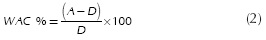Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Ingeniería e Investigación
versão impressa ISSN 0120-5609
Ing. Investig. vol.36 no.1 Bogotá jan./jun. 2016
https://doi.org/10.15446/ing.investig.v36n1.52855
DOI: http://dx.doi.org/10.15446/ing.investig.v36n1.52855
Improvements in the synthesis of zeolites with low Si/Al ratio from Venezuelan sodium silicate for an environmentally friendly process
Mejoras en la síntesis de zeolitas con baja relación Si/Al a partir de un silicato de sodio de Venezuela para un proceso ambientalmente amigable
A. García1, C. M. López2, L. V. García3, J. Casanova4, and M. R. Goldwasser5
1 Adriana L. Garcia López: Chemical Eng. Doctoral student in engineering sciences, Universidad Central de Venezuela (UCV), Venezuela. MSc in Chemical Eng (UCV) Affiliation: Aggregate Professor, Escuela de Ingeniería Química UCV, Venezuela.
Email: adriana.ucv@gmail.com.
2 Carmen M. Lopez. Degree in Chemistry, UCV, Venezuela. Doctor en Ciencias, UCV, MSc in Chemical Eng (UCV) Venezuela. Affiliation: Titular Professor, Escuela de Química, UCV, Venezuela. Email: carmen.lopez@ciens.ucv.ve.
3 Luis García: Chemical Eng, UCV, Venezuela. Doctor en Ciencias, UCV, Venezuela. Affiliation: Professor, Universidad Técnica Particular de Loja, Ecuador.
Email: garciaberfon@gmail.com.
4 Johliny Casanova: Chemical Eng. Universidad Simón Bolívar (USB), MSc in Chemical Eng (USB). Affiliation: Assistant Professor, Escuela de Ingeniería Química UCV, Venezuela.
Email johliny@yahoo.com.
5 Mireya R. Goldwasser. Degree in Chemistry, UCV, Venezuela. PhD. in Chemical Eng. Imperial College, -England. Affiliation: Titular Professor, Escuela de Química, UCV, Venezuela.
E-mail: mireya.goldwasser@ciens.ucv.ve.
How to cite: García, A., López, C. M., García, L. V., Casanova, J., & Goldwasser, M. R. (2016). Improvements in the synthesis of zeolites with low Si/ Al ratio from Venezuelan sodium silicate, for an environmentally friendly process. Ingeniería e Investigación, 36(1), 62-69. DOI: http://dx.doi.org/10.15446/ing.investig.v36n1.52855.
ABSTRACT
LTA and FAU zeolites were successfully synthesized from a Venezuelan sodium silicate solution, by hydrothermal crystallization under autogenous pressure at 100 °C, with 2 - 24 h crystallization times. The synthesized materials were characterized by XRD, BET specific surface area and SEM. A series of synthesis tests were performed to study the influence of the molar composition of the starting mixture over zeolites crystallization. The effect of crystallization time for a particular synthesis mixture composition was studied for both zeolites types. The reuse as alkaline medium of the mother liquor separated during filtration, and the effect of the aging before crystallization were additionally studied. The experimental results are in agreement with the crystallization mechanism proposed for zeolites synthesis in liquid phase. The use of a 2SiO2: Al2O3: 6.Na2O: 240H2O synthesis mixture composition allows obtaining LTA zeolite within 2 h of crystallization. For FAU zeolite, no aging period was needed when starting with a 4SiO2: Al2O3: 6.6Na2O:264H2O composition. It was possible to synthesize both zeolites with high purity and crystallinity and with adequate water adsorption properties.
Keywords: LTA zeolites, FAU zeolites, hydrothermal synthesis, zeolite synthesis, environmentally friendly process.
RESUMEN
Zeolitas LTA y FAU fueron sintetizadas con éxito a partir de una solución de silicato de sodio venezolano, por cristalización hidrotérmica bajo presión autógena a 100 ° C, a tiempos de cristalización de 2- 24 h. Los materiales sintetizados se caracterizaron por DRX, área específica BET y SEM. Se realizaron una serie de pruebas de síntesis para estudiar la influencia de la composición molar de la mezcla de partida sobre la cristalización. Se estudió el efecto del tiempo de cristalización para una composición de mezcla de síntesis particular para ambos tipos zeolitas, la reutilización como medio alcalino del licor madre separado durante la filtración, y el efecto del envejecimiento antes de la cristalización. Los resultados experimentales están de acuerdo con el mecanismo propuesto de cristalización para la síntesis de zeolitas en fase líquida. El uso de una composición de 2SiO2: Al2O3: 6.Na2O: 240H2O en la síntesis permite la obtención de zeolita LTA a partir de 2 h de cristalización. Para la zeolita FAU, no se requirió de período de envejecimiento cuando se parte de la composición 4SiO2: Al2O3: 6.6Na2O: 264H2O. Fue posible sintetizar ambas zeolitas con alta pureza y cristalinidad y propiedades adecuadas de adsorción de agua.
Palabras clave: Zeolita LTA, zeolita FAU, síntesis hidrotérmica, síntesis de zeolitas, proceso ambientalmente amigable.
Received: June 28th 2015 Accepted: February 26th 2016
Introduction
Zeolites were originally defined as microporous crystalline aluminosilicates, composed of TO4 tetrahedral (T = Si, Al) with an atomic ratio O: (Al + Si) = 2 The presence of Al3+ induces a negatively charged framework requiring the presence of extra-framework cations within the structure for electro neutrality. The extra-framework cations are exchangeable giving rise to the rich ion-exchange chemistry of these materials, which together with cations nature highly influence the adsorption properties of zeolites.
Natural zeolites are found as widespread components in basic volcanic rocks; however, contamination with others minerals is frequent (Gottardi and Galli, 1985) which can limit its application in adsorption processes or catalytic reactions. Zeolite synthesis at laboratory level - give rise to formation of pure phases, allowing a wide variety of structures, including some not known to occur naturally. An extensive information concerning zeolites can be found in open literature (Breck, 1974; Martinez and Corma, 2014).
The hydrothermal synthesis of aluminosilicate zeolites involves several elementary steps by which a mixture of Si and Al species, metal cations, organic molecules and water, are converted via an alkaline supersaturated solution into a microporous crystalline aluminosilicate. Besides the complexity of the crystallization mechanism, meta-stability of zeolites is a factor that could contribute to create some uncertainty on the synthesized product. The chemistry of the initial mixture, mainly the nature of initial precursors and their molar ratios, are of considerable importance. Therefore, the use of very specific precursor materials is required to achieve formation of a certain zeolite phase (Zaarour et al., 2014). Zeolites are extensively used as adsorbents, catalysts and ion-exchange agents; those particularly aluminum rich, such as A and X zeolites, have been widely used in natural gas drying and CO2 and sulfur removal (Velu et al., 2002).
Several authors have reported the synthesis of Linde Type-A (LTA) and Faujasite type (FAU) zeolites by using different silicon and aluminum sources at temperatures between 70 and 120 °C, from aluminosilicate gels with several compositions. The range of synthesis mixture compositions used is as follows: SiO2 /Al2O3 = 2 - 5, H2O / Na2O = 10 - 100 and Na2O /SiO2 = 1.2 - 16 (Pak and Mohammadi, 2006; Bayati et al., 2008, Jacas et al., 2012; Xing-Dong et al., 2013; Ge et al., 2012) for LTA synthesis and SiO2 /Al2O3 = 2 and 20, with Na2O / SiO2 = 1 - 4 and H2O / Na2O = 20 - 70 for FAU synthesis (Valtchev et al., 2007; Xiong et al., 2001; Yang et al., 2001; Li et al., 2002).
Venezuela's natural gas reserves have been estimated at 197.089 MMMCF in 2013 (197,089,000,000 cubic feet) (PDVSA, 2013). Removal of the water associated with natural gas will reduce potential corrosion, hydrate formation and freezing in pipelines. Molecular sieves conformed by synthetic zeolites such as LTA and FAU, exhibit adequate properties for removal of water and impurities present in natural gas streams (Gandhidasan et al., 2001). The Venezuelan trade of imported zeolites represents a remarkable amount, generating a high cost. Therefore, in Venezuela, zeolites syntheses as water adsorbents for natural gas dehydration is a subject of particular relevance, especially if the zeolites are synthesized from national raw materials.
The present work deals with the synthesis of LTA and FAU type zeolites by the hydrothermal synthesis method, using a Venezuelan produced commercial sodium silicate. The effect of varying the composition of the starting mixture and the crystallization time was studied. Additionally, the influence of reusing the mother liquor separated from filtration as an alkaline medium for the synthesis of LTA zeolite, and the influence of the aging time before crystallization of FAU zeolites, are also discussed.
Experimental
The following starting materials were used for the synthesis: sodium silicate solution 27 wt % SiO2, 11 wt % Na2O and 62 wt % H2O (from GLASSVEN); caustic soda 50 wt % NaOH (from INDUCHEM); aluminum hydroxide 56 wt % Al2O3 (from Aldrich), and distilled water.
An aqueous sodium aluminate solution was firstly prepared by dissolving the aluminum hydroxide with a sodium hydroxide solution under heating. The composition of the resultant sodium aluminate solution was 6.5 wt % Al2O3; 18.5 wt % Na2O and 75 wt % H2O. This solution was then added with continuous stirring to a sodium silicate solution until a homogeneous gel was obtained. In the case of FAU zeolite, prior to crystallization, the synthesis mixture was subjected to 24 h aging period at room temperature. The formed gel was then transferred to covered plastic containers without agitation and heated at 100 °C for a specific crystallization time. The solids were recovered by iltration, washed with distilled water and dried at 100 °C overnight. In some LTA zeolites synthesis, the mother liquor separated from filtration of the synthesized zeolite was used as alkaline solution for subsequent syntheses.
X-ray diffraction (XRD) analyses were performed with a Brucker diffractometer D-8 using Cu ka radiation (X = 1.54 A) operated at 40 kV, 50 mA and scanning speed of 2 ° 29 / min. Crystallinity (% Cryst) was calculated according to Equation (1), taking diffraction lines between 20 ° and 40 ° 29 angles. 100 % crystallinity was assigned to the sample with the highest summary of intensities of chosen lines.
The BET specific surface area (BETSA) expressed as m2g-1of the samples was obtained with a Micromeritics Tristar 3000 at a liquid nitrogen temperature, using the BET equation. All samples were pre-treated overnight at 250 °C under vacuum. Scanning electron micrographs (SEM) were taken on a Hitachi S - 2400 microscope operated at 40 keV and 50 mA. Samples were Pt-coated before analyses on an Eiko Engineering Instrument. Local crystal composition was determined by electron probe microanalysis on an EDX detector Kevex 7000 System.
In order to determine the water adsorption capacity (WAC %), an amount of the original sample (weight A) was heated in a furnace at 250 °C for 6h to obtain the dry sample weight (weight "D"). The mass difference between original and dry sample (A-D) represents the amount of adsorbed water. Then, WAC % was expressed as grams of adsorbed water by 100 grams of dry zeolite, and calculated according to Equation (2).
Results and Discussion
Effect of the synthesis mixture composition
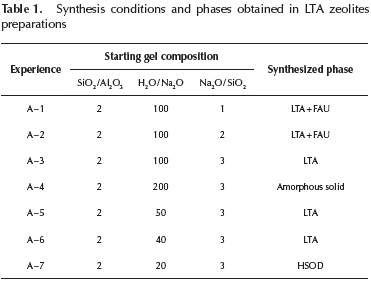
LTA type zeolite: Composition of the synthesis mixtures used for LTA zeolites preparation is indicated in Table 1. Since the Si/Al molar ratio of LTA zeolite framework is equal to 1, the SiO2 / Al2O3 molar ratio in the starting mixture and the crystallization time were set as 2 and 24 h respectively. Final phases determined by XRD analyses are also shown in Table 1.
Synthesis conditions reported by Bayati et al. (2008) were taken as reference for A - 1 sample preparation (Table 1). However, the XRD pattern of this sample indicates the presence of a mixture of LTA and FAU phases, as shown in Figure 1a. An increase in Na2O / SiO2 ratio up to 2, produces a decrease in the intensity of diffraction lines characteristics of FAU zeolites (sample A - 2, Figure 1b). An additional increase in Na2O / SiO2 to 3 produces a pure LTA phase, with diffraction lines characteristics of this zeolite (Figure 1c).
The above results indicate that LTA phase is favored in higher alkaline media, such as that generated when using an important diluted synthesis mixture (H2O / Na2O = 100). However, a highly diluted synthesis mixture, H2O / Na2O = 200, leads to formation of an amorphous solid (sample A - 4, XRD pattern not shown), indicating a delay in the zeolites crystallization.
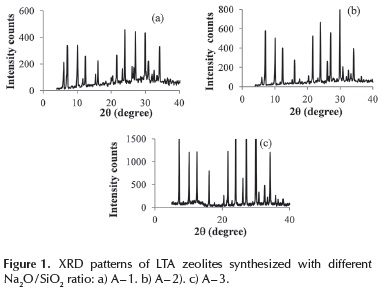
Samples A - 5, A - 6 and A - 7 were prepared to evaluate the effect of decreasing dilution of the synthesis mixture. LTA diffraction lines were only observed for A - 5 and A - 6 (Figures 2a and 2b). A reduction of H2O / Na2O ratio to 20, leads to the formation of hydroxysodalite (sample A - 7, Figure 2c). It is worth noting that the hydroxysodalite has applications as membranes for separation of H2 and CH4 mixtures and for alcohols dehydration (Nabavi et al., 2014; Khajavi et al., 2010).
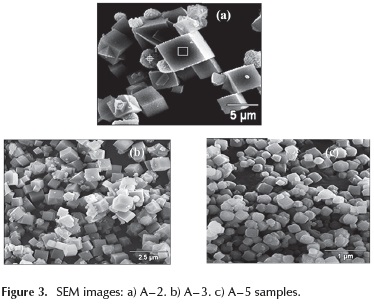
SEM micrographs were performed to A - 2, A - 3 and A - 5 in order to identify the effect of the molar composition over the crystallite size and morphology. As shown in Figure 3, all samples exhibited cubic crystallite aggregates, characteristic of LTA zeolite. For A - 1 and A - 2 samples, cubic crystallite aggregates of similar sizes (∼5|μm) (A - 1 not shown) were observed, with well-defined edges surrounded by smaller crystallite agglomerates probably of FAU type zeolite. According to the XRD pattern, only a minor proportion of these crystallites were present on the A - 2 sample. On the A - 3 sample, smaller cubic crystal aggregates (∼1 μm) are formed, due to the higher Na2O / SiO2 ratio employed in the synthesis mixture.
Due to its higher alkalinity, this mixtures accelerates the nucleation rate increasing production of smaller crystallite aggregates. A - 5 and A - 6 (not shown) samples show the smaller crystallite size aggregates (∼0.5|μm); these samples were synthesized using a smaller H2O / Na2O ratio and the same Na2O / SiO2 ratio, compared to that of the A - 3 sample. The effect on the size of crystallite aggregates by decreasing H2O / Na2O ratio is similar though less noticeable compared to the decrease observed with variations in Na2O / SiO2 ratio.This suggests that the nucleation rate is more influenced by changes in Na2O / SiO2 ratio. Moreover, the cubic crystallites in the last three samples presented beveled edges. For all samples, the Si/ Al ratio as determined by EDX analyses was close to 1.0.
The most accepted mechanism regarding zeolites crystallization establishes that the nuclei of zeolite crystals begin to form in the gels liquid phase or at the interface of gel phases. The solid and liquid phases of aluminosilicate gels are connected by the solubility equilibrium. Increasing solubility leads to an increase of ions concentration in the liquid phase, such as silicates, aluminates or aluminosilicates.
As a result, the probability of condensation reactions between ions increases, producing a raise in the crystal nuclei formation (Cundy & Cox, 2005). T. Wakihara et al. (2004) reported the use of atomic force microscopy, in order to study the mechanism of faujasite crystal growth. The authors provide evidence for the liquid phase mechanism, since their results show that the crystallization proceeds by mutual transference of aluminosilicate species between the solution and the solid phase.
The results of the experiments carried out for LTA zeolite seem to agree with the mechanism described in the preceding paragraph. An increase in the media alkalinity increases the ions concentration leading to the formation of LTA zeolite, thereby promoting nucleation and growth of crystals of this zeolite. Similarly, the highest dilution of the synthesis mixture decreases the concentration of ionic species in the liquid phase with a decrease in the crystallization rate. At lower water contents in the synthesis mixture, and thus greater concentration of ionic species in the liquid phase, a similar effect to that obtained at higher alkalinity was observed, favoring formation of LTA zeolite. Reduction of H2O / Na2O ratio to 20, changes not only the concentration but also the nature of the present species leading to formation of hydroxysodalite.
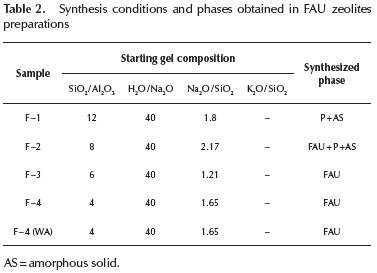
FAU type zeolite: Table 2 shows the composition of the reactants mixture for FAU zeolites synthesis. The freshly prepared mixture of F - 1 to F - 3 experiments were aged at room temperature for 24 h, while that of F - 4(WA) experiment was carried out without previous aging before hydrothermal treatment at 100 °C for 18 h.
In faujasite synthesis experiments, the mixture molar ratios were limited by the initial compositions of the silicate and aluminate solutions. The relatively high Na2O content of these solutions due to decreasing SiO2 /Al2O3 to values lower than 4, produces Na2O / SiO2 ratios higher than 2, giving rise to formation of LTA zeolite (experiments not shown).
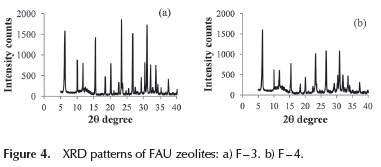
In this series of experiments, the sample prepared with the highest Si/Al ratio, showed an XRD pattern characteristic of P zeolite (XRD pattern not shown). Decreasing values of SiO2 /Al2O3 < 8, produces a solid with XRD lines characteristics of FAU and P zeolites (sample F - 2, XRD not shown). Solids prepared with SiO2 / Al2O3 ratio of 6 and 4 showed a typical XRD pattern of FAU structure with high intensity lines free of the presence of other extra lines, indicating good purity and crystallinity (samples F - 3 and F - 4, Figure 4a and 4b).
It is worth mentioning that the tested compositions for the synthesized zeolites were taken from successful preparations reported in scientiic literature. However, only with two synthesis mixture compositions was it possible to obtain FAU zeolite. This result is ascribed to differences in the starting silicon source. It was possible to obtain the LTA zeolite in a wider range of mixture compositions maintaining SiO2 / Al2O3 = 2; with Na2O / SiO2 = 1- 3 and H2O / Na2O = 100 - 40. In this case, the higher alkalinity of the medium can be suitable to avoid the silicate polymerization due to the lower SiO2 / Al2O3 ratio employed.
Effect of aging time in the synthesis of FAU zeolite
Most used procedures for FAU zeolites synthesis recommend aging the synthesis mixture at room temperature before zeolite crystallization in order to obtain faujasites with high purity and crystallinity (Ginter et al., 1992; Masoudian et al., 2013).
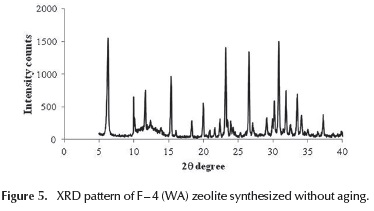
In order to evaluate the effect of aging period at room temperature, the F - 4(WA) sample was synthesized without aging (WA) before crystallization.
This solid showed a typical faujasite XRD pattern, quite similar to that obtained for F - 4 (Figure 5). This result is important due to the fact that it significantly reduces the time of the zeolite preparation. This aspect becomes more interesting from the perspective of commercial zeolite production.
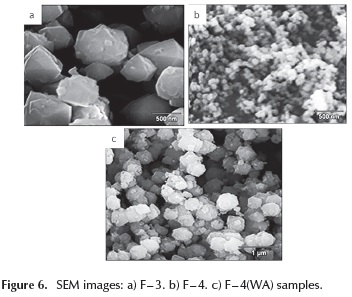
Figure 6 shows SEM micrographs of F - 3, F - 4 and F - 4(WA) samples. The typical morphology of octahedral crystals was observed for all samples, with differences in the crystallite size of aggregates. In Figure 6, it can be seen that F - 3 crystallite aggregates are larger compared to those of F - 4 sample. In the later sample, the rate of nucleation of the crystals must be bigger due to the higher Na2O / SiO2 ratio in the synthesis mixture. Moreover, the larger size of crystallite aggregates for F - 4 (WA) sample compared to F - 4 sample, suggests that the aging time also favors the nucleation rate of faujasite crystals.
Effect of using the mother liquor separated from the filtration of LTA zeolite synthesis.
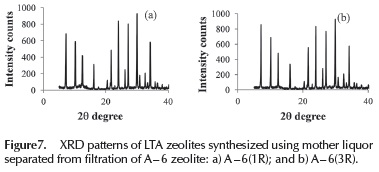
A synthesis experiment was carried out using the mother liquor separated from filtration of the A - 6 sample, as alkaline solution for dissolving the aluminum hydroxide and subsequent formation of the aluminate solution. The first obtained sample was named A6 - 1R. This procedure was then repeated twice to produce A6 - 2R and A6 - 3R samples, using mother liquors of A6 - 1R and A6 - 2R synthesis respectively. According to the XRD patterns shown in Figure 7, a zeolite with high purity was obtained for all above preparations. Zeolite crystals may be present into mother liquor separated from filtration, acting as seeding, favoring the nucleation and crystallization of LTA zeolite.
It is worth noting that with the use of mother liquor, it was possible to reduce the amounts of sodium hydroxide and water used in the synthesis. Besides, it represents a way of consuming the volume of solutions generated in zeolites syntheses. This synthesis strategy is considered environmentally gentle (Ng et al., 2013).
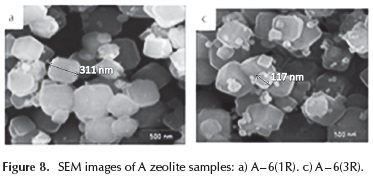
Figure 8 shows - SEM micrographs of A6 - 1R, and A6 - 3R samples. The morphology and size of the observed crystal aggregates are similar in these samples and comparable to those of the A-6 sample prepared from the original solutions. In all cases, cubic crystals with beveled edges were obtained. However, it is worth mentioning that for A6-2R and A6 - 3R samples, very small zeolite crystallites were obtained, probably caused by the effect of dissolution of zeolite crystals seeding dissolved in the mother liquor.
Effect of crystallization time
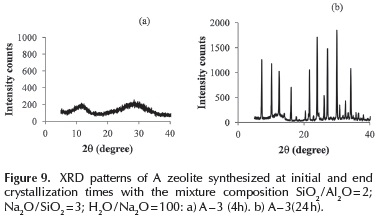
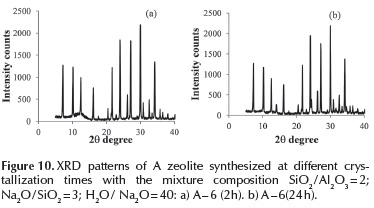
LTA type zeolite: To investigate the effect of crystallization time, a series of experiments were performed by varying the crystallization time, using gel compositions corresponding to A - 3 and A - 6 samples. The XRD patterns of solids, initially and at the end of the crystallization, are shown in Figures 9 and 10. Under the synthesis conditions of the A - 3 sample, LTA zeolite crystallization starts about 4 - 8 h. The intensity of XRD lines does not vary appreciably after 16 h for similar crystallinity samples of 100 %. Experiments performed under A - 6 sample conditions, with a lower dilution (H2O / Na2O = 40), LTA zeolite crystallized within 2 h with only insignificant changes in the intensity of XRD lines after longer times (Cristallinity 100 %).
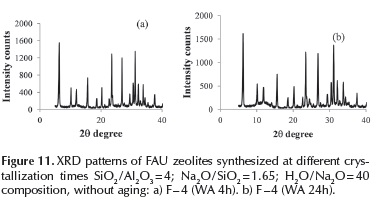
FAU type zeolite: The effect of crystallization time was investigated for F4 (WA) synthesis, without aging. XRD patterns corresponding to samples obtained after 4 and 24 h, (Figure 11) corroborate FAU zeolite synthesis for all times, without signiicant variation in the intensities of the diffraction lines.
SEM micrographs of 4 and 24 h samples are shown in Figure 12. These results are very interesting from an industrial point of view due to the fact that zeolite preparation time was significantly since aging was not necessary at room temperature and a pure and crystalline zeolite was obtained after 4 h crystallization.
Samples characterization
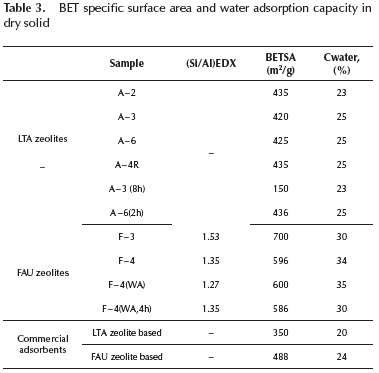
Table 3 shows BET specific surface areas and WCA % values, for some of the synthesized samples. For FAU zeolites, the Si/Al ratio from EDX analysis is also presented. For comparison, the corresponding values for two commercial adsorbents, based on A and X zeolites, are also included.
BET specific surface area and water adsorption capacity values are similar to those previously reported (Corona et al., 2009; Zhang et al., 2013), being higher for synthesized samples compared to commercial adsorbents, attributed to the fact that the later ones contain, besides the zeolite, a certain proportion of binder required to confer the materials final shape.
Conclusions
A series of LTA zeolites were synthesized from a Venezuelan sodium silicate product with a SiO2 /Al2O3 = 2, Na2O/SiO2 = 3 - 1 and H2O / SiO2 = 40 - 100 synthesis mixture, at 100 °C, 2 - 24 h. It was observed that the crystallization rate increases with an increase in alkalinity and a decrease in dilution of the initial mixture, in agreement with the proposed mechanism for liquid phase zeolites crystallization.
Reuse of the mother liquor separated from the zeolite filtration, involves the possibility of better utilization of the large volumes of solution associated to zeolites synthesis, besides economy in NaOH use.
Morphology of LTA zeolites was dependent on the synthesis mixture composition for lower alkalinity and higher dilution, whereas compositions of higher alkalinity and lower dilution allow preparation of cubic crystals with beveled edges.
Synthetic faujasites were obtained from national sodium silicate, in a narrow synthesis mixture composition such as SiO2/Al2O3 = 6 - 4; Na2O / SiO2 = 1.21- 1.65 and H2O / Na2O = 40. It was possible to obtain FAU zeolite without aging period and with low crystallization time (4 h). This result is interesting from a commercial point of view, since it reduces the total time of zeolite preparation. Differences in size of zeolite crystal aggregates were observed depending on synthesis conditions. However, these differences do not affect the zeolite water adsorption capacity, which is higher compared to that of commercial adsorbents used as references.
It has been shown that LTA and FAU zeolites could be successfully synthesized from a Venezuelan sodium silicate, indicating that the use of a national silicon source is suitable for the manufacture of commercial adsorbents, which can be used in gas drying processes such as natural gas dehydration.
Acknowledgments
The authors acknowledge the financial support of the Venezuelan "Fondo Nacional de Ciencia, Tecnología e Innovación" (PEII 2012000165) and "Consejo de Desarrollo Científico y Humanístico, Universidad Central de Venezuela" (AIA 08 - 8490 -2012 and AIA 03 - 8475 -2012), and of the Glassven and Induchem companies for samples of starting materials provided for the synthesis.
References
Bayati B., Babaluo A., Karimi R. (2008). Hydrothermal synthesis of nanostructure NaA zeolite: The effect of synthesis parameters on zeolite seed size and crystallinity. Journal of the European Ceramic Society, 28 (14) 2653-2657. DOI: 10.1016/j.jeurceramsoc.2008.03.033. [ Links ]
Breck D. (1974). Zeolite Molecular Sieves: structure, chemistry, and use New York: Wiley. [ Links ]
Corona O., Hernández M, Hernández F., Rojas F., Portillo R., Lara V., Carlos F. (2009). Propiedades de adsorción en zeo-litas con anillos de 8 miembros I. Microporosidad y superficie externa. Revista Materia, 14, (3) 918-931. DOI: 10.1590/S1517-70762009000300004. [ Links ]
Cundy C., Cox P. (2005). The hydrothermal synthesis of zeolites: Precursors, intermediates and reaction mechanism. Microporous and Mesoporous Materials. 82 (1-2) 1-78. DOI: 10.1002/chin.200537220. [ Links ]
Gandhidasan P., Al-Farayedhi A., Al-Mubarak A. (2001). Dehydration of natural gas using solid desiccants. Energy, 26 (9) 855-868. DOI: 10.1016/S0360-5442(01)00034-2. [ Links ]
Ge Q., Shao J., Wang Z., Yan Y. (2012). Effects of the synthesis hydrogel on the formation of zeolite LTA. Microporous and Mesoporous Materials, 151, 303-310. DOI: 10.1016/j.micromeso.2011.10.019. [ Links ]
Ginter D., Bell A., Radke C. (1992). The effects of gel aging on the synthesis of NaY zeolite from colloidal silica. Zeolites, 12 (6) 742-749. DOI: 10.1016/0144-2449(92)90125-9. [ Links ]
Gottardi G. and Galli E. (1985). Natural Zeolites. Minerals and Rocks. Berlin: Heidelberg Springer Verlag. DOI: 10.1007/978-3-642-46518-5. [ Links ]
Jacas A., Ortega P., Velasco M,, Camblor M,, Rodríguez M. (2012). Síntesis de zeolita LTA sobre soportes de corindón: Evaluación preliminar para la eliminación de metales pesados. Boletín de la Sociedad Española de Cerámica y Vidrio, 51(5) 249-254. DOI: 10.3989/cyv.352012. [ Links ]
Khajavi S., Sartipi S., Gascon J., Jansen J., Kapteijn F. (2010), Thermostability of hydroxy sodalite in view of membrane applications. Microporous and Mesoporous Materilas, 132 (3) 510-517. DOI: 10.1016/j.micromeso.2010.03.035. [ Links ]
Li S., Tuan V,, Falconer J., Noble R. (2002). X-type zeolite membranes: preparation characterization and pervaporation performance. Microporous and Mesoporous Materilas, 53 (1-3) 59-70. DOI: 10.1016/S1387-1811(02)00324-4. [ Links ]
Martínez C, Corma A. (2013). Zeolites. Reference Module in Chemistry, Molecular Science and Chemical Engineering, Comprehensive Inorganic Chemistry II. Vol 5: Porous Material and Nano materials, 103-131. DOI: 10.1016/b978-0-08-097774-4.00506-4. [ Links ]
Masoudian S., Sadighi S., Abbasi A. (2013). Synthesis and characterization of high aluminum zeolite X from technical grade materials. Bulletin of Chemical Reaction Engineering & Catalysis, 8 (1) 54-60. DOI: 10.9767/bcrec.8.1.4321.54-60. [ Links ]
Nabavi M., Mohammadi T., Kazemimoghadam M. (2014). Hydrothermal synthesis of hydroxy sodalite membranes. Ceramics International, 40 (4) 5889-5896. DOI: 10.1016/j.ceramint.2013.11.033. [ Links ]
Ng, E. Zou, X. Mintova S and S. Suib. (2013). New and Future Developments in Catalysis. Hybrid Materials, Composites and Organocatalysts, Amsterdam : Elsevier. DOI: 10.1016/B978-0-444-53876-5.00013-1. [ Links ]
Pak A., Mohammadi T. (2006). Zeolite NaA membranes synthesis. Desalination, 200 (1-3) 68-70. DOI: 10.1016/j.desal.2006.03.245. [ Links ]
PDVSA. (2013) Annual management report. Retrieved from: http://www.pdvsa.com/interface.sp/database/fichero/free/8978/1644.PDF Consulted:07/02/2015. [ Links ]
Valtchev V., Rigolet S., Bozhilov K. (2007). Gel evolution in a FAU-type zeolite yielding system at 90 °C. Microporous and Mesoporous Materials, 101 (1-2) 73-82. DOI: 10.1016/j.micromeso.2006.10.016. [ Links ]
Velu S., Ma X., Song C. (2002). Zeolite based adsorbents for desulfurization of jet fuel by selective adsorption. Fuel Chemistry Division Preprints 47 (2) 447-448. [ Links ]
Wakihara T., Sugiyama A., Okubo T. (2004). Crystal growth of faujasite observed by atomic force microscopy. Micropo-rous and Mesoporous Materials, 70, (1-3) 7-13. DOI: 10.1016/j.micromeso.2004.02.016. [ Links ]
Xing-dong L., Yi-pin W., Xue-min C., Yan H., Jin M. (2013). Influence of synthesis parameters on NaA zeolite crystals. Powder Technology. 243, 184-193. DOI: 10.1016/j.pow-tec.2013.03.048. [ Links ]
Xiong G., Yu Y., Feng Z., Xin Q., Xiao F., Li C. (2001). UV Raman Spectroscopic study on the synthesis mechanism of zeolite X. Microporous and Mesoporous Materials 42 (2-3), 317-323. DOI: 10.1016/S1387-1811(00)00340-1. [ Links ]
Yang S., A. Navrotsky, B. Phillips. (2001). An in situ calorimetric study of the synthesis of FAU zeolite. Microporous and Mesoporous Materials, 46 (2-3) 137-151. DOI: 10.1016/S1387-181 1(01)00268-2. [ Links ]
Zaarour M., Dong B., Naydenova I., Retoux R., Mintova S. (2014). Progress in zeolite synthesis promotes advanced applications. Microporous and Mesoporous Materials, 189, 11-21.DOI: 10.1016/j.micromeso.2013.08.014. [ Links ]
Zhang X., Tang D., Zhang M., Yang R. (2013). Synthesis of NaX zeolite: Influence of crystallization time, temperature and batch molar ratio SiO2/Al2O3 on the particulate properties of zeolite crystals. Powder Technology, 235, 322-328. DOI: 10.1016/j.powtec.2012.10.046. [ Links ]