No. 4, 2004, pp. 413-423. [ Links ]













