Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Ingeniería e Investigación
Print version ISSN 0120-5609
Ing. Investig. vol.33 no.3 Bogotá Sept./Dec. 2013
A. R. García-Soto1, G. Rodríguez-Niño2 and C. A. Trujillo3
1 Andrés Ricardo García Soto. BSc in Chemical Engineering, Universidad Nacional de Colombia, Bogotá. MSc, Universidad Nacional de Colombia, Bogotá. Research Assistant, Universidad Nacional de Colombia, Colombia. E-mail: argarcias@unal.edu.co
2 Gerardo Rodríguez Niño. Chemical Engineer, Universidad Nacional de Colombia, Bogotá. M. Sc. and Ph. D., Universidad Nacional de Colombia, Colombia. Affiliation: Professor, Universidad Nacional de Colombia, Colombia. E-mail: grodriguezn@unal.edu.co
3 Carlos Alexander Trujillo, BSc Chemistry, Universidad Nacional de Colombia, Bogotá. PhD, Universidad Nacional de Colombia, Bogotá. Professor, Universidad Nacional de Colombia, Colombia. E-mail: catrujillo@unal.edu.co
How to cite: García Soto, A. R., Rodríguez Niño, G., Trujillo, C. A., LTA zeolite synthesis: optimising synthesis conditions by using the modified sequential simplex method., Ingeniería e Investigación, Vol. 33, No. 3, December 2013, pp. 22 - 27.
ABSTRACT
This paper presents research concerning the simultaneous effect of the most influential synthesis variables in the preparation of Zeolite LTA. The simplex method was used for ascertaining optimal synthesis conditions, where the relative crystallinity (regarding a commercial sample) of synthesised zeolite was defined as response function. Crystallinity was measured by X-ray diffraction. Optimum conditions were found after 24 trials, giving 95.4% relative crystallinity and Na2O/SiO2=2.58, SiO2/Al2O3=1.45 and H2O/Na2O=46.92 molar ratios; 11.7 h crystal growth time, 6.1 h crystallisation time (reaction) and 78oC reaction temperature were found to be optimal synthesis conditions. A computer programme for simulating a zeolite phase diagram was designed to verify whether the molar ratios indicated by the optimisation algorithm were leading to the desired zeolite type. The results were compared to those in previous work where the fixed factor method was used.
Keywords: Zeolite LTA, crystallinity, simplex method, optimisation.
RESUMEN
Este artículo presenta un estudio del efecto simultáneo de las variables de síntesis más significativas en la obtención de Zeolita LTA. Se aplicó el método simplex modificado para determinar las condiciones óptimas de síntesis, definiendo como función y objetivo la cristalinidad relativa de la zeolita obtenida y evaluada mediante la difracción de rayos X. Se requirieron 24 ensayos para que el método convergiera de manera óptima, logrando así, una cristalinidad de 95,4 %, obtenida para las relaciones molares Na2O/SiO2=2,58, SiO2/Al2O3=1,45 y H2O/Na2O=46,92, condiciones de operación de 11,7 h de maduración, 6,1 h de cristalización y 78oC de temperatura de reacción. Se diseñó un programa de computador para simular el diagrama de fases zeolíticas, el cual indicaba si las relaciones molares propuestas por el algoritmo de optimización conducían a la zona de obtención de la zeolita deseada. Los resultados obtenidos, aplicando el método simplex, fueron comparados con un trabajo previo en el que se usó el método de una variable fija en el tiempo.
Palabras clave: Zeolita LTA, cristalinidad, método simplex y optimización.
Received: February 11th 2013 Accepted: October 15th 2013
Introduction
Zeolites form part of a wide group of aluminosilicate materials, having a crystal nature and well-defined, homogeneous cavities. Due to their crystal structure, implying uniformity regarding the size of their pores, zeolites are commonly used as molecular sieves. According to the International Zeolite Association, zeolite A, also known as Lynde type A (LTA), has around 4 Å pore size; it is small compared to other types of zeolite.
Many zeolite synthesis studies have been carried out in laboratory conditions (Cundy and Cox, 2003; Pina et al., 2004; Malekpour, 2008), trying to reproduce their natural growth mechanism. Milton et al., (1959), based on previous works (Barrer, 1933), first prepared zeolite A for using it as a dehydrating agent and separation media for light paraffines and gaseous mixtures. The synthesis of this kind of material involves the hydrothermal reaction of silica and alumina gels in a basic medium (Breck, 1974), at 70oC to 100oC, at atmospheric pressure and in excess water (Szostak, 1998).
Zeolite formation mechanisms can be considered as consisting of three stages: maturation (when depolymerisation and reordering of the materials in the gel occurs), nucleation (when crystalline precursors are formed) and crystal growth (Aurbach et al., 2003; Weitkamp and Puppe, 1999; Herrmann, 2005). The chemical representation (the chemical formula) for a cell unit is Na12[(AlO2)12(SiO2)12]·27H2O, implying that Zeolite LTA has 12 tetrahedra in every cell unit, occupied by 12 sodium atoms and 27 water molecules. Typical reaction gel composition is as follows: 3.165 Na2O : Al2O3 : 1.926 SiO2 : 128 H2O (Thompson and Franklin, 2003).
Zeolite LTA synthesis has been studied in the present work, taking into account the effect of the most significant synthesis variables regarding product crystallinity. Based on our results and those available in the literature (Herrmann, 2005; Milton, 1959; Thompson and Franklin, 2003; Tosheva and Valtchev; 2005), it was determined that the following parameters had the strongest influence on zeolite crystallinity: the concentration of silicon dioxide (SiO2), aluminium oxide (Al2O3), sodium oxide (NaO2) and water on the reaction gel, medium pH, maturation time, crystallisation time and temperature. It has been found that shaking speed has had no significant effect on synthesis (Bayati et al., 2008); some studies have thus reported that synthesis can be carried out even without agitation. However, others have indicated that shaking speed can be set between 65 and 85 rpm (Breck, 1974).
A method for the independent evaluation of a variable (while allowing others to be fixed) has been used in previous work; however, the simultaneous effect of synthesis variables is not properly considered in such classical approach. The sequential simplex optimisation method is a simple and efficient method allowing the simultaneous effect of two or more variables on an objective variable to be considered, when no mathematical relationship between them is known (Spendley et al., 1962).
A simplex consists of a geometrical figure having k+1 vertexes, where k is the number of dimensions of a factorial space, representing the number of independent variables in an experiment. The optimisation method is sequential, which means that is only possible to achieve a new stage closer to the optimal, once the response of a previous step is known. Every assay in an experiment represents a set of experimental variables and the worst assay is rejected according to the response of the objective variable. The search-path towards optimal conditions then represents an evolutionary mechanism which always tries to develop a better response than those in previous assays.
The sequential simplex optimisation method proceeds as follows:
- The vertex (assay) having the worst response is reflected over the centroid of the remaining k vertexes (with α=1, reflection coefficient);
- If the new vertex response is better than that obtained in previous vertexes, an expansion of this new vertex is applied (γ=2, expansion coefficient) to extend movement in the direction of reflection;
- If the new vertex response is just better than the worst previous vertex, but not better than that of the remaining vertexes, contraction is applied (0<β<1, contraction coefficient) to reduce step size for the new simplex; and
- Nelder and Mead (Olsson and Nelson, 1975; Zhao et al., 2009; Burmen et al., 2006) have presented the simplex modified method involving reflection, expansion and contraction operations as well as a contraction movement with change of direction (δ= -0,5). The movement is particularly useful when a new response is worse than any previous ones.
These steps led to the formation of a new simplex having a new vertex setting a new essay's experimental conditions. The simplex method has had valuable applications in R&D where multi-parametric operations are involved: chromatography, (Romero, 1997; Bakeas and Siskos, 1996) chemical synthesis (Sener et al., 1996; Bayraktar et al., 2003), spectroscopy (Pergantis et al., 1994; Koch et al., 1997), molecular simulation (Ruiz et al., 1996) and system modelling (Xiong and Jutan, 2003).
This article presents such methodology as used in optimising Zeolite LTA synthesis conditions, taking its relative crystallinity as the objective function.
Experimental procedure
Characterising raw materials
Raw materials were supplied by Productos Químicos Panamericanos de Colombia (PQP):sodium metasilicate (Na2SiO3) and sodium aluminate (NaAlO2), both commercial grade. Deionised water was also used. The raw materials' composition was determined by x-ray fluorescence (XRF).
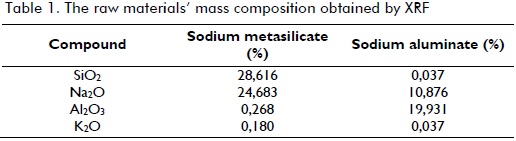
Determining reaction mixture composition
The following nomenclature was used for molar compositions for the reaction gel: x Na2O : Al2O3 : and SiO2 : z H2O, where x, y and z represent molar quantities. A ternary diagram can be used to represent the Na2O - Al2O - SiO2 system where the formulation of a reaction mixture could lead to a specific zeolite type (see Figure 1).
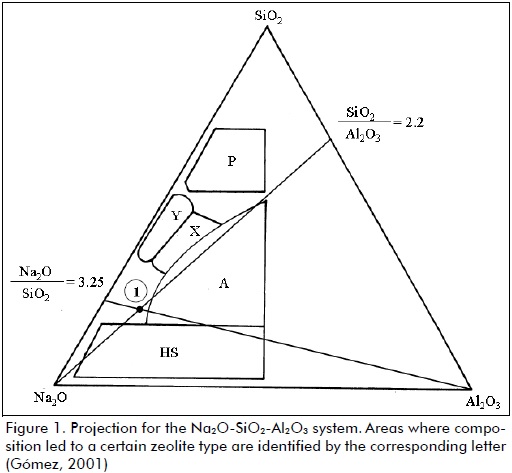
A computer programme was created to simulate the ternary diagram presented in Figure 1. Entering Na2O/SiO2, SiO2/Al2O3 and H2O/Na2O reagent compositions (as shown in Table 1) and molar ratios in the programme enabled determining whether the proposed molar ratios led to the desired zeolite by means of mass balance.
Optimisation problem statement
Product quality (expressed as obtained zeolite crystallinity) was set as objective function. Crystallinity was considered to depend on the following parameters: starting gel composition (Na2O - Al2O - SiO2 - H2O), pH, maturation time (tm), crystallisation time (tc), reaction temperature (Tc) and shaking speed (ω) (Sathupunya et al., 2003; Xu et al., 2005; Pak and Mohammadi, 2006). Mixture composition can be written in terms of molar ratios. Medium pH depends on mixture composition and therefore was not considered as an optimisation variable. Agitation speed was set at a constant 60 rpm. Optimisation could finally be stated as follows:
To maximise:
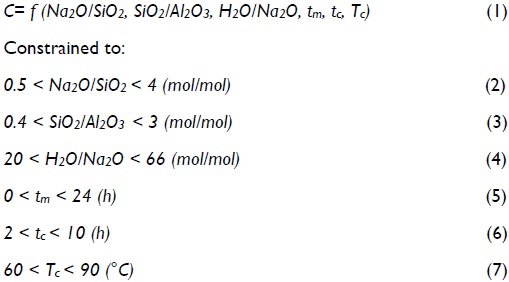
Zeolite LTA synthesis
The synthesis involved using 100 cm3 stainless steel reactors. Calculations were made using the computer programme to load the reactor to 70% total volume with the reaction mixture.
Starting mixture was prepared and subsequent synthesis was carried out as follows: sodium metasilicate and sodium aluminate gels were separately prepared with half the water required by the mass balance. The mixtures were vigorously shaken for 10 minutes at room temperature and then mixed, adding the silicate gel, over the aluminate gel. The final mixture was shaken for 10 minutes again and left at room temperature for the necessary maturation time. The mixture was then loaded on the reactor, which was connected a rotatory axis, allowing the entire reactor to rotate inside an oven at controlled temperature.
After the reactor had been loaded, temperature and rotation speed were set and the synthesis or crystallisation period started. The reactor was then unloaded and rapidly cooled. The suspension was filtered and washed with deionised water until pH between 9 and 10 was obtained. The wet solid was then dried at 110oC for 15 hours. The zeolite dust so obtained was analysed by x-ray diffraction (XRD) to determine relative crystallinity.
The prepared zeolite was characterised using an X'Pert HighScore Plusdiffractometer having the following specifications:
2θ: 0-50 dg range,
λ: CuA1 (1,54056 Å) wave length,
0.02 dg step size, and
2 s time step.
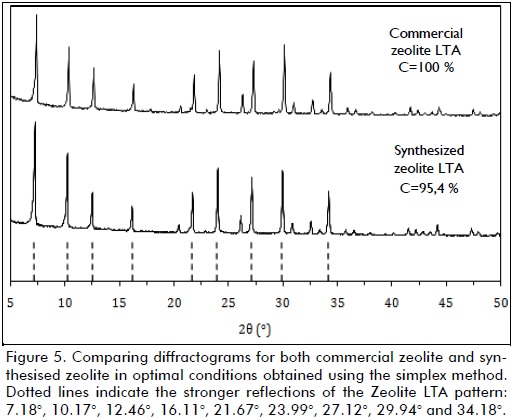
Sample diffractograms were examined and compared to patterns in preloaded databases using DRXWin software (a graphic and analytic computer tool for XRD pattern analysis). Zeolite LTA was found to adequately represent the experimentally found XRD patterns.
Commercial produced Zeolite LTA (AMTEX S.A) was used as reference sample. The samples' crystallinity was determined following ASTM D5357-03 (standard test method for determining zeolite sodium A relative crystallinity by X-ray diffraction).
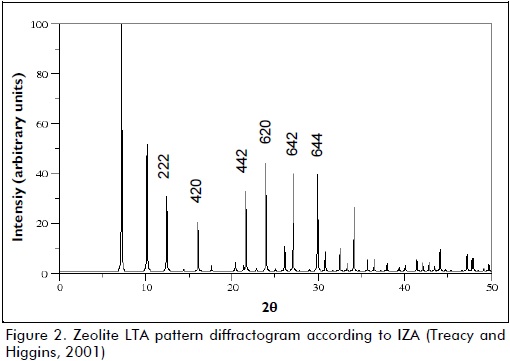
The procedure involved determining the integral intensity of the six peaks shown in Figure 2 for both the experimentally obtained samples and the commercial reference sample. Relative crystallinity percentage (C) was computed as being the ratio between the areas of counts under the selected peaks, thus:

Sxand Srwere added areas under the selected peaks in an experimental sample and in the reference sample, respectively.
Using the optimisation algorithm
An initial simplex had to be set to start the optimisation algorithm; this meant setting the first k+1 assays.
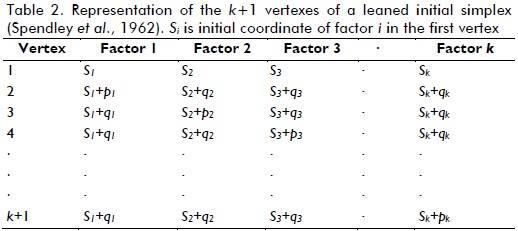
At least three methods for setting the first simplex have been developed (Walters et al., 1991). Spendley et al., (1962) have proposed a leaned initial simplex, where coplanar vertexes (or collinear vertexes for a three vertex simplex) and degeneration of thehyper-tetrahedral figureareavoided. Table 2 shows how that first simplex was set.
where:
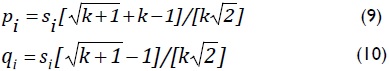
This initial simplex formulation warranted no simplex degeneration because the matrix determinant resulting from factor values from vertex 2 to k+1 would always be different to zero. Mathematically it means that all vertexes would be linearly independent.
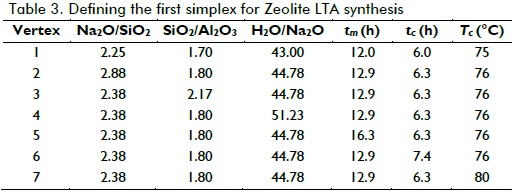
For this work, the first vertex was set as the middle point of the whole factorial space; this meant that the middle point of each factor's range was included in constrains (Eq. (2) to (7)). Moreover, the step size of each variable, si, was chosen as 1/5 the length of each range. Table 3 shows the first simplex, which was obtained by following Eq. (9) and (10).
Results and Discussion
Twenty-four assays were required to attain the optimal point, whereas all parameters converged on their optimal values leading to the highest crystallinity possible in accordance with the given synthesis conditions. Figure 3 shows how synthesised zeolite crystallinity tended towards the optimum during the experiments.
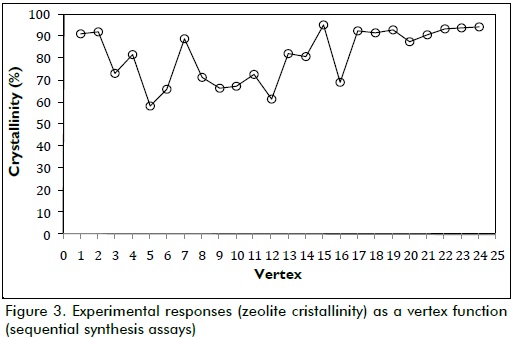
Figure 3 shows that the two suggested starting assays yielded good crystallinity responses, meaning that choosing the midpoint of whole factorial space, like the starting vertex, led the algorithm to a value very near the optimum. The algorithm moved away from the optimum area in vertex 3 and moved to vertex 14, the smallest value being found in vertex 5 (58.5%). If these points were analysed on a response surface, it could be said that the first two assays just reached the surface top, very near the optimum, and then they went down to the bottom, involving an exploratory search by combining synthesis variables.
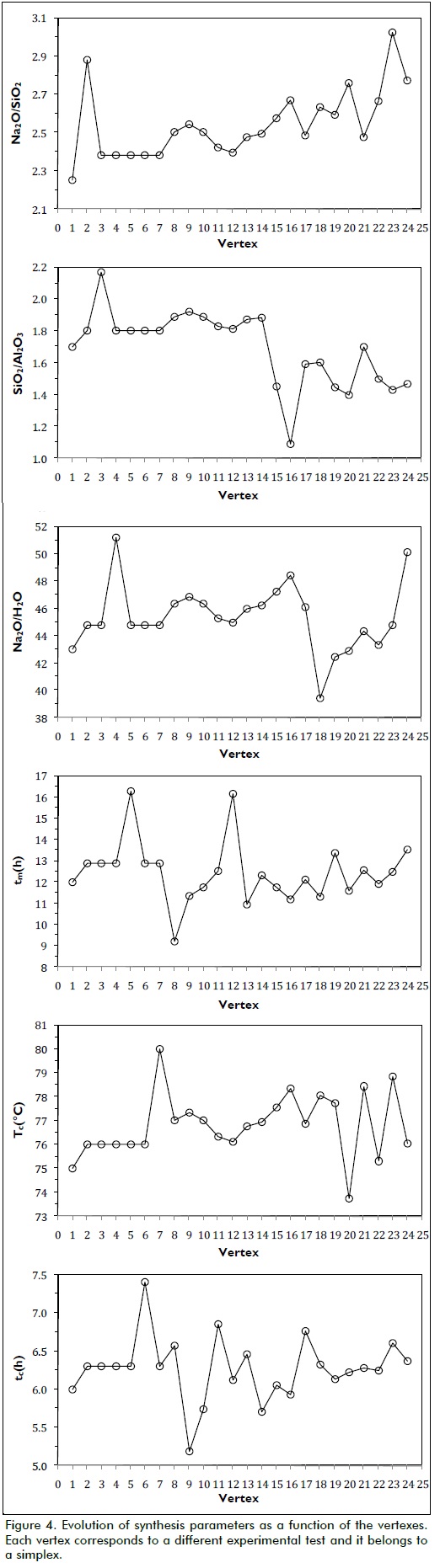
As no satisfactory response was found, the algorithm started to ascend again (from vertex 10 to 14) toward the response surface top, optimal response being obtained at vertex 15, which yielded 95.4% relative crystallinity. The sequential algorithm made a search around the nearest area to the optimum (from vertex 16 to 24, i.e. 9 further experiments) trying to improve the response. As the optimal response in vertex 15 did not increase, the algorithm was stopped and no more experiments were carried out. Vertex 15 was chosen as synthesis optimum. Figure 5 shows the diffractograms obtained for both the commercial Zeolite LTA used as reference and synthesised zeolite in optimal conditions according to vertex 15 parameters.
Regarding the effect of the compositions, Figure 4 shows that the SiO2/Al2O3 ratio was the most dynamic in terms of response evolution. It started taking average values of 1.80 in the first 14 vertexes and then decreased sharply to the corresponding optimal value, which was 1.45, stabilising itself at an average value of 1.50. Regarding zeolite stability, it is known that when the Si/Al ratio is very low, it generates a strong anionic framework inside a structure, allowing a strong interaction with highly polar components such as water. Therefore, by reducing the SiO2/Al2O3 molar ratio in the presence of excess water, it could facilitate the formation of precursor crystal nuclei which grow homogeneously during the later crystallisation step.
Maturation time was found to be significant in the product's crystallinity. Figure 4-d shows that the highest maturation times were 16.3 and 16.1 h for vertexes 5 and 12, respectively. The lowest crystallinity was obtained in both cases; however, acceptable crystallinity values were achieved when this time lasted 12 to 13 h. Regarding crystallisation time, acceptable results were achieved while this lasted 6.0 to 6.5 h, the optimum being reached in 6.1 h.
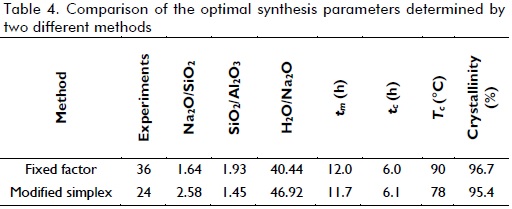
The method's convergence was effective, taking into account that the optimum was reached with just 15 experiments. This was strongly attributed to the values for variables in the initial simplex which, once established, did not undergo drastic changes. There were reasons to believe that the optimum reached was local. First, the algorithm always moved within a limited range of values, considering that the limits set for the variables when formulating the optimisation problem were broader than these. Moreover, the size of each variable pitch (1/5 of the length of the range) proved to be relatively small, which did not allow an examination of the variables in other areas of the response surface where it may have contained other optimal values. Previous studies by our group found other values for the synthesis variables, in some cases differing considerably from those found by the above simplex method.
Zeolite LTA synthesis has been carried out in previous studies, where the fixed factor method was used; Table 4 compares the results obtained by both methods.
Conclusions
This study showed the efficiency of the modified simplex method as being an important optimisation technique for determining the best conditions for Zeolite LTA synthesis in the laboratory. It involved only 24 trials, evaluated at different levels of optimisation variables, for assessing the joint influence of all variables in the search for the optimum. Furthermore, another comparative advantage of the simplex method was observed during target response evolution: if experiments were performed with simplex movements generating unsatisfactory responses, the same algorithm was able to correct itself and return to the right direction towards the optimum, without generating significant change regarding the found optimum.
Just one local optimum was found, perhaps due to the small pitch size for each variable, which led the algorithm to explore a limited area of values within the possible entire search space.
It was very important to design the computer programme to evaluate whether the compositions generated by the optimisation method indeed corresponded to compositions for obtaining Zeolite LTA. Thus, it always guaranteed that the algorithm worked with appropriate composition values, thereby obtaining the desired type of zeolite.
When comparing the modified simplex method to the fixed variable method (i.e. preliminary experiments for this work), the former required only 24 trials, 12 less than the latter, although the optimum had been reached in test number 15, giving 95.4% crystallinity. The simplex method synthesis temperature became significantly reduced from 90oC, suggested by the pertinent literature to be 78oC.
Acknowledgments
The research for this paper was financed by the Administrative Department of Science, Technology and Innovation (Colciencias), in Colombia, through project 110152128366, CT 448-2011.
References
Aurbach, S., Carrado, K., Dutta, P., Handbook of zeolites science and technology., USA, CRC Press, 2003, pp. 91-128. [ Links ]
Bakeas, E. B., Siskos, P. A., A four factor simplex optimization of a serially coupled open tublar columns gas chromatographic system., Journal of High Resolution Chromatography, Vol. 19, No. 5, May., 1996, pp. 277-283. [ Links ]
Bayati, B., Babaluo, A. A., Karimi, R., Hydrothermal synthesis of nanostructure Na-A zeolite: the effect of synthesis parameters on zeolite seed size and crystallinity., Journal of European Ceramic Society, Vol. 28, May., 2008, pp. 2653-2657. [ Links ]
Bayraktar, A. T., Porte, C., Delacroix, A., Moreno, C. M., Catalytic oxidation of sodium sulfite in a gas-liquid tubular ejector: optimization of the operational conditions by simplex method., Chemometrics and Intelligent Laboratory Systems, Vol. 65, No. 1, Jan., 2003, pp. 113-117. [ Links ]
Breck, D., Zeolite molecular sieves: structure, chemistry and use., New York, Wiley, 1974. [ Links ]
Burmen, A., Puhan, J., Tuma, T., Grid restrained Nelder-Mead algorithm., Computational Optimization and Applications, Vol. 34, Mar., 2006, pp. 359-375. [ Links ]
Cundy, C. S., Cox, P. A., The hydrothermal synthesis of zeolites: history and development from the earliest days to the present time., Chemical Reviews, Vol. 103, Feb., 2003, pp. 663-701. [ Links ]
Gómez, J. M., Síntesis, caracterización y aplicaciones catalíticas de zeolitas básicas., a PhD thesis presented at Universidad Complutense de Madrid, Madrid, 2001. [ Links ]
Herrmann, R., Schwieger, W., Scharf, O., Stenzal, C., Toufar, H., Zchmachtl, M., Ziberi, B., Grill, W., In situ diagnostics of zeolite crystalization by ultrasonic monitoring., Microporous and Mesoporous Materials, Vol. 80, Jan., 2005, pp. 1-9. [ Links ]
Koch, I., Harrington, C. F., Reimer, K. J., Cullen, W. R., Simplex optimization of conditions for the determination of antimony in environmental samples by using electrothermal atomic absorption spectrometry., Talanta, Vol. 44, May., 1997, pp. 1241-1251. [ Links ]
Malekpour, A., Millanib, M. R., Kheirkhah, M., Synthesis and characterization of a NaA zeolite membrane and its applications for desalination of radioactive solutions., Desalination, Vol. 225, Sep., 2008, pp. 199-208. [ Links ]
Milton, R. M., Molecular sieve adsorbents., U.S. Patent, 2,882,243., April 1959. [ Links ]
Olsson, D. M., Nelson, L. S., The Nelder-Mead simplex procedure for function minimization., Technometrics, Vol. 17, No. 1, Feb., 1975, pp. 45-51. [ Links ]
Pak, A., Mohammadi, T., Zeolite Na-A membrane synthesis., Desalination, Vol. 200, Nov., 2006, pp. 68-70. [ Links ]
Pergantis, S. A., Cullen, W. R., Wade, A. P., Simplex optimization of conditions for the determination of arsenic in environmental samples by using electrothermal atomic absorption spectrometry., Talanta, Vol. 41, Feb., 1994, pp. 205-209. [ Links ]
Pina, M., Arruebo, M., Fleta, F., Bernal, M., Coronas, J., Menéndez, M., Santamaría, J., A semi-continuous method for the synthesis of Na-A zeolite membranes on tubular supports., Journal of Membrane Science, Vol. 244, Sep., 2004, pp. 141-150. [ Links ]
Romero, E., Optimización del análisis mediante crotomatografía líquida de metabolitos de la planta Uncaria tomentosa (uña degato) utilizando el método simplex secuencial., Tecnología en Marcha, Vol. 18, No. 2, Feb., 1997, pp. 91-94. [ Links ]
Ruiz, M. B., Otto, P., Smeyers, Y. G., The simplex method for geometry optimization in half-projected Hartree-Fock calculations of excited states., Journal of Molecular Structure, Vol. 365, Jun., 1996, pp. 151-165. [ Links ]
Sathupunya, M., Gulari, E., Wonghasemjit, S., Na-A (LTA) zeolite synthesis directly from alumatrane and silatrane by sol-gel microwave techniques., Journal of European Ceramic Society, Vol. 23, Jul., 2003, pp. 1293-1303. [ Links ]
Sener, E., Karagözler, A. E., Pekel, A. T., Simplex optimization of reaction variables in the production of cobalt(III) acetate in a bipolar packed-bed reactor., Analytica Chimica Acta, Vol. 335, Jul., 1996, pp. 35-40. [ Links ]
Spendley, W., Hext, G. R., Himsworth, F. R., Sequential application of simplex designs in optimization and evolutionary operation., Technometrics, Vol. 4, No. 4, Nov., 1962, pp. 441-461. [ Links ]
Szostak, R., Molecular sieves: principles of synthesis and identification., 2nd ed., New York, Van Nostrand Reinhold, 1998. [ Links ]
Thompson, R., Franklin, K., Synthesis of Zeolite LTA., Zeolites, Vol. 11, Apr., 2003, pp. 577. [ Links ]
Tosheva, L., Valtchev, V. P., Nanozeolites: synthesis, crystallization mechanism, and applications., Chemistry of Materials, Vol. 17, May., 2005, pp. 2494-2513. [ Links ]
Treacy, M. M., Higgins, J. B., Collection of simulated XRD Powder Patterns for Zeolites., Published on behalf of the Structure Commission of the International Zeolite Association, Fourth Revised Edition, 2001, Elsevier. [ Links ]
Walters, F., Parker, L., Morgan, S., Deming, S., Sequential Simplex Optimization., Boca Raton, Florida, CRC Press Inc., 1991, pp. 122-131. [ Links ]
Weitkamp, J., Puppe, L., Catalysis and zeolites: fundamental and applications., Berlin, Springer, 1999, pp. 16-23. [ Links ]
Xiong, Q., Jutan, A., Continuous optimization using a dynamic simplex method., Chemical Engineering Science, Vol. 58, 2003, pp. 3817-3828. [ Links ]
Xu, X., Boa, Y., Yang, W., Liu, J., Lin, L., Synthesis, characterization and single gas permeation properties of Na-A zeolite membranes., Journal of Membrane Science, Vol. 249, Mar., 2005, pp. 51-64. [ Links ]













