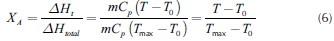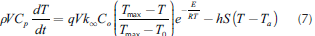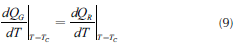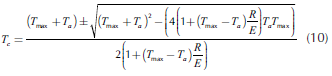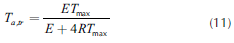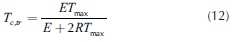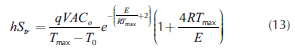Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Ingeniería e Investigación
Print version ISSN 0120-5609
Ing. Investig. vol.35 no.2 Bogotá May/Aug. 2015
https://doi.org/10.15446/ing.investig.v35n2.46258
DOI: http://dx.doi.org/10.15446/ing.investig.v35n2.46258.
Stability criteria and critical runway conditions of propylene glycol manufacture in a continuous stirred tank reactor
Criterios de estabilidad y condiciones críticas de runaway de la producción de propilenglicol en un reactor continuo de tanque agitado
S. M. López-Zamora1, I. Dobrosz-Gómez2, and M. Á. Gómez-García3
1 Sandra Milena López Zamora. Chemical Engineer, Universidad Nacional de Colombia, Manizales, Colombia. M.Sc. in Chemical Engineering. Affiliation: Universidad Nacional de Colombia, Manizales, Colombia. Grupo de Investigación en Procesos Reactivos Intensificados con Separación y Materiales Avanzados (PRISMA). E-mail: smlopezz@unal.edu.co.
2 Isabel Dobrosz Gómez. Chemist, Lodz University of Technology, Lodz, Poland. M. Sc. Eng., Lodz University of Technology, Lodz, Poland. PhD., Lodz University of Technology, Lodz, Poland. Affiliation: Grupo de Investigación en Procesos Reactivos Intensificados con Separación y Materiales Avanzados (PRISMA ) Colombia. E-mail: idobrosz-gomez@unal.edu.co.
3 Miguel Ángel Gómez. Chemical Engineer, Universidad Nacional de Colombia, Manizales, Colombia. M.Sc. in Chemical Engineering, Universidad Nacional de Colombia, Bogotá, Colombia. PhD in Chemical Engineering, Université Louis Fasteur, Strasbourg I, France. Affiliation: Grupo de Investigación en Procesos Reactivos Intensificados con Separación y Materiales Avanzados (PRISMA) Colombia. E-mail: magomez@unal.edu.co.
How to cite: López-Zamora, S. M., Dobrosz-Gómez, I., & Gómez-García, M. Á. (2015). Stability criteria and critical runway conditions of propylene glycol manufacture in a continuous stirred tank reactor. Ingeniería e Investigación, 35(2), 56-60. DOI: http://dx.doi.org/10.15446/ing.investig.v35n2.46258.
ABSTRACT
The present paper presents the development of a new method for the analysis of the steady state and the safety operational conditions of the propylene oxide hydrolysis with excess of water in a Continuous Stirred Tank Reactor (CSTR). At first, for the industrial operational typical values, the generated and removed heat balances were examined. Next, the effect of coolant fluid temperature on the critical ignition and extinction temperatures (TCI and TCE, respectively) was analyzed. The influence of the heat exchange parameter (hS) on coolant and critical temperatures was also studied. Finally, the steady state operation areas were defined. The existence of multiple stable states was recognized when the heat exchange parameter was in the range of 6.636 < hS kJ/(min.K) < 11.125. The Unstable operation area was located between the TC| and TCE values, restricting the reactor operation area to the low stable temperatures.
Keywords: Stability criteria, propylene oxide, propylene glycol, runaway conditions.
RESUMEN
En este trabajo se presenta un nuevo método para el análisis del estado estable y de las condiciones operativas seguras para la hidrólisis del óxido de propileno con exceso de agua en un reactor continuo de tanque agitado (CSTR). Inicialmente, para valores típicos de operación industrial, se examinaron los balances de calor generado y removido. Luego, se analizó el efecto de la temperatura del fluido de enfriamiento en las temperaturas de ignición y extinción (TC| y TCE, respectivamente). También se estudió el efecto del parámetro de transferencia de calor (hS) sobre las temperaturas críticas y del fluido de enfriamiento. Finalmente, se definieron las áreas de operación estables. Se reconoció la existencia de múltiples estados estables cuando el parámetro de transferencia de calor está en el intervalo de 6.636 < hS kJ/(min.K) < 11.125. El área de operación inestable está localizada entre los valores de TCI y TCE, restringiendo la operación del reactor al área de bajas temperaturas estables.
Palabras clave: Criterios de estabilidad, óxido de propileno, propilen glicol, condiciones de runaway.
Received: October 15th 2014 Accepted: March 4th 2015
Introduction
Propylene glycol is an organic compound obtained by the hydrolysis of propylene oxide with excess of water. It is a colorless and odorless liquid. Nowadays it is produced on a large scale, and primarily used in the production of polymers (saturated polyester resins) and in the food processing (E-number E1520). Propylene glycol presents anti-freeze properties and it is used as fluid for heat transfer at low temperatures; as solvent in paints and cleaners; and as an intermediate for the synthesis of different chemical products such as alkyds (The Dow Chemical Company 2011).
The hydrolysis of propylene oxide with water is an exothermic reaction. Under certain conditions, it presents multiple steady states and/or runaway behavior, making the reactive operation potentially risky. For safety, it is very important to determine how stable the steady state is. Different methodologies for the safety operation of reactors and analysis of runaway conditions have been proposed. As a first approximation, thermal runaway prediction was performed by a lineal stability and overall energy balances analysis. It was established that critical conditions depend on the temperature curve. Thus, cooling in a chemical reactor can be controlled by thermal conduction in the reactive mixture (Semenov 1928; Kamenetskii 1969). More recently, the concept of parametric sensitivity analysis was proposed by Bilous & Amundson (1956). They suggested that when the system operates in the parametric sensible area, slight changes in the inlet parameters of the reactor generate excessive changes in the outlet parameters. Thus, different criteria based on the geometric conditions of temperature and conversion curves have been developed. For the Continuous Stirred Tank Reactor (CSTR) systems some examples can be pointed out: (i) Luo et al. (2000) studied the polymerization of phenol-formaldehyde reaction and (ii) Ojeda et al. (2014) studied the cyclo-trimethylene-triamine production process.
In the case of propylene oxide hydrolysis, Švandová et al. (2005) discussed the relation between software tools, primarily designed for mathematical modeling and simulation, and chemical reactors analysis. They identified the Hazard and Operability (HAZOP) zone for the hydrolysis reaction. Additionally, Vadapalli & Seader (2001) employed ASPEN Plus software for the study of multiple steady-states of this reaction, and Molnár et al. (2003) presented the sensitivity analysis of a CSTR with cooling jacket.
In this work, a new method for the analysis of steady state and safety operational conditions of the hydrolysis of propylene oxide with excess of water is proposed. Thus, at first, the generated and removed heat balances are analyzed. Next, the effect of coolant fluid in the critical ignition and extinction temperatures is presented. The influence of the heat exchange parameter (hS) on coolant and critical temperatures is also studied. Finally, the steady state operation areas are defined.
Mathematical model
For the propylene oxide hydrolysis, Fogler (2008) reports a first order kinetic (n=1) with respect to propylene oxide and zero order one with respect to water Equations (1-2).
The overall energy balance in a CSTR implies that the heat generation rate (QG), Equation (3), minus the heat removal rate (QR), Equation (4), equals the heat accumulation (Equation(5)).
If the reactive system operates adiabatically, conversion and reactive temperature, for a time t, can be correlated by the ratio between the transient heat release (AHt) and the overall heat release (AHto l), as shown in Equation (6). Thus, the overall balance can be rewritten as follows (Equation(7)):
Uncontrolled reaction conditions (runaway) can occur when the heat generated in the process is higher than the removed one. In this case, the exothermic system goes through a quick increase in temperature (runaway condition). Semenov (1928) defined the sufficient, necessary, and critical runaway conditions as given by Equations (8) and (9).
Thus, considering steady state, from Equations (7) and (9), the critical temperature can be defined (Equation (10)), while the transition points, Equations (11)-(13), can be obtained by equaling the two roots of Equation (10). In fact, it presents two possible numerical solutions: the low one, which corresponds to the critical ignition temperature and the high one, which is the critical extinction temperature.
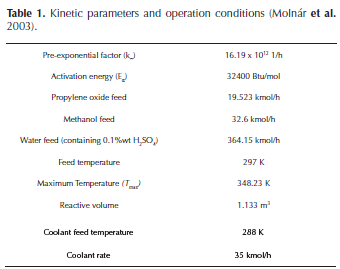
Thus, the analysis of the reactive system stability was performed based on the definitions presented above. Kinetic parameters and typical industrial operation conditions are given in Table 1.
Results and discussion
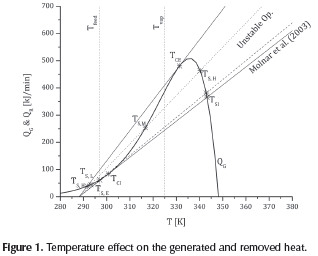
The desirable operation conditions, defined from the transition point, the critical ignition and extinction temperatures, and the generated and removed heat curves are presented in Figure 1. The Figure also includes the overall energy balance expressed as the removed heat (straight lines) and generated heat (Qc), at a coolant temperature of 288.15 K. As a reference, the feed temperature of the reactive mixture (Tfeed) and the vaporization temperature of propylene oxide (Tvap= 324.75 K) were indicated. It is important to notice that operation at temperatures higher than the vaporization one should be avoided.
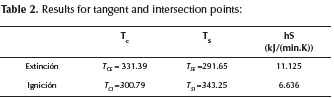
Two tangent and two intersection points (TCE, TCI, TSE, TSI) can be defined from Figure 1. They correspond to the critical hS slope values (Table 2). Thus, unstable operation conditions will correspond to the values 6.636 < hS kJ/ (min.K) < 11.125. If the reactive system operates at these conditions, the existence of multiple steady states can be expected. In this region, three intersection points can be obtained: TS,L, TS,M and TS,H(Figure 1).
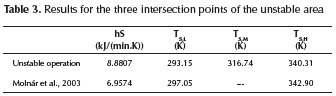
For an intermediate hS value of 8.8807, the intersection points were calculated (dotted line). These values are included in Table 3 and compared with the results reported by Molnár et al. (2003), obtained using a CSTR transient model. From the analysis of the TSMvalue, one can see that a slight change in temperature can take the system into a higher (runaway) or lower operation temperature. Even if the higher operation state implies higher productivity, it is also related to a non-safety operation. Hence, operational values proposed by Molnár etal. (2003) can be considered as stable ones, taking into consideration that: firstly, operation temperature is lower than the ignition critical point (TS,L<TCl) and secondly it is near the intersection ignition point (TS,H∼TSl).
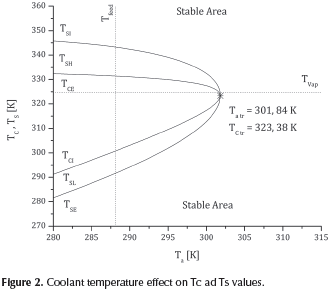
Figure 2 presents the effect of coolant temperature on the intersection and tangent points (TCl, TCE, TSI, TSE). Slight rise in coolant temperature results in an increase in TSE, TSL and TCI and in a decrease in TCE, TSH, and TSI. All lines converge to the transition point (Figure 2). Reactive system should operate in the stable state area without surpassing vaporization temperature.
Thus, different operation areas can be defined:
(i) A stable high temperature area (TSH), limited by the final ignition temperature stable point (TSI) and extinction critical temperature (TCE),
(ii) A stable low temperature area (TSL), restricted by the final stable extinction temperature point and the critical ignition temperature (TCl);
(iii) An unstable area between critical extinction (TCE) and ignition temperatures (TCl); and
(iv) A stable area external to the final extinction (TSE) and ignition temperature points (TSI).
In order to avoid non-safety or non-controlled conditions, the reactive system should operate in the (iv) area.
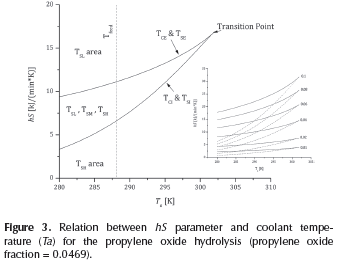
Figure 3 displays the effect of coolant temperature on the slope of removed heat (hS). Maximum hS value corresponds to the transition point (hStr=16.74 kJ/(min.K)). This is a watershed of temperature: it increases as propylene oxide fraction increases (see inserted figure in Figure 3). The value of hS increases as the T increases. When TC>TC,tr,, the value of TC is TCE. If TC<TC,tr, TC is equal to TCl. When TS>TC,tr, the value of TS is TSl. When TS<TC,tr, TS is equal to TSE. Unstable operation area is located between the critical and stable ignition and extinction temperatures. High hS values are recommended in order to assure operation at stable area. Thus, for an analogous parameter, UA, Fogler (2008) (UA = 126.6 kJ/(min.K)) and Molnár et al., (2003) (UA = 240 kJ/(min.K)) guaranteed in their analysis that the removed heat will be higher than the generated one. However, no explanation related to the selection of these values was included. From the results of this study, it is possible to argue that in order to work in the stable low temperature area (TSl), the hS values should be higher than 9.38 kJ/(min.K).
Conclusions
A new method for the analysis of a steady state and safety operational conditions of the hydrolysis of propylene oxide to propylene glycol in a CSTR was developed. Thus, the existence of multiple stable states was recognized when the parameter hS, related to the reactor heat exchange, was in the range of 6.636 < hS kJ/(min.K) < 11.125.
The effect of feed temperature on the critical and stable ignition and extinction temperature locus was also considered and the transition point was calculated. This point separates the stable working area from the unstable one and allows defining different operational conditions. Unstable operation area was located between TCl and TCE, indicating that the operating point should be in the external area and under the vaporization temperature of propylene oxide. It restricts the reactor operation area to the stable low temperatures.
References
Bilous, O. & Amundson, N. R. (1956). Chemical Reactor Stability. AlChE Journal, 2, pp.117-126. DOI: 10.1002/aic.690020124. [ Links ]
Fogler, H.S. (2008). Elementos de ingeniería de las reacciones químicas (4ta Ed.) Pearson Education. [ Links ]
Kamenetskii, F. (1969). Diffusion and Heat Transfer in Chemical Kinetic (2da Ed.). P. Press, ed., New York. [ Links ]
Luo, K. M. et al. (2000). The critical runaway condition and stability criterion in the phenol-formaldehyde reaction. Journal of Loss Prevention in the Process Industries, 13(2), pp.91-108. DOI: 10.1016/S0950-4230(99)00071-6. [ Links ]
Molnár, A., Markos, J. & Jelemensky, L. (2003). Safety analysis of CSTR towards changes in operating conditions. Journal of Loss Prevention in the Process Industries, 16, pp.373380. DOI: 10.1016/S0950-4230(03)00069-X. [ Links ]
Ojeda, J. C. et al. (2014). Análisis de la Sensibilidad Paramétrica del Proceso de Producción de Ciclo-Trimetileno-Triamina. Información tecnológica, 25(4), pp.153-162. DOI: 10.4067/S0718-07642014000400017. [ Links ]
Semenov, N. N. (1928). Zur Theorie des Verbrennungsprozes-ses. Zeitschrift fur Physik, 48(7-8), pp.571-582. DOI: 10.1007/BF01340021. [ Links ]
Švandová, Z. et al. (2005). Steady States Analysis and Dynamic Simulation as a Complement in the Hazop Study of Chemical Reactors. Process Safety and Environmental Protection, 83(5), pp.463-471. DOI: 10.1205/psep.04262. [ Links ]
The Dow Chemical Company. (2011). Hoja de datos técnicos. [ Links ]
Vadapalli, A. & Seader, J. D. (2001). A generalized framework for computing bifurcation diagrams using process simulation programs. Computers & Chemical Engineering, 25, pp.445-464. DOI: 10.1016/S0098-1354(01)00624-X. [ Links ]